Abstract
To be activated by cell surface G protein-coupled receptors, heterotrimeric G proteins must localize at the cytoplasmic surface of plasma membranes. Moreover, some G protein subunits are able to traffic reversibly from the plasma membrane to intracellular locations upon activation. This review will highlight new insights into how nascent G protein subunits are assembled and how they arrive at plasma membranes. In addition, recent reports have increased our knowledge of activation-induced trafficking of G proteins. Understanding G protein assembly and trafficking will lead to a greater understanding of novel ways that cells regulate G protein signaling.
Heterotrimeric G proteins, composed of α, β and γ subunits, function to transduce signals from agonist bound heptahelical G protein-coupled receptors (GPCR) to intracellular effector proteins. G protein signaling pathways mediate a vast number of physiological responses, and dysregulation of these pathways contributes to many diseases, including cancer, heart disease, hypertension, endocrine disorders, and blindness (1–5). Extracellular ligands that bind to and activate GPCRs to initiate G protein signaling pathways include small molecule neurotransmitters, peptide hormones, chemokines, lipids, and environmental stimuli such as light, odorants, and tastes. Due to such ubiquitous importance, GPCRs are major targets for pharmaceutical therapeutics.
For G proteins, the accepted mechanism of action is visualized as a continuous cycle of activation and inactivation of the G protein α subunit (Gα). Agonist binding to a GPCR at the extracellular cell surface induces a conformational change in the GPCR that allows it to directly promote GDP release from the inactive Gα, which is in the heterotrimeric (αβγ) complex. Next, GTP binds to Gα, and Gα and the βγ dimer (Gβγ) dissociate giving rise to signaling competent GTP-bound Gα and free Gβγ. With the exception of the β5 subunit (6), β subunits (Gβ) and γ subunits (Gγ) appear to irreversibly associate and exist as βγ dimers, whether as free Gβγ or Gβγ bound to Gα. To complete the G protein cycle, Gα hydrolyzes its bound GTP and then GDP-bound Gα reassociates with Gβγ (1).
To be activated by a cell surface GPCR, G proteins must be located at the intracellular surface of the cell’s plasma membrane (PM). As peripheral membrane associated proteins, G proteins thus require mechanisms that allow tight membrane binding. G proteins undergo covalent modification by several different lipids, myristoylation and/or palmitoylation for Gα and isoprenylation for Gγ (Table 1), and these attached lipids play an essential role in serving as hydrophobic anchors to localize the G protein subunits to membranes (7). However, the cellular pathways by which G protein subunits are assembled and reach the PM after their synthesis are not well understood. Moreover, it has become increasingly clear that G protein localization is dynamic, and activation can promote a reversible redistribution of Gα and Gβγ to discrete membrane subdomains or different regions of the cell. This review will focus on recent work that provides new insight into the cell biology of G proteins, including chaperone proteins that facilitate folding of nascent Gβ and assembly of Gβγ, mechanisms and pathways involved in targeting of nascent Gα and Gβγ to the PM, and activation-induced trafficking of G proteins.
Table 1.
Sites of G protein lipid modifications. The N-terminal sequences of several Gα and the C-terminal sequences of two Gγ are shown. Myristate links through an amide bond to an N-terminal glycine after removal of the initiating methionine as indicated by G. Palmitate attaches via a thioester bond to cysteine (in bold italics) residues near the N-terminus of Gα. γ1 and γ2 are isoprenylated through a thioether bond to a cysteine, indicated by C. After isoprenylation the C-terminal three amino acids are removed (↓), and the new C-terminus is carboxyl methylated. This is a representative listing of G protein subunits. In humans, 16 genes encode Gα (plus additional splice variants), 5 genes encode Gβ (Gβ proteins are not known to be lipid-modified), and 12 genes encode Gγ.
| Gαsubunits | N-termini of αsubunits | Lipid modification |
|---|---|---|
| αi1 | MGCTLSAEDKAAVERSKMID- | Myristoylation,Palmitoylation |
| αo1 | MGCTLSAEERAALERSKAIE- | Myristoylation,Palmitoylation |
| αZ | MGCRQSSEEKEAARRSRRID- | Myristoylation,Palmitoylation |
| αt | MGAGASAEEKHSREL- | Myristoylation |
| αs | MGCLGNSKTEDQRNEEDAQR- | Palmitoylation |
| αq | MTLESIMACCLSEEAKEARR- | Palmitoylation |
| α14 | MAGCCCLSAEEKESQRISAE- | Palmitoylation |
| α16 | MARSLRWRCCPWCLTEDEKA- | Palmitoylation |
| α12 | MSGVVRTLSRCLLPAEAGAR- | Palmitoylation |
| α13 | MADFLPSRSVLSVCFPGCVL- | Palmitoylation |
| Gγsubunits | C-termini of γsubunits | Lipid modification |
| γ1 | -KGIPEDKNPFKELKGGC↓VIS | Farnesylation |
| γ2 | -TPVPASENPFREKKFFC↓AIL | Geranylgeranylation |
SYNTHESIS OF β AND γ, DIMER FORMATION, AND ISOPRENYLATION
Although Gβ and Gγ subunits interact to form the irreversible Gβγ dimer rapidly after synthesis (beginning within 2.5 min) in cultured cells (8), new evidence has demonstrated that this critical process of βγ dimer formation is regulated by proteins that act as chaperones for Gβ. The chaperone protein CCT (chaperonin containing tailless-complex polypeptide 1) is involved in the proper folding of proteins having the seven-bladed β-propeller structure, such as occurs in Gβ, and, using in vitro transcription/translation systems, it has been shown recently that the CCT complex binds newly synthesized Gβ (9, 10). The CCT complex is necessary to prevent the formation of Gβ aggregates (9). Moreover, Gγ does not bind to the CCT complex (10), suggesting that CCT is specifically involved in the folding of Gβ.
Another protein involved in the folding of Gβ is the phosducin-like protein (PhLP1), and it appears that PhLP1 functions in concert with the CCT complex (Figure 1). Previous work suggested that PhLP1 functions to inhibit G protein signaling by binding free Gβγ; however, it is now clear that a major role of PhLP1 is to facilitate the formation of Gβγ by promoting folding of Gβ. In Dictyostelium discoideum cells deficient in the phosducin-like protein PhLP1 or in Hela and HEK 293 cells depleted of PhLP proteins by siRNA, the level of Gβ subunit was strongly reduced and G protein signaling was impaired (11, 12). Conversely, over-expression of wild type PhLP1 increased the quantity of Gβ subunits in cultured cells (12). Nascent GGβ but not Gγ, was shown to interact with PhLP1 by co-immunoprecipitation (12). Previous studies had demonstrated that PhLP1 binds to CCT and regulates the ability of CCT to catalyze the folding of newly synthesized proteins (13, 14), and indeed PhLP1 could be immunoprecipitated in a ternary complex containing CCT and Gβ subunits. Taken together, the above studies suggest that PhLP1 functions as a co-chaperone to facilitate CCT-mediated folding of Gβ.
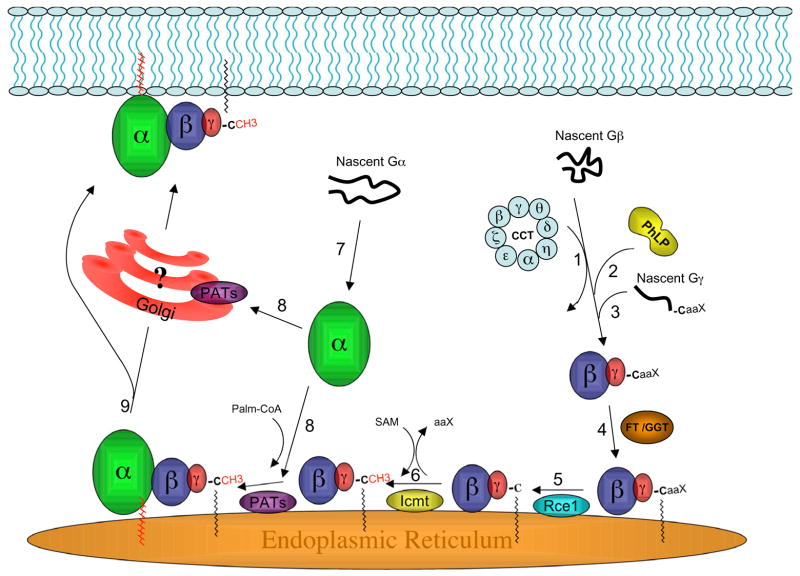
Figure 1. Model of G protein assembly and trafficking to the plasma membrane.
Recent studies have suggested a model for the plasma membrane targeting of nascent G protein subunits. The following key steps are indicated in the figure and further discussed in the text: 1) The CCT chaperone complex binds to nascent Gβ and promotes proper folding. 2) PhLP1 binds to form a ternary complex, and then PhLP1 phosphorylation stimulates the release of PhLP1-Gβ. 3) Nascent Gγ then binds to form PhLP1-Gβγ. It is not clear when and where PhLP1 and Gβγ dissociate. In addition, proteins such as DRiP78 may facilitate folding of Gγ. 4) Gγ is farnesylated or geranylgeranylated by a cytoplasmic farnesyl transferase or geranylgeranyl transferase (FT/GGT), and then Gβγ is targeted to the cytoplasmic surface of the ER. 5) An ER-localized protease (Rce or ras converting enzyme) specifically cleaves the C-terminal three amino acids (i.e, the –aaX of the CaaX motif) from isoprenylated Gγ. 6) The new isoprenylcysteine carboxyl terminus of Gγ is methylated by a specific ER-localized carboxyl methyl transferase (Icmt). 7) Similar to Gβ, chaperone proteins may exist to promote proper folding and membrane targeting of Gα. Ric-8, or similar proteins, has been speculated to play such a role for Gα; however, this remains to be defined. 8) Unanswered questions include: exactly where in the cell Gα and Gβγ interact to form the heterotrimer and where Gα is palmitoylated. Specific palmitoyl acyl transferases (PATs) have not been clearly defined for Gα, although many PATs are localized at Golgi or ER membranes. Based on several studies (see text), we speculate that the ER is a site of heterotrimer formation and Gα palmitoylation. 9) Lastly, in this model the newly formed heterotrimer moves from an intracellular location to the PM. The details of this process are mostly unknown. Some evidence supports a Golgi-independent pathway, but a role for the Golgi cannot be ruled out.
An analysis of the role of phosphorylation of PhLP1 in Gβγ assembly has provided some mechanistic insight (15). Mutation of N-terminal casein kinase 2 (CK2) phosphorylation sites in PhLP1 prevented PhLP1 from promoting the expression of Gβγ in cells (12, 16); however, expression of the mutant PhLP1 strongly increased binding of nascent Gβ to CCT (15). Using a pulse chase analysis, it was observed that expression of wt PhLP1, but not CK2 phosphorylation deficient PhLP1, stimulated the release of nascent Gβ from CCT. Lukov, et al. have presented the most complete model to date for the assembly of Gβγ (15). The key steps in the model are: 1) Newly synthesized Gβ cannot fold properly on its own and thus binds the chaperone CCT; 2) PhLP1 binds forming a ternary complex; 3) Phosphorylation of PhLP1, likely by CK2, releases a PhLP1-Gβ complex in which the Gβ subunit is now properly folded into its seven-bladed propeller structure; and 4) The Gγ subunit then binds to form PhLP1-Gβγ. How and when Gβγ is dissociated from PhLP1 is unclear. It’s possible that Gβγ binding to a membrane surface, likely the ER, and/or binding to Gα promotes PhLP1 release, since the PhLP1 binding site on Gβγ overlaps with both a basic membrane association region and the Gα-binding surface (15,17). Although aspects of this model need to be confirmed and refined, these novel demonstrations that Gβγ formation is a regulated process (9–12, 15, 16) add a new layer of complexity for understanding the regulation of G protein signaling. Particularly intriguing is the speculation that cells may regulate phosphorylation of PhLP1 to control levels of G protein Gβγ subunits under varying circumstances.
Another key question is to understand where in the cell newly synthesized Gβ, likely as a PhLP1-Gβcomplex, interacts with Gγ and whether Gβγ dimer formation precedes or follows isoprenylation of Gγ. Previous work with purified subunits showed that Gβ could bind to either isoprenylated or non-processed Gγ, although there was a preference for non-processed Gγ (18). In addition, pulse-chase studies in cultured cells are consistent with Gβγ formation in the cytoplasm, i.e., before attachment of the hydrophobic isoprenyl group (8). Consistent with this, when PhLP1 is depleted in Dictyostelium discoideum, Gγ is not isoprenylated, as measured indirectly by a detergent partitioning assay, suggesting that Gγ isoprenylation is dependent on PhLP1-mediated Gβγ formation (11). Moreover, Gβ protein levels are decreased when Gγ protein levels are reduced by depletion of TCP-1 (tailless complex polypeptide-1), one of the CCT subunits, or by expression of dominant negative phosphorylation-deficient PhLP1 (15, 16). These results indicate that the formation of the Gβγ dimer stabilizes the Gγ protein. Thus, most studies support a model in which nascent Gβγ is assembled in the cytoplasm, and subsequently the Gγ subunit of the Gβγ dimer is isoprenylated.
Does Gγ, like Gβ, also require auxiliary proteins for its proper folding and stability? Because Gγ is a small protein (~70 amino acids) consisting mostly of two helices, it may not require as much help as Gβ to attain its proper structure. However, a recent report provided the first evidence that a chaperone protein exists for Gγ (19). It was found that an ER-resident protein termed DRiP78 interacts with Gγ, and Gβ can compete with DRiP78 for binding to Gγ. Furthermore, siRNA-mediated knockdown of DRiP78 resulted in reduced protein levels of overexpressed γ2 or γ3 in HEK 293 cells. Intriguingly, DRiP78 also interacted with PhLP, as analyzed by BRET studies with overexpressed proteins, suggesting the possibility that DRiP78 and PhLP could coordinate in the formation of Gβγ dimers. It will be important in future studies to look more closely at the role of DRiP78 or similar proteins in the stability of endogenous Gγ. In addition, the ER location of DRiP78 suggests that Gβγ assembly could take place at the ER rather than the cytoplasm.
Processing of Gγ involves not only lipid modification, but also subsequent modification of the isoprenylated C-terminus. Gγ subunits are members of a specific set of proteins in eukaryotic cells that contain covalently attached C-terminal isoprenyl groups (15-carbon farnesyl or 20-carbon geranylgeranyl). The protein isoprenyl transferases, which attach isoprenyl groups to proteins, have been well characterized (20). A carboxyl-terminal CaaX motif (where C= cysteine, a=aliphatic amino acid, and X= any amino acid) is a feature common to Gγ subunits and other known isoprenylated proteins such as Ras (21, 22). The X residue specifies which isoprenyl group will be linked to the cysteine via a thioether bond. Among 12 human Gγ, γ1, γ9 and γ11 have serine in the X position and are farnesylated, and the rest of them have leucine and are modified with a geranylgeranyl group (Table 1). This process occurs in the cytoplasm as has been shown for other isoprenylated proteins like Ras (23). Isoprenylated proteins are then targeted to the cytoplasmic surface of the ER, through an unknown mechanism, for further processing (Figure 1). Mutation studies have shown that isoprenyl modification at the C-terminal CaaX motif is a prerequisite for ER targeting (22, 24–26). At the ER the –aaX is cleaved by a protease called Ras Converting Enzyme-1 (RCE-1) (27, 28). Upon removal of the C-terminal three amino acids (i.e., -aaX), the C-terminal isoprenylated cysteine is methylated by the isoprenyl cysteine carboxyl methyl transferase (Icmt) (28, 29). Although the isoprenyl group provides a hydrophobic membrane anchor, the physiological role for the –aaX proteolysis and carboxyl methylation is not clear. However, for proteins that are modified by the less hydrophobic farnesyl rather than geranylgeranyl, the carboxyl methylation appears to provide an added hydrophobicity that is important for efficient membrane binding. Consistent with this, farnesylated Gγ failed to localize to membranes when overexpressed in cells lacking Icmt, while geranylgeranylated Gγ were not affected in their ability to localize to endomembranes (24).
SYNTHESIS OF α SUBUNITS AND LIPID MODIFICATION
In contrast to the strong evidence implicating CCT and PhLP1 in folding and assembly of Gβγ, less is known regarding potential chaperones for Gα subunits. Pulse-chase experiments in cells indicate that newly synthesized Gα are produced on free ribosomes in the cytoplasm (8, 30), but whether additional proteins promote folding and/or eventual interaction with Gβγ is not clear. However, such a role has been speculated recently for a protein called Ric-8, which has been demonstrated to function as a non-GPCR guanine-nucleotide exchange factor (GEF) for certain Gα (31). Recently, it has become clear that G proteins are involved in regulating asymmetric cell division, and three parallel studies showed that loss-of-function Ric-8 mutants in Drosophila caused defects in gastrulation, neuroblast differentiation, spindle orientation and asymmetric division (32–34). The surprising finding from these studies was that αi, αo and Gβ were mislocalized in the Ric-8 mutant; instead of localizing to plasma membranes as in wild type Drosophila cells, αi, αo and Gβ were found in the cytoplasm. A diminution of the amount of αi as well as the Drosophila Gβ, β13F, was also detected by western blot and immunofluorescence in Ric-8 mutants compared to wild type cells. Moreover, β13F did not interact with αi in Ric-8 mutant cells. These results argue that Ric-8 is somehow involved in the targeting of αi, αo and Gβ to plasma membranes (32–34), and it was speculated that Ric-8 might function as a chaperone that binds αi and promotes assembly of a G protein heterotrimer (33). Since Drosophila Gβ and Ric-8 did not interact with each other in these studies and mammalian Ric-8 did not interact with mammalian Gβγ (31), the mis-localization of Gβ in the Ric8 mutant is likely a consequence of the unavailability of αi and αo to interact with Gβγ (32–34); as discussed below in this review Gα and Gβγ require interaction with each other for proper plasma membrane targeting. An additional study in C. elegans showed that Ric-8 was required for cortical localization of one Gα, GPA-16, but not for another one termed GOA-1 (35). Further elucidation of the role of Ric-8 or similar proteins in assembly and plasma membrane localization of G proteins is eagerly awaited. It is tempting to speculate that all Gα require interaction with specific proteins that would facilitate folding, heterotrimer assembly, and plasma membrane targeting.
The critical membrane binding determinant for Gα is lipid modification by myristoylation and/or palmitoylation (Table 1). Myristoylation only occurs on Gα of the αi family, including αi, αo, αz, and αt subunits. Myristoylation, attachment of the 14-carbon fatty acid myristate to a glycine at the free N-terminus, is catalyzed by N-myristoyl transferase (NMT). The glycine at position 2 becomes the extreme N-terminal residue after removal of the initiating methionine. In addition to the key glycine, other N-terminal residues are important for recognition by NMT; particularly important is a serine or threonine at position 6 (36). Myristoylation is an irreversible co-translational modification, and thus constitutes the earliest event that promotes membrane targeting for Gα of the αi family.
Palmitoylation, on the other hand, is a reversible post-translational modification occurring on all Gα, with the exception of αt. The 16-carbon fatty acid palmitate is attached via a thioester bond to one or more cysteine residues within the N-terminal 20 amino acids (Table 1). The mechanisms of palmitoylation have long been unclear and controversial in terms of much debate as to whether palmitoylation is an enzymatic or non-enzymatic reaction. However, numerous reports over the last several years have identified a family of palmitoyl acyltransferases (PATs). These enzymes were initially identified in and purified from yeast. One called Erf2 palmitoylates Ras in vitro (37) and another called Akr1 palmitoylates the casein kinase Yck2 (38). This family of PATs are also termed DHHC proteins because they all contain a conserved Asp-His-His-Cys motif (for review see (39)). Seven DHHC genes have been identified in yeast and at least twenty-two exist in the human genome, but their physiological and pathological importance have not been well described. Defining the palmitoylated proteins that are substrates for specific DHHC PATs is the focus of current studies in this field. In terms of specificity for Gα, a recent study showed that overexpressed DHHC-3 and 7 could enhance palmitoylation of co-expressed αs (40), whereas another report showed that DHHC-9/GCP16, which shows specificity for H-Ras and N-Ras, had no PAT activity for αi (41). As mentioned above, palmitoylation is reversible, and an acyl-protein thioesterase (APT1) that can depalmitoylate Gα has been identified (42, 43). APT1 was able to depalmitoylate both αi and αs in purified preparations, and co-expression of APT1 with αs in HEK293 cells resulted in a faster turnover of palmitate on αs compared to αs in the absence of APT1 co-expression (43). Furthermore, an APT1 was identified in S. cerevisiae and shown to be responsible for virtually all of the depalmitoylating activity in yeast. Accordingly, yeast mutants lacking the APT1 gene failed to depalmitoylate the yeast Gα, Gpa1. Unfortunately, APT1 deletion strains showed no defect in the G protein-mediated pheromone response. Thus, a clear physiological role for depalmitoylation remains to be defined (42). Lastly, a novel site and type of palmitoylation of αs has been detected by mass spectrometry. In this study, palmitate was attached to the N-terminal glycine via a stable amide bond but the role of this modification is unknown (44).
PLASMA MEMBRANE TARGETING OF G PROTEINS
As described above, both Gα and Gγare covalently modified by lipids, and these modifications are essential for membrane targeting. However, our current understanding suggests that PM targeting of G proteins is a complex process requiring assembly of the heterotrimer, specific trafficking pathways, and additional potential membrane binding motifs in the G protein subunits.
One lipid modification alone may not provide enough energy to keep a protein anchored to a cellular membrane, and a lipid modification often occurs in conjunction with another membrane targeting signal (45–48). In this two-signal model, the other signal can be a second lipid modification, an interaction with a plasma membrane protein or a polybasic motif in the sequence of the targeted protein (49). For example, in αi, αo, and αz, two membrane targeting signals are myristoylation and palmitoylation; co-translational myristoylation is considered the first signal while palmitoylation is the second signal (7). Moreover, the two-signal model definition can be extended to include the idea that more than two membrane targeting signals can function together.
The interaction of Gα with Gβγ, i.e., formation of the heterotrimer, appears to function as a key additional signal for PM targeting. Several recent studies, discussed below, using expression of G protein subunits in cultured cells or genetic deletion of select subunits are consistent with a model in which Gα or Gβγ alone are not properly targeted to the PM but instead require interaction with each other. First, overexpression of Gα or Gβγ in cultured cells often results in inefficient PM localization of the individual subunits, but co-expression of Gα and Gβγ leads to very strong PM localization of both Gα and Gβγ. Although it had been well documented that co-expression of Gβγ could increase the amount of membrane-bound Gα in various expression systems, including insect cells (50), more recent observations indicated that, in a reciprocal manner, Gαwas necessary for efficient membrane targeting of Gβγ. This was demonstrated by showing that overexpression of several different combinations of Gβ and Gγ followed by detection by fluorescence microscopy resulted in weak localization of the Gβγ at the PM and an accumulation of the majority of the Gβγ at intracellular structures, predominantly ER (24, 26, 51). In contrast, co-expression of αs, αq or αi resulted in strong PM localization of different Gβγ (24, 26, 51). A second line of evidence demonstrating a reciprocal role for Gα and Gβγ in the subcellular localization of each other is that when one subunit, either Gα or Gβγ, is intentionally mistargeted the other subunit also mislocalizes. When β1γ2 was targeted to the cytoplasmic surface of mitochondria via a mitochondria targeting signal fused to γ2, co-expressed wild type αz was also found localized to mitochondria (52). Using an identical strategy, αs was targeted to mitochondria, and co-expressed wild type β1γ2 could be recruited to that organelle (26).
Third, a role for heterotrimer formation in PM targeting of Gα and Gβγ has been shown through the generation of mutant subunits that are defective in binding to their partner. When a mutant Gβγ in which the Gβ subunit contains several mutations that disrupt binding to Gα was expressed in HEK293 cells, this Gα binding-defective Gβγ remained at endomembranes even when a Gα was co-expressed (26). Similarly, Gα mutants containing mutations in N-terminal residues that contact Gβfail to localize to the PM when expressed in cells. Such Gβγ binding-defective mutants of αs and αq appeared to be predominantly cytoplasmic (53), whereas a Gβγ binding-defective αz was localized to endomembranes (52). This difference in localization of Gβγ binding-defective αz versus Gβγ binding-defective αs and αq is likely due to co-translational myristoylation of αz (Table 1). Moreover, there appears to be cooperation between Gα palmitoylation and interaction with Gβγ for proper PM targeting of G proteins. Gβγ binding-defective αs and αq are poorly palmitoylated (53), and palmitoylation site mutants of several Gα fail to promote PM localization of co-expressed Gβγ (26). In other words, palmitoylation of Gα is necessary for PM localization of the heterotrimer, and, on the other hand, interaction with Gβγ is necessary for palmitoylation of Gα.
Lastly, and possibly most compelling, genetic deletion of Gα or Gβγ in model organisms confirm the reciprocal role of Gα or Gβγ for proper PM localization of each subunit. In the yeast S. cerevisiae, Gβγ is not able to properly localize at the PM in a null mutant for the yeast Gα, Gpa1 (54). When two critical Gα, GOA-1 and GPR-16, were disrupted by RNAi in C. elegans embryos, the Gβ GPB-1 failed to localize at the PM but instead was detected intracellularly (55). Similar results were also obtained using a third model system – the Drosophila eye. αq, which mediates light-dependent signaling of rhodopsin in Drosophila photoreceptors, is predominantly found in a membrane fraction; however, in fractions prepared from a mutant that expresses very low amounts of an eye-specific Gβ, αq shows a substantial shift into a cytosolic fraction (56, 57). Conversely, Drosophila eye-specific Gβ shifts from being equally distributed between a membrane and cytosolic fraction in wild type flies to being 80% in the cytosolic fraction in mutant flies having negligible amounts of αq (57). Taken together, an abundance of recent data supports a model in which a key determinant of PM localization of G proteins is the proper formation of the heterotrimer. As discussed later in this review, these results beg the question of where in the cell Gα and Gβγ interact since this model seems incompatible with heterotrimer formation after the subunits reach the PM. Instead, the above results suggest that Gα and Gβγ would interact before reaching the PM.
Polybasic stretches of amino acids can also act as an additional signal in conjunction with lipid modifications to promote membrane targeting in a multitude of proteins (46, 47, 58). In the case of Gβγ, while membrane association is due primarily to the attachment of the isoprenyl group on the Gγ subunit, structural studies of transducin Gβγ (β1γ1) indicate that there is a region of positive electrostatic potential on a surface of β1 that surrounds the site of farnesylation (59). It has been shown that β1γ1 binds more strongly to vesicles formed from the acidic lipid phosphatidylserine (PS) than to vesicles formed from the neutral lipid phosphatidylcholine (PC) (60, 61), supporting a role for ionic interactions in membrane association of β1γ1. When calculations of electrostatic interactions between β1γ1 and 2:1 PC/PS phospholipid membranes were made, it was found that electrostatic interactions are predicted to enhance the membrane partitioning of β1γ1 by about an order of magnitude (62). This is not as strong as the electrostatic contribution of other basic proteins (58, 63, 64), but still significant. The residues that form the basic surface patch on β1 are conserved in β2, β3, and β4, suggesting that the electrostatic contribution is a common feature among these Gβ as well. When the sequences of Gβ isoforms from a wide range of species were examined through homology modeling, prominent basic surface patches were found in sequences that possessed at least fifty percent identity to β1 (62). Taken together, the demonstrated electrostatic attraction between β1γ1 and PC/PS membranes combined with the conservation of basic residues among other Gβ isoforms and across related species strongly suggests that this polybasic patch plays a role in regulating the membrane association of Gβγ. We can speculate that the positive patch on Gβ may have a greater influence on membrane binding of Gβγ combinations in which the Gγ is farnesylated, i.e., γ1, γ9, and γ11, compared to Gβγ containing a more hydrophobic geranylgeranylated Gγ.
In the case of Gα, a polybasic motif can be detected in the N-termini of nonmyristoylated subunits through homology modeling and electrostatic surface maps (65). Helical wheel diagrams show that this motif maps to one face of the helix at the opposite side of the residues contacting Gβγ. Consequently these positively charged residues are free to interact with the negatively charged interface of the plasma membrane whether or not Gα is bound to Gβγ. Indeed, substitution of N-terminal basic residues results in decreased membrane localization of αs and αq (M.C. and P.W, unpublished results). Also, given that this positively charged motif is much less pronounced in members of the myristoylated αi family, it was proposed that the polybasic motif substitutes for myristoylation as a membrane targeting signal in the non-myristoylated Gα (65). These results, combined with the fact that other proteins, such as growth-associated protein-43 (GAP43) and RGS proteins (66–69), that undergo palmitoylation also have α-helical polybasic motifs in their N-termini, suggest the likelihood that this motif serves as a membrane targeting signal for Gα. Based on the above evidence, the two-signal model for G proteins can be extended to state that for αi family members myristoylation is the first membrane targeting signal and interactions with Gβγ and palmitoylation are critical additional signals. For the non-myristoylated Gα (Table 1), Gβγ binding and the polybasic region of the N-terminus can be considered first signals with palmitoylation comprising the critical second signal. On the other hand, for Gβγ isoprenylation likely functions as the first membrane targeting signal with polybasic surfaces in Gβ and interaction with Gα as key second signals.
In summary, it is clear that G protein localization at the PM is a complex process, requiring lipid modifications, polybasic motifs, and chaperone proteins. Moreover, a number of recent studies suggest a model in which the heterotrimer is assembled before the subunits reach the PM.
ROLE OF ORGANELLES IN G PROTEIN TRAFFICKING
It has become increasingly clear that G proteins are not simply directly transferred to the PM after synthesis in the cytoplasm, but instead utilize intracellular organelles as intermediates in trafficking. A strong indication of this is the organelle localization of enzymes involved in lipid modification and processing of Gα and Gβγ. As mentioned above, after Gγ is isoprenylated, likely as a Gβγ dimer, by cytoplasmic isoprenyl transferases, further CaaX processing of –aaX proteolysis and carboxyl methylation is carried out by ER-localized enzymes. Thus, Gβγ localize to the cytoplasmic surface of the ER as a key step in their eventual targeting to the PM (Figure 1).
Intracellular organelles may also be sites of palmitoylation of Gα and assembly of the heterotrimer, two interrelated processes. Although a specific DHHC enzyme that serves as a PAT for any Gα has not been clearly identified yet, a number of studies are consistent with a model for intracellular organelle palmitoylation of Gα. Localization studies of all members of the DHHC family indicate that most of the DHHC proteins are found at intracellular organelles, mainly the ER and Golgi, at least when overexpressed (70). DHHC PATs have been identified for yeast and mammalian Ras, and they localize to the ER and Golgi, respectively (37, 41). Lastly, DHHC-3 can enhance palmitoylation of αs when co-expressed, and overexpressed DHHC-3 is found predominantly at the Golgi (70, 71). On the other hand, PM-enriched fractions contain a PAT activity that can palmitoylate certain Gα (72), suggesting that a relevant Gα PAT may exist at the PM. Identifying specific PATs for Gα and determining where they are localized and how they influence PM targeting are current challenges that will lead to great insight into G protein trafficking.
The assembly of the G protein heterotrimer has been proposed to occur on intracellular organelles. The reciprocal requirement for Gα and Gβγ interaction for PM targeting is most consistent with a model in which assembly occurs prior to the subunits reaching the PM. Based on the colocalization of a palmitoylation site mutant of Gα with Gβγ in a region of cells containing the Golgi, it was proposed that Gα and Gβγ first interact together at this organelle and that the Golgi would be the site of palmitoylation of Gα (24). On the other hand, the expression of a dominant negative mutant of Sar1, a small GTPase involved in ER to Golgi trafficking, or the treatment of cells with the Golgi disruptor Brefeldin A perturbed neither the PM targeting of αs, αq and αz nor the palmitoylation of αq, αi and αz (30, 73, 74). Moreover, a recent report did not detect Golgi localization of either a non-palmitoylated αs or αq mutant or of co-expressed Gβγ (73). Thus, the lack of requirement for an intact Golgi and the observed lack of Gα and Gβγ localization at the Golgi in some studies (30, 73, 74) suggest an alternative trafficking pathway that does not require the Golgi. Although ER-restricted palmitoylation of Gα and assembly of the heterotrimer has not been demonstrated, we propose the ER as a likely site (Figure 1). A recent report provided a possible explanation to some of discrepancies of whether or not the Golgi is required for G protein trafficking to the PM. The authors showed that overexpressed Gα and Gβγ displayed Golgi-independent PM localization; however, when a GPCR was also overexpressed, PM targeting of overexpressed Gα and Gβγ was Golgi-dependent (75). The implication was that when Gα and Gβγ assembled into an intracellular complex with a GPCR, the complex followed the typical secretory pathway, as would be expected for a transmembrane GPCR. Thus, G proteins may utilize different trafficking pathways depending upon additional proteins that might pre-form signaling complexes. An alternative explanation for the requirement for organelles, such as ER and potentially Golgi, in the PM targeting of G proteins is that these organelles are not required to move G proteins along the typical secretory pathway, but instead the importance of the organelles is that they are sites for critical enzymes such as DHHC PATs. In this scenario, if a key DHHC PAT was localized to the Golgi, secretory pathway disruptors would not affect the ability of a Gα to be palmitoylated because the DHHC PAT may dynamically redistribute to other organelles that can be accessed by Gα.
Recently, a protein called Gα-Interacting Vesicle (GIV) associated protein was identified in GC pituitary cells. As shown using a pull down assay, this GIV interacts via its C terminus with αi3. GIV interacts also with other members of the αi family and with αs. Using fluorescent microscopy, GIV has been detected in vesicles in the cytoplasm and enriched in COPI vesicles co-localizing with αi; GIV was also associated with the cis Golgi region and detected close to the ER. More precisely, GIV has been detected in a specific fraction of liver extract enriched in ER/Golgi transport vesicles. Interestingly it interacts weakly with a GTP-bound form of αs, suggesting that GIV would be a partner of the inactive GDP-bound αs. Based on these results, the authors proposed that αs would regulate vesicle trafficking through its interaction with GIV (76), but it is also possible that, conversely, GIV would be involved in the trafficking of inactive αs. Experiments taking advantage of advances in live cell imaging may help define G protein trafficking pathways. In addition, most of the studies to date have used overexpression of proteins, and much more difficult studies of endogenous proteins are required to increase our understanding of organelle involvement in G protein trafficking to the PM. Figure 1 presents a model showing our current state of knowledge regarding the trafficking of nascent G proteins to the PM.
PLASMA MEMBRANE DISTRIBUTION OF THE THREE SUBUNITS
Another level of organization in the localization of G proteins is their organization into cell surface microdomains termed lipid rafts. Due to the enrichment in cholesterol and glycosphingolipids of these membrane domains, they resist solubilization by non-ionic detergent and display low-buoyant density in sucrose density gradients (77). Caveolae are a subset of these domains, and they are defined morphologically by flask-shaped invaginations of the plasma membrane and biochemically by the residence of caveolin (78–80).
There is substantial evidence that Gα localize to lipid raft and caveolae membrane microdomains (81). Numerous studies using immunofluorescence microscopy, electron microscopy, and density gradient fractionation have demonstrated that various Gα, namely αi, αs, and αq, are significantly enriched in lipid rafts and caveolae in several cell lines and tissues (82–87). Dual acyl chains, composed of either tandem palmitoylation or myristoylation plus palmitoylation, are well characterized signals for targeting proteins to these microdomains (88–90), and thus lipid modifications play a critical role in localizing Gα to membrane microdomains (91, 92). In addition, lipid rafts contain a PAT activity capable of palmitoylating αi (93). Another key mode of targeting Gα to microdomains may be interactions with other microdomain-associated proteins; in particular, interaction with caveolin could allow differential targeting of Gα to caveolae versus caveolin-lacking lipid rafts. Interaction of Gα with caveolin has been demonstrated (92, 94, 95), but others have not observed direct binding of Gα to caveolin (83, 94). Overall, while the existence of an interaction between Gα and caveolin seems debatable, it appears clear that at least a portion of Gα localize in caveolae and lipid rafts.
On the other hand, the situation with Gβγ is less clear. Gβγ has been found present but not enriched in microdomains with techniques that utilize triton X-100 extraction (8, 85, 94, 96), but Gβγ has been found in these domains to a greater extent using detergent-free methods (86). In contrast, purified Gβγ did not incorporate into detergent resistant membranes (DRM), i.e., lipid rafts, of reconstituted sphingolipid- and cholesterol-rich liposomes (91). Another study found that in reconstituted PS/SM/Ch (phosphatidyl serine/sphingomyelin/cholesterol) membranes, tryptophans and tyrosines of Gβγ are less accessible to quenching by iodide ions over a large concentration range compared to when Gβγ is bound to PS/PC (phosphatidyl serine/phosphatidyl choline) membranes. This indicates that the presence of lipid rafts protects a portion of tryptophan and tyrosine residues from anionic quenchers. Moreover, when Gβγ is bound to PS/SM/Ch membranes, it is more susceptible to trypsin digestion than when it is bound to PS/PC membranes, implicating a difference in the disposition of Gβγ when it is bound to membranes containing lipid rafts. It was speculated that while excluded from lipid rafts, Gβγ may localize close to the domain interface, which occludes a portion of the protein (97). The reason for the differences in lipid raft localization of Gβγ in extracts from cells versus reconstitution approaches is unclear; it is possible that Gβγ may transiently associate with lipid rafts or have a weaker association with them than Gα does (91).
Localization of G proteins to membrane microdomains may serve as a mode of regulation of G protein signaling pathways. The colocalization of G proteins, GPCRs, and effectors in microdomains may enhance signaling by increasing their local concentration and promoting interactions among them (98–100). If this were the case for a particular signaling pathway, then disruption of lipid rafts and caveolae would disrupt signaling of the pathway. For example, in platelets, thrombin stimulation causes a translocation of αq to lipid rafts, and cyclodextrin (CD) treatment, which depletes cholesterol, impairs this recruitment of αq to rafts along with the production of phosphatidylinositol second messengers and consequent activation of platelets (101). CD treatment also leads to a loss of PIP2 and αq from caveolin-enriched membrane fractions in A431 cells (102). This redistribution is associated with a decrease in EGF- or bradykinin-stimulated inositol phosphate production. On the other hand, cholesterol depletion had no effect on bradykinin-stimulated phospholipase A2 activation (103). This suggests that the effects of cholesterol depletion may differ among signaling pathways, and microdomains may serve to modulate signaling specificity.
Alternatively, membrane microdomains may serve to negatively regulate signaling. In this model, caveolae and lipid rafts would sequester G proteins from other proteins of the pathway that are not present in these microdomains, thus inhibiting interactions among components. For example, recent results using reconstitution of purified proteins suggest that lipid rafts could inhibit association of PLCβ2 and Gβγ and the subsequent activation of PLCβ2 (97). In rat salivary epithelial A5 cells, isoproterenol-stimulated cAMP accumulation was slightly but significantly increased when cells were depleted of cholesterol via treatment with CD (104). In cardiac myocytes, the β2-adrenergic receptor (β2AR) and αi are found in caveolae, while the majority of the β1AR and αs are not (105). Filipin treatment, another technique for cholesterol depletion, increases β2AR physiological signaling and has no affect on β1AR signaling (106). Treatment with pertussis toxin, which inactivates αi, in conjunction with filipin caused an even greater increase in β2AR signaling, implying that the filipin-induced increase is due to enhanced activation of αs and not a decrease in interaction with αi in caveolae. These results could imply that lipid rafts serve as a mode of inhibiting interactions of proteins in the αs signaling pathway. An alternative explanation is that since lipid rafts may facilitate internalization, then disruption of lipid rafts would lead to increased receptors, αs, and adenylyl cyclase at the membrane. This is another possible mechanism of regulation of G protein signaling by membrane microdomains (98–100, 106).
In conclusion, it seems clear that at least some portion of G proteins are targeted to membrane microdomains, and the degree of enrichment in lipid rafts and/or caveolae may vary among G protein families and among cell types. This localization can serve as a means of modulating signaling specificity and may either positively or negatively regulate interactions among G proteins, receptors, and effectors, at least for some signaling pathways.
G PROTEIN TRAFFICKING AFTER RECEPTOR STIMULATION
Once G proteins reach the PM, they do not necessarily statically reside there. Instead, similarly to the well-studied internalization of GPCRs, G proteins, at least select subunits, can undergo activation-dependent translocation from the PM to the cytoplasm followed by recycling back to the PM (Figure 2). The trafficking of G proteins after receptor activation has been mostly based on observations of αs redistribution after agonist stimulation of the β2-AR. However, new reports have described receptor-dependent trafficking of αq and Gβγ as well as provided new data about αs trafficking, and these new findings are highlighted below. Additionally, the vertebrate photoreceptor-specific G protein transducin, consisting of αt and β1γ1, has been well-documented to translocate off of membranes upon activation; this has been well-reviewed recently (107) and will be only briefly discussed in this section.
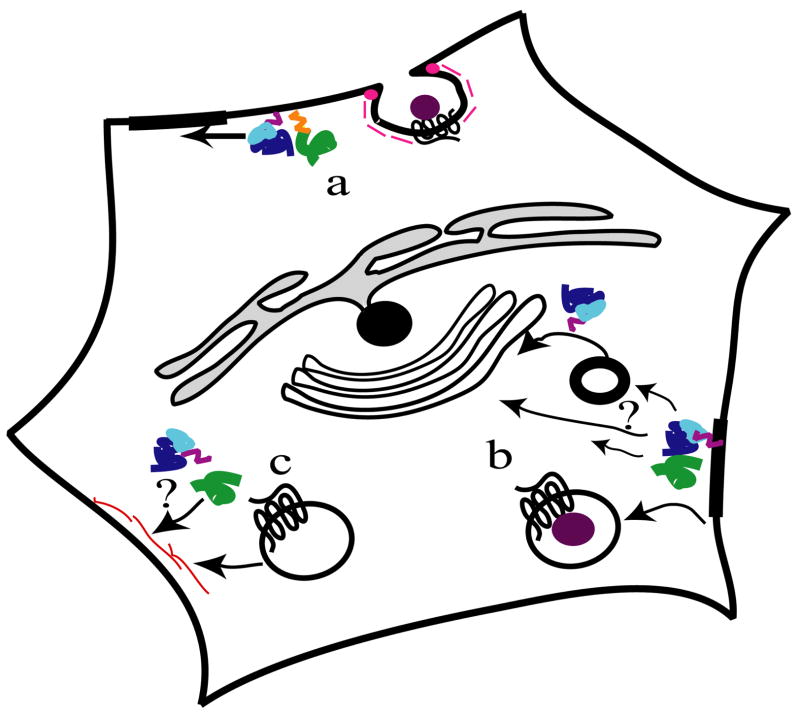
Figure 2. Model for agonist-induced trafficking of GPCRs and G proteins.
a. Following the addition of the agonist, a typical GPCR, e.g., β2-AR, internalizes following a classical endocytic pathway depending on dynamin and clathrin (pink). αs (green) is enriched in rafts (thick line) and its palmitoylation state is modified (orange). b. αs internalizes (in some cases αq, too). This internalization is dependant on cholesterol, a lipid raft constituent (82). The location of αs after internalization is not clear, maybe diffuse in the cytoplasm or accumulating in small vesicles containing raft markers. GPCR is detected in endosomes. β and γ subunits (blue) also can internalize and would accumulate in vesicles or close to the Golgi. c. The removal of the agonist induces the recycling of GPCR and also α, β and γ subunits to PM. For β2-AR and a number of other GPCRs, the recycling follows an actin-dependent pathway (red). αq recycling is dependent on an actin-dependant molecular motor myosin III Ninac and Gβγ in Drosophila photoreceptor cells (56, 115).
Activation of αs by mutational activation, cholera toxin-mediated ADP-ribosylation, or agonist-stimulated β2-AR causes αs to translocate off of the PM, as assayed by fractionation, immunofluorescence microscopy and live cell imaging (82, 108–113). Most studies agree that the β2-AR-dependent release of αs from the PM is fairly rapid, observable within 1–5 min after agonist addition (82, 111–113), and, in addition, β2-AR and αs show a similar time course of internalization (111, 112). The exact subcellular location of internalized αs remains to be well-defined. Mutationally activated αs appears diffusely localized throughout the cytoplasm by immunofluorescence and is found predominantly in a soluble cytosolic fraction (109, 110, 112, 114). β2-AR-activated αs also appears by immunofluorescence to be somewhat diffusely localized throughout the cytoplasm (110, 112), but fractionation studies reveal only a very small increase in soluble αs upon agonist stimulation (112, 113). A possible explanation for the lack of soluble αs after receptor activation is that the internalized αs is actually bound to membrane vesicles; indeed recent studies using live cell imaging of αs-GFP or αs-CFP indicate that internalized αs is, at least partially, localized to vesicle structures (82, 111, 113).
Compared to αs, there has been much less evidence for activation-induced internalization of other Gα. However, two recent reports provide strong evidence for αq redistribution in Drosophila eyes (56, 115). Using subcellular fractionation and electron microscopy, αq was shown to internalize when rhodopsin is activated with light (56, 115). Consistent with demonstrations of αq translocation in Drosophila eyes, earlier reports showed that endogenous αq/11 was also detected inside cultured cells when the angiotensin II receptor or thyrotropin-releasing hormone receptor-1 were stimulated with their respective ligands (116–118). In the Drosophila studies and the study with the angiotensin II receptor in HEK293 cells, αq/11 redistribution into the cytoplasm occurred in 5–20 min, on a similar timescale as for αs described above (56, 115, 117). On the other hand, αq/11 translocation in response to agonist activation of thyrotropin-releasing hormone receptor-1 was much slower, requiring more than 2 hours of stimulation, and showed an intracellular punctate pattern of internalization (116). Small shifts into the cytoplasm and soluble fractions have also been observed for mutationally activated αq (110, 119). Clearly, at least under some conditions, αq, in addition to αs, can redistribute from the PM upon activation.
Interestingly, Gβγ also appears to be capable of receptor-dependent redistribution from the PM, as revealed by recent live cell imaging studies. When β1γ7 was visualized with the technique of bimolecular fluorescence complementation (BiFC) and was co-expressed with αs-CFP, β1γ7 showed a similar co-internalization with αs into apparent vesicle structures upon β2-AR stimulation (111). In another series of recent studies in which either Gβ or Gγ was fused to YFP, β1γ11 rapidly (< 20 seconds) translocated from the PM to a Golgi region of CHO cells upon agonist stimulation of M2 muscarinic acetylcholine receptors (120). β1γ5 showed significantly less and slower translocation (120, 121). γ5 is geranylgeranylated, whereas γ11 contains a less hydrophobic farnesyl group. However, this only partially explained the difference in translocation between β1γ11 and β1γ5; the authors provided evidence to support a model in which a high affinity interaction of the γ subunit with a GPCR inhibits Gβγ translocation, but a lower affinity Gγ-GPCR interaction allows rapid translocation of Gβγ (121). In addition, the same group showed that co-expression of β1γ11 with αs, αq, αo, or αi allowed different GPCRs to stimulate PM to Golgi translocation of γ1γ11 (122). The kinetics of the internalization of β1γ11 depended on which Gα was co-expressed and correlated with the rate of GTP/GDP exchange for the particular Gα (122). The recent studies described above suggest that Gβγ can redistribute in the cell in response to GPCR activation. Additional studies should help clarify a number of important questions, such as whether Gβγ translocates to the Golgi (120–122) or other subcellular locations (111).
The mechanisms of the trafficking of G protein subunits after receptor stimulation are poorly understood. There is a strong correlation between activation, depalmitoylation and internalization, particularly in the case of αs: mutational activation of αs or activation of αs via the β2-AR induces a fast turn over of the palmitate group attached to αs as observed by pulse/chase and palmitate labeling experiments (123, 124). GPCR regulated changes in palmitoylation have also been observed for other Gα (123–127), suggesting that it is a general phenomenon. Consistent with the importance of rapid turnover of palmitate on αs for activation-induced internalization, replacing the N-terminus of αs with other PM targeting motifs produces an αs that does not internalize in response to activation (110); however, a causative role for depalmitoylation of Gα controlling internalization remains to be formally established.
Exactly how activation of a Gα leads to more rapid turnover of its attached palmitate is not completely understood. Studies with purified proteins indicate that association of Gβγ with αs inhibits αs depalmitoylation, and thus it appears that a key factor in activation-induced depalmitoylation is the decreased affinity of interaction of the activated Gα with Gβγ (7, 128). This may simply allow access of a palmitoyl thioesterase. It is worthwhile mentioning that Gαand Gβγ may not always fully dissociate upon activation. Although this is contrary to the G protein dogma, several recent studies using a variety of experimental approaches, including co-precipitation, FRET, and Gα-Gβγ fusions, have obtained results consistent with the proposal that G protein signaling can occur in the absence of subunit dissociation (129–132). As the mechanisms of activation-induced trafficking of G proteins are explored further, it will be important to consider that Gα and Gβγ may remain associated. Lastly, several studies have determined that downstream signaling pathways do not influence receptor-dependent changes in Gα redistribution or palmitate turnover (56, 115, 133). Thus, at least the initial events involved in depalmitoylation and release from the PM of αs and αq are a direct result of activation (GTP binding) of the Gα.
Light-driven translocation of vertebrate transducin is also clearly dependent on activation but does not involve depalmitoylation since αt is the only Gα that does not undergo palmitoylation (Table 1). For transducin the key to its translocation ability is that αt is modified by myristate only and γ1, of transducin Gβγ, is modified by farnesyl. In the transducin heterotrimer, these two lipids together provide tight binding to membranes. However, upon activation-dependent dissociation either myristate or farnesyl does not provide strong membrane binding, and αt and β1γ1 redistribute off of membranes (107, 134–136).
Several studies have indicated that internalization of Gαdoes not require internalization of the activating GPCR and occurs independently of typical endocytic pathways used by GPCRs. First, αs can be activated independently of GPCR activation, e.g., by mutational activation or cholera toxin, and exhibits redistribution off the PM. Second, αs and αq retain internalization in response to GPCR activation under conditions in which the internalization of the GPCR has been blocked by mutation of the GPCR, expression of a dominant negative dynamin, or treatment of cells with hypertonic sucrose, a disruptor of clathrin-coated pit mediated endocytosis (111, 112, 115, 117). In contrast, a dominant negative dynamin prevented β2-AR-stimulated internalization of αs-GFP in C6 glioma cells (82). Dynamin can be involved in both clathrin-coated pit-mediated endocytosis and lipid raft-mediated endocytosis, and these differing results in terms of the requirement for dynamin in Gαinternalization may indicate that Gα utilize different mechanisms for internalization depending upon the cell type. Indeed, a role for caveolae/lipid rafts is indicated by a recent report (82). αs, and also αq, were enriched in a detergent resistant membrane fraction after the addition of agonists (82, 137); the depletion of membrane cholesterol blocked the internalization of αs (82), and internalized αs-GFP co-localized with the lipid raft marker cholera toxin B (82). Lastly, internalized αs appears not to associate with early endosomes (82, 111), consistent with translocation independent of a GPCR endocytic pathway. In summary, activated αs, and possibly αq, traffic from the PM into a cell’s interior following a pathway that is independent of GPCR endocytic pathways and may involve lipid rafts.
After internalization, αs and αq recover localization at the PM when the GPCR agonist is removed, suggesting an active recycling process (56, 112, 115). Recycling to the PM occurred somewhat slowly, taking approximately 1 hour for either internalized αs in HEK293 cells or internalized αq in Drosophila eyes, and this time course of αs recycling to the PM was identical to the time course of β2-AR PM recycling (112). Very little is known about the mechanisms of recycling of internalized G proteins, although the first clues were provided recently. Internalized αs-CFP was shown to colocalize with Rab11 containing vesicles in HEK293 cells (111), suggesting the possibility that αs utilizes these vesicles to recycle to the PM (111). Moreover, one of the studies using Drosophila eyes showed that recovery of internalized αq at the PM was much slower in mutant flies that possessed much reduced levels of Gβγ (56). Thus, it seems that both the initial targeting of a Gαto the PM, as well as its recycling, requires interaction with Gβγ. Lastly, the other recent study using Drosophila eyes showed that PM recycling of αq was specifically defective in flies lacking the photoreceptor specific myosin III NINAC (115). It will be important to determine whether similar motor proteins also play a role in trafficking/recycling of G proteins in cells other than photoreceptor cells.
In summary, observations of activation-induced G protein trafficking have recently moved beyond only αs, and live cell imaging studies and studies using model systems have begun to provide novel insights into the trafficking itineraries and mechanisms of G proteins after GPCR activation at the PM. The physiological function of activation-induced G protein translocation remains elusive. In photoreceptors, translocation of G proteins likely plays a role in light adaptation (138). Activation-induced G protein internalization may be a general mechanism contributing to desensitization and also may provide a regulatable mechanism for transporting G proteins to diverse subcellular locations to carry out signaling functions.
CONCLUSION
A number of exciting recent studies have increased our understanding of how G proteins are assembled and targeted to the PM and how G proteins redistribute in response to activation. Yet, many important questions remain to be answered, including a detailed mechanistic understanding of G protein trafficking and its functional consequences. G proteins typically need to be localized at the cytoplasmic surface of plasma membranes to couple an activated GPCR to downstream effectors, and thus understanding how G proteins arrive at the PM is of fundamental importance. However, it is becoming increasingly clear that G proteins have signaling roles at subcellular locations in addition to the PM (3). A recent study demonstrated that a constitutively active form of Gpa1, a yeast Gα, can be found at endosomes where it can activate a phosphatidylinositol 3-kinase (139). In addition, a series of studies have implicated Gβγ in functioning at the Golgi to direct a protein kinase D and protein kinase C-dependent pathway that promotes the formation of Golgi transport vesicles (140–142). Not only can G proteins signal at various intracellular membranes, Gβγ appears to function in the nucleus where it can regulate the glucocorticoid receptor (143). Thus, it will be essential to understand how G proteins localize to diverse subcellular locations, and understanding mechanisms of G protein trafficking will certainly provide insight into novel G protein signaling functions.
Acknowledgments
Research in the authors’ laboratory is supported by NIH grants GM56444 and GM62884, and an Established Investigator Award from the American Heart Association.
References
- 1.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–81. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel AM, Weinstein LS. Inherited diseases involving g proteins and g protein-coupled receptors. Annu Rev Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- 3.Spiegelberg BD, Hamm HE. Roles of G-protein-coupled receptor signaling in cancer biology and gene transcription. Curr Opin Genet Dev. 2007;17:40–4. doi: 10.1016/j.gde.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Melien O. Heterotrimeric G proteins and disease. Methods Mol Biol. 2007;361:119–44. doi: 10.1385/1-59745-208-4:119. [DOI] [PubMed] [Google Scholar]
- 5.Malbon CC. G proteins in development. Nat Rev Mol Cell Biol. 2005;6:689–701. doi: 10.1038/nrm1716. [DOI] [PubMed] [Google Scholar]
- 6.Sondek J, Siderovski DP. Ggamma-like (GGL) domains: new frontiers in G-protein signaling and beta-propeller scaffolding. Biochem Pharmacol. 2001;61:1329–37. doi: 10.1016/s0006-2952(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 7.Wedegaertner PB. Lipid modifications and membrane targeting of G alpha. Biol Signals Recept. 1998;7:125–35. doi: 10.1159/000014538. [DOI] [PubMed] [Google Scholar]
- 8.Rehm A, Ploegh HL. Assembly and intracellular targeting of the betagamma subunits of heterotrimeric G proteins. J Cell Biol. 1997;137:305–17. doi: 10.1083/jcb.137.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota S, Kubota H, Nagata K. Cytosolic chaperonin protects folding intermediates of G{beta} from aggregation by recognizing hydrophobic {beta}-strands. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0600195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells CA, Dingus J, Hildebrandt JD. Role of the chaperonin CCT/TRiC complex in G protein betagamma-dimer assembly. J Biol Chem. 2006;281:20221–32. doi: 10.1074/jbc.M602409200. [DOI] [PubMed] [Google Scholar]
- 11.Knol JC, Engel R, Blaauw M, Visser AJ, van Haastert PJ. The phosducin-like protein PhLP1 is essential for G{beta}{gamma} dimer formation in Dictyostelium discoideum. Mol Cell Biol. 2005;25:8393–400. doi: 10.1128/MCB.25.18.8393-8400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukov GL, Hu T, McLaughlin JN, Hamm HE, Willardson BM. Phosducin-like protein acts as a molecular chaperone for G protein betagamma dimer assembly. Embo J. 2005;24:1965–75. doi: 10.1038/sj.emboj.7600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Benito J, Bertrand S, Hu T, Ludtke PJ, McLaughlin JN, Willardson BM, Carrascosa JL, Valpuesta JM. Structure of the complex between the cytosolic chaperonin CCT and phosducin-like protein. Proc Natl Acad Sci U S A. 2004;101:17410–5. doi: 10.1073/pnas.0405070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin JN, Thulin CD, Hart SJ, Resing KA, Ahn NG, Willardson BM. Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc Natl Acad Sci U S A. 2002;99:7962–7. doi: 10.1073/pnas.112075699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukov GL, Baker CM, Ludtke PJ, Hu T, Carter MD, Hackett RA, Thulin CD, Willardson BM. Mechanism of assembly of G protein beta gamma subunits by CK2-phosphorylated phosducin-like protein and the cytosolic chaperonin complex. J Biol Chem. 2006;281:22261–22274. doi: 10.1074/jbc.M601590200. [DOI] [PubMed] [Google Scholar]
- 16.Humrich J, Bermel C, Bunemann M, Harmark L, Frost R, Quitterer U, Lohse MJ. Phosducin-like protein regulates G-protein betagamma folding by interaction with tailless complex polypeptide-1alpha: dephosphorylation or splicing of PhLP turns the switch toward regulation of Gbetagamma folding. J Biol Chem. 2005;280:20042–50. doi: 10.1074/jbc.M409233200. [DOI] [PubMed] [Google Scholar]
- 17.Savage JR, McLaughlin JN, Skiba NP, Hamm HE, Willardson BM. Functional roles of the two domains of phosducin and phosducin-like protein. J Biol Chem. 2000;275:30399–407. doi: 10.1074/jbc.M005120200. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JB, Casey PJ. The role of prenylation in G-protein assembly and function. Cell Signal. 1996;8:433–7. doi: 10.1016/s0898-6568(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 19.Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor interacting protein 78 acts as a molecular chaperone for Ggamma subunits prior to assembly with Gbeta. J Biol Chem. 2007 doi: 10.1074/jbc.M608846200. [DOI] [PubMed] [Google Scholar]
- 20.Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47:681–99. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Wright LP, Philips MR. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J Lipid Res. 2006;47:883–91. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Gelb MH, Scholten JD, Sebolt-Leopold JS. Protein prenylation: from discovery to prospects for cancer treatment. Curr Opin Chem Biol. 1998;2:40–8. doi: 10.1016/s1367-5931(98)80034-3. [DOI] [PubMed] [Google Scholar]
- 23.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–77. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 24.Michaelson D, Ahearn I, Bergo M, Young S, Philips M. Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell. 2002;13:3294–302. doi: 10.1091/mbc.E02-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu HW, Casey PJ. Enzymology and biology of CaaX protein prenylation. Recent Prog Horm Res. 1999;54:315–42. discussion 342-3. [PubMed] [Google Scholar]
- 26.Takida S, Wedegaertner PB. Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gbetagamma. J Biol Chem. 2003;278:17284–90. doi: 10.1074/jbc.M213239200. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez L, Magee AI, Marshall CJ, Hancock JF. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. Embo J. 1989;8:1093–8. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fung BK, Yamane HK, Ota IM, Clarke S. The gamma subunit of brain G-proteins is methyl esterified at a C-terminal cysteine. FEBS Lett. 1990;260:313–7. doi: 10.1016/0014-5793(90)80132-3. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc Natl Acad Sci U S A. 1998;95:11175–80. doi: 10.1073/pnas.95.19.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fishburn CS, Herzmark P, Morales J, Bourne HR. Gbetagamma and palmitate target newly synthesized Galphaz to the plasma membrane. J Biol Chem. 1999;274:18793–800. doi: 10.1074/jbc.274.26.18793. [DOI] [PubMed] [Google Scholar]
- 31.Tall GG, Krumins AM, Gilman AG. Mammalian Ric-8A (synembryn) is a heterotrimeric Galpha protein guanine nucleotide exchange factor. J Biol Chem. 2003;278:8356–62. doi: 10.1074/jbc.M211862200. [DOI] [PubMed] [Google Scholar]
- 32.David NB, Martin CA, Segalen M, Rosenfeld F, Schweisguth F, Bellaiche Y. Drosophila Ric-8 regulates Galphai cortical localization to promote Galphai-dependent planar orientation of the mitotic spindle during asymmetric cell division. Nat Cell Biol. 2005;7:1083–90. doi: 10.1038/ncb1319. [DOI] [PubMed] [Google Scholar]
- 33.Hampoelz B, Hoeller O, Bowman SK, Dunican D, Knoblichl JA. Drosophila Ric-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nat Cell Biol. 2005;7:1099–105. doi: 10.1038/ncb1318. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Ng KH, Qian H, Siderovski DP, Chia W, Yu F. Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat Cell Biol. 2005;7:1091–8. doi: 10.1038/ncb1317. [DOI] [PubMed] [Google Scholar]
- 35.Afshar K, Willard FS, Colombo K, Siderovski DP, Gonczy P. Cortical localization of the Galpha protein GPA-16 requires RIC-8 function during C. elegans asymmetric cell division. Development. 2005;132:4449–59. doi: 10.1242/dev.02039. [DOI] [PubMed] [Google Scholar]
- 36.Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J Biol Chem. 2001;276:39501–4. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- 37.Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–73. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- 38.Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–8. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–27. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–96. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Swarthout JT, Lobo S, Farh L, Croke MR, Greentree WK, Deschenes RJ, Linder ME. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem. 2005;280:31141–8. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- 42.Duncan JA, Gilman AG. Characterization of Saccharomyces cerevisiae acyl-protein thioesterase 1, the enzyme responsible for G protein alpha subunit deacylation in vivo. J Biol Chem. 2002;277:31740–52. doi: 10.1074/jbc.M202505200. [DOI] [PubMed] [Google Scholar]
- 43.Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS) J Biol Chem. 1998;273:15830–7. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- 44.Kleuss C, Krause E. Galpha(s) is palmitoylated at the N-terminal glycine. Embo J. 2003;22:826–32. doi: 10.1093/emboj/cdg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunphy JT, Linder ME. Signalling functions of protein palmitoylation. Biochim Biophys Acta. 1998;1436:245–61. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- 46.Hancock JF, Patterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–6. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 48.Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of G alpha z requires two signals. Mol Biol Cell. 1998;9:1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 50.Hepler JR, Kozasa T, Smrcka AV, Simon MI, Rhee SG, Sternweis PC, Gilman AG. Purification from Sf9 cells and characterization of recombinant Gq alpha and G11 alpha. Activation of purified phospholipase C isozymes by G alpha subunits. J Biol Chem. 1993;268:14367–75. [PubMed] [Google Scholar]
- 51.Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gbeta gamma isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Galphas and Galphaq. J Biol Chem. 2001;276:23945–53. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- 52.Fishburn CS, Pollitt SK, Bourne HR. Localization of a peripheral membrane protein: Gbetagamma targets Galpha(Z) Proc Natl Acad Sci U S A. 2000;97:1085–90. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evanko DS, Thiyagarajan MM, Wedegaertner PB. Interaction with Gbetagamma is required for membrane targeting and palmitoylation of Galpha(s) and Galpha(q) J Biol Chem. 2000;275:1327–36. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- 54.Song J, Hirschman J, Gunn K, Dohlman HG. Regulation of membrane and subunit interactions by N-myristoylation of a G protein alpha subunit in yeast. J Biol Chem. 1996;271:20273–83. doi: 10.1074/jbc.271.34.20273. [DOI] [PubMed] [Google Scholar]
- 55.Gotta M, Ahringer J. Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat Cell Biol. 2001;3:297–300. doi: 10.1038/35060092. [DOI] [PubMed] [Google Scholar]
- 56.Kosloff M, Elia N, Joel-Almagor T, Timberg R, Zars TD, Hyde DR, Minke B, Selinger Z. Regulation of light-dependent Gqalpha translocation and morphological changes in fly photoreceptors. Embo J. 2003;22:459–68. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elia N, Frechter S, Gedi Y, Minke B, Selinger Z. Excess of Gbetae over Gqalphae in vivo prevents dark, spontaneous activity of Drosophila photoreceptors. J Cell Biol. 2005;171:517–26. doi: 10.1083/jcb.200506082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray D, Hermida-Matsumoto L, Buser CA, Tsang J, Sigal CT, Ben-Tal N, Honig B, Resh MD, McLaughlin S. Electrostatics and the membrane association of Src: theory and experiment. Biochemistry. 1998;37:2145–59. doi: 10.1021/bi972012b. [DOI] [PubMed] [Google Scholar]
- 59.Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379:369–74. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda T, Takao T, Shimonishi Y, Murata M, Asano T, Yoshizawa T, Fukada Y. Characterization of interactions between transducin alpha/beta gamma-subunits and lipid membranes. J Biol Chem. 1994;269:30358–63. [PubMed] [Google Scholar]
- 61.Seitz HR, Heck M, Hofmann KP, Alt T, Pellaud J, Seelig A. Molecular determinants of the reversible membrane anchorage of the G-protein transducin. Biochemistry. 1999;38:7950–60. doi: 10.1021/bi990298+. [DOI] [PubMed] [Google Scholar]
- 62.Murray D, McLaughlin S, Honig B. The role of electrostatic interactions in the regulation of the membrane association of G protein beta gamma heterodimers. J Biol Chem. 2001;276:45153–9. doi: 10.1074/jbc.M101784200. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Tal N, Honig B, Peitzsch RM, Denisov G, McLaughlin S. Binding of small basic peptides to membranes containing acidic lipids: theoretical models and experimental results. Biophys J. 1996;71:561–75. doi: 10.1016/S0006-3495(96)79280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Tal N, Honig B, Miller C, McLaughlin S. Electrostatic binding of proteins to membranes. Theoretical predictions and experimental results with charybdotoxin and phospholipid vesicles. Biophys J. 1997;73:1717–27. doi: 10.1016/S0006-3495(97)78203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosloff M, Elia N, Selinger Z. Structural homology discloses a bifunctional structural motif at the N-termini of G alpha proteins. Biochemistry. 2002;41:14518–23. doi: 10.1021/bi026729x. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein LS, Grillo AA, Loranger SS, Linder ME. RGS4 binds to membranes through an amphipathic alpha -helix. J Biol Chem. 2000;275:18520–6. doi: 10.1074/jbc.M000618200. [DOI] [PubMed] [Google Scholar]
- 67.Tu Y, Woodson J, Ross EM. Binding of regulator of G protein signaling (RGS) proteins to phospholipid bilayers. Contribution of location and/or orientation to Gtpase-activating protein activity. J Biol Chem. 2001;276:20160–6. doi: 10.1074/jbc.M101599200. [DOI] [PubMed] [Google Scholar]
- 68.Chen C, Seow KT, Guo K, Yaw LP, Lin SC. The membrane association domain of RGS16 contains unique amphipathic features that are conserved in RGS4 and RGS5. J Biol Chem. 1999;274:19799–806. doi: 10.1074/jbc.274.28.19799. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi N, Matsubara M, Titani K, Taniguchi H. Circular dichroism and 1H nuclear magnetic resonance studies on the solution and membrane structures of GAP-43 calmodulin-binding domain. J Biol Chem. 1997;272:7639–45. doi: 10.1074/jbc.272.12.7639. [DOI] [PubMed] [Google Scholar]
- 70.Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–83. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:369–77. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–9. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- 73.Takida S, Wedegaertner PB. Exocytic pathway-independent plasma membrane targeting of heterotrimeric G proteins. FEBS Lett. 2004;567:209–13. doi: 10.1016/j.febslet.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalo S, Linder ME. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell. 1998;9:585–97. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dupre DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hebert TE. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281:34561–73. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- 76.Le-Niculescu H, Niesman I, Fischer T, DeVries L, Farquhar MG. Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J Biol Chem. 2005;280:22012–20. doi: 10.1074/jbc.M501833200. [DOI] [PubMed] [Google Scholar]
- 77.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–44. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 78.Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–8. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 79.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 81.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–40. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 82.Allen JA, Yu JZ, Donati RJ, Rasenick MM. Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67:1493–504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- 83.Huang C, Hepler JR, Chen LT, Gilman AG, Anderson RG, Mumby SM. Organization of G proteins and adenylyl cyclase at the plasma membrane. Mol Biol Cell. 1997;8:2365–78. doi: 10.1091/mbc.8.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang WJ, Ying YS, Rothberg KG, Hooper NM, Turner AJ, Gambliel HA, De Gunzburg J, Mumby SM, Gilman AG, Anderson RG. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994;126:127–38. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–26. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–8. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–7. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 89.Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–63. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arni S, Keilbaugh SA, Ostermeyer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem. 1998;273:28478–85. doi: 10.1074/jbc.273.43.28478. [DOI] [PubMed] [Google Scholar]
- 91.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–8. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 92.Galbiati F, Volonte D, Meani D, Milligan G, Lublin DM, Lisanti MP, Parenti M. The dually acylated NH2-terminal domain of gi1alpha is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. Palmitoylation of caveolin-1 is required for the recognition of dually acylated g-protein alpha subunits in vivo. J Biol Chem. 1999;274:5843–50. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- 93.Dunphy JT, Greentree WK, Linder ME. Enrichment of G-protein palmitoyltransferase activity in low density membranes: in vitro reconstitution of Galphai to these domains requires palmitoyltransferase activity. J Biol Chem. 2001;276:43300–4. doi: 10.1074/jbc.M104275200. [DOI] [PubMed] [Google Scholar]
- 94.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–98. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 96.Rehm A, Ploegh HL. The betagamma subunits of heterotrimeric G proteins acquire detergent insolubility directly at the plasma membrane. FEBS Lett. 1997;416:39–44. doi: 10.1016/s0014-5793(97)01119-8. [DOI] [PubMed] [Google Scholar]
- 97.Scarlata S. Regulation of the lateral association of phospholipase Cbeta2 and G protein subunits by lipid rafts. Biochemistry. 2002;41:7092–9. doi: 10.1021/bi025625j. [DOI] [PubMed] [Google Scholar]
- 98.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–67. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann N Y Acad Sci. 2005;1047:166–72. doi: 10.1196/annals.1341.015. [DOI] [PubMed] [Google Scholar]
- 100.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–4. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 101.Bodin S, Giuriato S, Ragab J, Humbel BM, Viala C, Vieu C, Chap H, Payrastre B. Production of phosphatidylinositol 3,4,5-trisphosphate and phosphatidic acid in platelet rafts: evidence for a critical role of cholesterol-enriched domains in human platelet activation. Biochemistry. 2001;40:15290–9. doi: 10.1021/bi0109313. [DOI] [PubMed] [Google Scholar]
- 102.Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 103.Sabourin T, Bastien L, Bachvarov DR, Marceau F. Agonist-induced translocation of the kinin B(1) receptor to caveolae-related rafts. Mol Pharmacol. 2002;61:546–53. doi: 10.1124/mol.61.3.546. [DOI] [PubMed] [Google Scholar]
- 104.Miura Y, Hanada K, Jones TL. G(s) signaling is intact after disruption of lipid rafts. Biochemistry. 2001;40:15418–23. doi: 10.1021/bi015574a. [DOI] [PubMed] [Google Scholar]
- 105.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 106.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–6. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 107.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–8. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Ransnas LA, Svoboda P, Jasper JR, Insel PA. Stimulation of beta-adrenergic receptors of S49 lymphoma cells redistributes the alpha subunit of the stimulatory G protein between cytosol and membranes. Proc Natl Acad Sci U S A. 1989;86:7900–3. doi: 10.1073/pnas.86.20.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levis MJ, Bourne HR. Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992;119:1297–307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thiyagarajan MM, Bigras E, Van Tol HH, Hebert TE, Evanko DS, Wedegaertner PB. Activation-induced subcellular redistribution of G alpha(s) is dependent upon its unique N-terminus. Biochemistry. 2002;41:9470–84. doi: 10.1021/bi025533u. [DOI] [PubMed] [Google Scholar]
- 111.Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J Biol Chem. 2004;279:44101–12. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- 112.Wedegaertner PB, Bourne HR, von Zastrow M. Activation-induced subcellular redistribution of Gs alpha. Mol Biol Cell. 1996;7:1225–33. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu JZ, Rasenick MM. Real-time visualization of a fluorescent G(alpha)(s): dissociation of the activated G protein from plasma membrane. Mol Pharmacol. 2002;61:352–9. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- 114.Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gs alpha. Cell. 1994;77:1063–70. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 115.Cronin MA, Diao F, Tsunoda S. Light-dependent subcellular translocation of Gqalpha in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J Cell Sci. 2004;117:4797–806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- 116.Drmota T, Novotny J, Kim GD, Eidne KA, Milligan G, Svoboda P. Agonist-induced internalization of the G protein G11alpha and thyrotropin-releasing hormone receptors proceed on different time scales. J Biol Chem. 1998;273:21699–707. doi: 10.1074/jbc.273.34.21699. [DOI] [PubMed] [Google Scholar]
- 117.Miserey-Lenkei S, Lenkei Z, Parnot C, Corvol P, Clauser E. A functional enhanced green fluorescent protein (EGFP)-tagged angiotensin II at(1a) receptor recruits the endogenous Galphaq/11 protein to the membrane and induces its specific internalization independently of receptor-g protein coupling in HEK-293 cells. Mol Endocrinol. 2001;15:294–307. doi: 10.1210/mend.15.2.0600. [DOI] [PubMed] [Google Scholar]
- 118.Drmota T, Novotny J, Gould GW, Svoboda P, Milligan G. Visualization of distinct patterns of subcellular redistribution of the thyrotropin-releasing hormone receptor-1 and gqalpha/G11alpha induced by agonist stimulation. Biochem J. 1999;340(Pt 2):529–38. [PMC free article] [PubMed] [Google Scholar]
- 119.Hughes TE, Zhang H, Logothetis DE, Berlot CH. Visualization of a functional Galpha q-green fluorescent protein fusion in living cells. Association with the plasma membrane is disrupted by mutational activation and by elimination of palmitoylation sites, but not be activation mediated by receptors or AlF4. J Biol Chem. 2001;276:4227–35. doi: 10.1074/jbc.M007608200. [DOI] [PubMed] [Google Scholar]
- 120.Akgoz M, Kalyanaraman V, Gautam N. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279:51541–4. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 121.Akgoz M, Kalyanaraman V, Gautam N. G protein betagamma complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction. Cell Signal. 2006;18:1758–68. doi: 10.1016/j.cellsig.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Azpiazu I, Akgoz M, Kalyanaraman V, Gautam N. G protein betagamma11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the alpha subunit type. Cell Signal. 2005 doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wedegaertner P. Reversible Palmitoylation in G-Protein Signaling. In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling. Elsevier Science; USA: 2003. pp. 651–656. [Google Scholar]
- 124.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–52. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 125.Chen CA, Manning DR. Regulation of galpha i palmitoylation by activation of the 5-hydroxytryptamine-1A receptor. J Biol Chem. 2000;275:23516–22. doi: 10.1074/jbc.M003439200. [DOI] [PubMed] [Google Scholar]
- 126.Bhamre S, Wang HY, Friedman E. Serotonin-mediated palmitoylation and depalmitoylation of G alpha proteins in rat brain cortical membranes. J Pharmacol Exp Ther. 1998;286:1482–9. [PubMed] [Google Scholar]
- 127.Gurdal H, Seasholtz TM, Wang HY, Brown RD, Johnson MD, Friedman E. Role of G alpha q or G alpha o proteins in alpha 1-adrenoceptor subtype-mediated responses in Fischer 344 rat aorta. Mol Pharmacol. 1997;52:1064–70. doi: 10.1124/mol.52.6.1064. [DOI] [PubMed] [Google Scholar]
- 128.Iiri T, Backlund PS, Jr, Jones TL, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gs alpha by palmitate and the beta gamma subunit. Proc Natl Acad Sci U S A. 1996;93:14592–7. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evanko DS, Thiyagarajan MM, Takida S, Wedegaertner PB. Loss of association between activated Galpha q and Gbetagamma disrupts receptor-dependent and receptor-independent signaling. Cell Signal. 2005;17:1218–28. doi: 10.1016/j.cellsig.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 130.Bunemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci U S A. 2003;100:16077–82. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Klein S, Reuveni H, Levitzki A. Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc Natl Acad Sci U S A. 2000;97:3219–23. doi: 10.1073/pnas.050015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Levitzki A, Klein S. G-protein subunit dissociation is not an integral part of G-protein action. Chembiochem. 2002;3:815–8. doi: 10.1002/1439-7633(20020902)3:9<815::AID-CBIC815>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 133.Degtyarev MY, Spiegel AM, Jones TL. Increased palmitoylation of the Gs protein alpha subunit after activation by the beta-adrenergic receptor or cholera toxin. J Biol Chem. 1993;268:23769–72. [PubMed] [Google Scholar]
- 134.Kassai H, Aiba A, Nakao K, Nakamura K, Katsuki M, Xiong WH, Yau KW, Imai H, Shichida Y, Satomi Y, Takao T, Okano T, Fukada Y. Farnesylation of retinal transducin underlies its translocation during light adaptation. Neuron. 2005;47:529–39. doi: 10.1016/j.neuron.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kerov V, Chen D, Moussaif M, Chen YJ, Chen CK, Artemyev NO. Transducin activation state controls its light-dependent translocation in rod photoreceptors. J Biol Chem. 2005;280:41069–76. doi: 10.1074/jbc.M508849200. [DOI] [PubMed] [Google Scholar]
- 136.Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–67. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pesanova Z, Novotny J, Cerny J, Milligan G, Svoboda P. Thyrotropin-releasing hormone-induced depletion of G(q)alpha/G(11)alpha proteins from detergent-insensitive membrane domains. FEBS Lett. 1999;464:35–40. doi: 10.1016/s0014-5793(99)01666-x. [DOI] [PubMed] [Google Scholar]
- 138.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 139.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 140.Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 141.Diaz Anel AM, Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol. 2005;169:83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–55. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 143.Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein beta interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–96. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]