Abstract
The lipid requirements of the Torpedo californica nicotinic acetylcholine receptor (nAChR) were assessed by reconstituting purified receptors into lipid vesicles of defined composition and by using photolabeling with 3-trifluoromethyl-3-(m-[125I]iodophenyl) diazirine ([125I]TID) to determine functionality. Earlier studies demonstrated that nAChRs reconstituted into membranes containing phosphatidylcholine (PC), the anionic lipid phosphatidic acid (PA) and cholesterol (CH) are particularly effective at stabilizing the nAChR in the resting (closed) state that is capable of undergoing agonist-induced conformational transitions (i.e. functionality). The present studies demonstrate that: (1) there is no obligatory requirement for PC; (2) that increasing the CH content serves to increase the degree to which nAChRs are stabilized in the resting state, this effect saturates at ∼ 35 mol% (molar lipid percentage); (3) the effect of increasing levels of PA saturates at ∼12 mol% and in the absence of PA nAChRs are stabilized in the desensitized state (i.e. nonfunctional). Native Torpedo membranes contain ∼35 mol% CH but less than 1 mol% PA, suggesting that other anionic lipid may substitute for PA. We report that: (4) phosphatidylserine (PS) and phosphatidylinositol (PI), anionic lipids that are abundant in native Torpedo membranes, also stabilize the receptor in the resting state although with reduced efficacy (∼50-60%) compared to PA; (5) For nAChRs reconstituted into PA/CH membranes at different lipid-protein molar ratios, receptor functionality decreases rapidly below ∼65 lipids per receptor. Collectively, these results are consistent with a functional requirement of a single shell of lipids surrounding the nAChR and specific anionic lipid- and sterol- (CH) protein interactions.
The agonist-induced conformational transitions that underlie the function of the Torpedo nicotinic acetylcholine receptor (nAChR) have been shown to be highly dependent on the surrounding lipid environment, providing a model system for understanding lipid-protein interactions (1-3). The nAChR is the best characterized member of a superfamily of ligand-gated ion channels (LGIC) that includes muscle- and neuronal-type nAChRs, serotonin 5-HT3 receptors, gamma-amino butyric acid type A receptors (GABAA), and glycine receptors (4, 5). The Torpedo (muscle-type) nAChR is a pentameric multispanning transmembrane protein comprised of four homologous subunits with a subunit stoichiometry of 2α:β:γ:δ that are arranged pseudosymetrically around a central axis forming an ion-conducting channel. In native Torpedo (post-synaptic) membranes, the nAChR exists predominantly in a resting (nonconducting) state that upon binding agonist is converted to a transient open (ion conducting) state and in the continued presence of agonist to a desensitized (nonconducting) state (4, 5).
Torpedo nAChRs purified and reconstituted into synthetic lipid membranes comprised of either phosphatidylcholine (PC) or phosphatidylethanolamine (PE), the two most abundant phospholipids present in native Torpedo membranes (6), are stabilized in the desensitized (or a desensitized-like) state and are incapable of undergoing agonist-induced state transitions or conducting ions (7-9). However, inclusion of both cholesterol (CH) and phosphatidic acid (PA) with PC membranes (PC/PA/CH) completely restores nAChR functionality (7, 9, 10). Membranes containing PA or CH alone, that is PC/PA or PC/CH, are able to stabilize differing proportions of nAChRs in the resting state, though clearly the presence of both lipids is required for full functionality (9, 11). The amount of PA present in native Torpedo membranes is relatively low (∼0.3 mol% on a molar basis; in Ref. 6) suggesting at least two possibilities: 1) other anionic lipids such as phosphatidylserine (PS) and phophatidylinositol (PI) which are relatively abundant in Torpedo membranes (∼9 and 3 mol% respectively; in Ref. 6), are able to substitute for PA; 2) enriched levels of PA may be found within microdomains in the bilayer or at the lipid annulus which surrounds the nAChR protein. We hypothesize that the first case is operative based on the following information: 1) there is a high exchange rate between lipids present at the nAChR lipid annulus and the bulk lipid and the exchange rate is unaffected by the lipid-protein ratio (2); 2) progressive delipidation has no effect on the lipid composition of nAChR membranes (12, 13); 3) with detergent-induced delipidation, loss of agonist-induced state transitions only occurs when the lipid-protein ratio falls below 45:1, that is below the number of lipids required to completely surround the protein (13). These facts argue against any selective enrichment of PA molecules at the nAChR lipid-protein interface and suggest that the possible existence of PA microdomains in the bilayer are not sufficient to explain the effects of PA on nAChR functionality.
To gain a more detailed picture of the specific lipid requirements of the nAChR and to test two central hypotheses: 1) that other anionic lipids, in particular PS and PI, will support receptor functionality; 2) that a single shell of lipids surrounding the receptor protein is sufficient to fully support receptor functionality, we reconstituted purified nAChRs into synthetic lipid membranes of defined composition and lipid-protein molar ratios. Receptor functionality, as defined by the ability of the nAChR to undergo agonist-induced state transitions, was assessed by photolabeling with [125I]TID, a conformationally sensitive probe of the nAChR (9, 14). Our data show that zwitterionic lipids (e.g. PC) are not required for nAChR functionality, as measured by the ability of the receptors reconstituted into PC/CH membranes to fully retain the resting state and undergo agonist-induced state transitions. The data show that levels of CH that maximally support nAChR functionality (35 mol%) are reflective of those found in native Torpedo membranes. Significantly, the data indicate that other anionic lipids present in relatively high abundance in native Torpedo membranes (PS, PI) also support nAChR functionality although with reduced efficacy (50-60%) compared to PA. Along these lines, nAChRs reconstituted into membranes containing levels of anionic lipids (PS, PI, PA; 10, 5, 1.6 mol% respectively) and cholesterol (33 mol%) reflective of those found in native Torpedo membranes, have full functionality. For nAChRs reconstituted into PA/CH membranes at decreasing lipid-protein molar ratios, receptor functionality decreases rapidly below 65 lipids per receptor. Collectively, these results are consistent with a minimum functional requirement of a single shell of lipids surrounding the nAChR and specific anionic lipid- and sterol- (CH) protein interactions that act synergistically to affect receptor functionality.
EXPERIMENTAL PROCEDURES
Materials
Frozen Torpedo californica electric organs were obtained from Aquatic Research Consultants (San Pedro, CA). Synthetic lipids, dioleoyl phosphatidic acid (DOPA), dioleoyl phosphatidylcholine (DOPC), dioleoyl phosphatidylserine (DOPS), as well as 1-palmitoyl-2-linoleoyl phosphatidylinositol (Soy derived-PLPI) and cholesterol (CH) were from Avanti Polar Lipids, Inc. (Alabaster, AL). Carbamylcholine chloride, bromoacetylcholine bromide and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO); 3-trifluoromethyl-3-(m-[125I]iodophenyl) diazirine ([125I]TID; ∼10 Ci/mmol) was obtained from Amersham Biosciences (Piscataway, NJ) and stored in ethanol at −4°C. Sodium cholate was from USB Corporation (Cleveland, OH).
nAChR-enriched Membrane Preparation and Solubilization
nAChR-enriched membranes were prepared from frozen Torpedo californica electric organs as described previously (15) with modifications. Briefly, ∼1000 g. of frozen Torpedo californica electric organs were homogenized for 4 × 1min. in a Waring blender in 1.5 L of homogenization cocktail (10 mg pepstatin, 10 mg leupeptin, 150 mg benzamidine, 115 U aprotinin, 12.5 mg calpain I (ALLN), 12.5 mg calpain II (ALLM), 200 mg sodium azide and 10 mg phenylmethylsulphonylflouride (in 1 mL ethanol), in distilled water. The homogenate was centrifuged (5,500 rpm for 10 min using JA-10 rotor in a Beckman J2-HS centrifuge), and the supernatant was collected through 3 layers of gauze. The pellets were rehomogenized in 240 mL of homogenization cocktail for 4 × 1 min in a Virtis (Cyclone model) and centrifuged as above. The combined supernatants were centrifuged (9,000 rpm for 5 h) and the pelleted membranes resuspended in vesicle dialysis buffer (VDB, 10mM MOPS, 100 mM NaCl, 0.1 mM EDTA, 0.02% NaN3, pH 7.4) to achieve a protein concentration of ∼ 2 mg/mL (∼ 400 mL) and stored at 4°C. For detergent solubilization, an equal volume of 2% sodium cholate in VDB was added to membrane suspensions (final concentration, 1 mg/mL protein; 1% cholate) and stirred for 90 min. at 4° C and then centrifuged (28,000 rpm for 1 h in a Ti-35 rotor in a Beckman XL-80 ultracentrifuge) to sediment insoluble material. The supernatant was treated with 20 μL of diisopropylflourophosphate (DFP), stirred for 15 min and applied to the affinity column as described next.
nAChR Purification and Reconstitution
nAChRs were affinity purified on a bromoacetylcholine bromide-derivatized Affi-Gel 10 column (Bio-Rad) as described previously (16). Briefly, the affinity column was prepared by coupling 50 mL of Affi-gel 10 to cystamine, reduction of the cystamine disulphide, and then sulfhydryl coupling to bromoacetylcholine bromide (1.5 g). The column was then equilibrated with ∼15 column volumes of the lipid mixture of choice in 1% cholate in VDB (0.2 mL/min; >15 h). The solubilized material was slowly applied to the affinity column (0.3 mL/min, ∼24 h, at 4°C). The column was then washed extensively with the defined lipid solution (0.2-0.9 mg/mL lipid) in 1% cholate in VDB (15 column volumes; >15 h). This extensive wash ensures complete exchange of endogenous lipids for the defined lipids mixture (13, 9). nAChRs were eluted from the column using the defined lipid solution (0.2 mg/mL; 0.05 mg/mL for low lipid-protein ratio samples) containing 10 mM carbamylcholine. Peak protein fractions (A280 × 0.6; in Ref. 16) were pooled and the lipid-protein molar ratio adjusted to 400 to 1 except were specified. To remove Carb and reconstitute nAChRs into membranes containing the defined lipid mixture, pooled fractions were dialyzed against 2L of VDB (4 d with buffer change once every 24 h). The reconstituted nAChRs were aliquoted (0.25 mg per tube) and stored at −800C. The final lipid composition of each reconstituted membrane was assessed by thin layer chromatography (TLC) using precoated silica gel K60 F254 TLC plates (5 × 20 cm; EM Sciences, Gibbstown, NJ). The TLC solvent was chloroform, methanol, water (65:24:4) and lipids visualized by iodine vapor. The final lipid composition of each reconstituted sample did not vary qualitatively from that of the control lipid mixture and no endogenous lipids were detected. For low lipid-protein ratio samples, protein (17) and phosphate assays (18) of select samples were used to verify a centrifugation based standard curve in which samples were centrifuged for 2 h at 18,000 rpm in JA-20 rotor and the ratio of the amount of material in the supernatant vs. pellet plotted as a function of the estimated lipid-protein ratio (r2 = 0.9895).
Photolabeling of Reconstituted nAChR Membranes with [125I]TID
The nAChR conformation and agonist-induced state transitions were assessed by the technique of hydrophobic photolabeling with 3-trifluoromethyl-3-(m-[125I]iodophenyl) diazirine ([125I]TID) as described in detail elsewhere (9, 14). Briefly, an aliquot of affinity-purified nAChRs reconstituted into lipid vesicles of defined composition (250 μg; 0.227 mg/mL in VDB) were incubated for 1 h at room temperature with [125I]TID at a final concentration of ∼ 0.4 μM, with or without 400 μM Carb. Incubations were performed in glass test tubes and under reduced lighting conditions. The samples were then irradiated with a 365 nm UV lamp (Spectroline EN-280L) for 7 minutes at a distance of less than 1 cm and pelleted by centrifugation (18,000 rpm for 1 h in JA-20 rotor). Pellets were solubilized in electrophoresis sample buffer and subjected to SDS-PAGE. For nAChRs at low lipid-protein ratios, material in both the centrifugal pellet and supernatant were subjected to SDS-PAGE (see lipid-protein ratio standard curve in the previous section).
SDS-Polyacrylamide Gel Electrophoresis
SDS-PAGE was performed according to the method of Laemmli (19) with 1.0 mm thick gels, containing separating gels composed of 8% polyacrylamide/0.33% bis-acrylamide. Following electrophoresis, gels were stained with Coomassie Blue R-250 (0.25% (w/v) in 45% methanol, 10% acetic acid, 45% H2O) and destained (25% methanol, 10% acetic acid, 65% H2O) to visualize nAChR subunit bands. Polyacrylamide gels were dried and exposed to Kodak X-OMAT LS film with an intensifying screen at −80°C (2-18 h exposure). [125I]TID incorporation into nAChR subunits was quantified by cutting out the stained receptor bands from the dried 8% acrylamide gel and the amount of 125I cpm present in each band determined by γ-counting in a Packard Cobra II gamma counter (5 min counting time/ band). Included in the γ-counting of the γ-subunit, was a proteolytic fragment of the γ–subunit designated γ' (Fig. 1A; Ref. 9). For each [125I]TID labeling experiment, the amount 125I cpm incorporated into individual nAChR subunits under a given condition (i.e in the presence or absence of carb) was graphically represented (bar graph) as the average of at least three determinations (that is from at least three gel lanes). Due to the radioactive decay of 125I (half-life is ∼60 d), total cpm levels in nAChR subunits vary significantly for [125I]TID samples labeled at different times and between different batches of [125I]TID. Therefore as a normalization function (which allows comparison of different samples), for each [125I]TID labeling experiment, the ratio of the amount of [125I]TID labeling in the γ– and α–subunit (γ/α ratio) in the absence and presence of agonist was calculated. The nAChR contains two α-subunits, and therefore the value representing half the amount of incorporation into the α-subunits was used to calculate the γ/α ratio on a mole per mole basis. Finally, the reported γ/α ratio's represent the mean value determined from at least three different [125I]TID labeling experiments (that is from three different bar graphs) with error bars indicating the standard error when represented graphically.
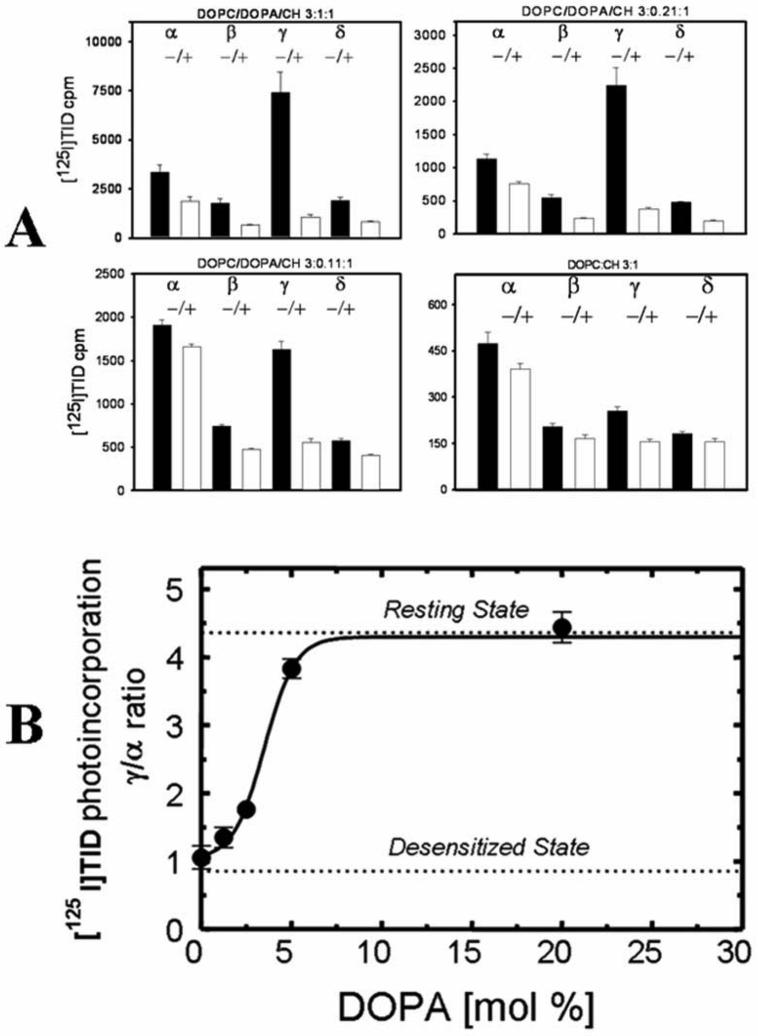
Figure 1. Effect of cholesterol levels in DOPC/DOPA membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
Affinity-purified nAChRs were reconstituted into membranes comprised of DOPC, DOPA and increasing amounts of cholesterol. The molar ratio of each lipid mixture was 3:1:[X] respectively. Reconstituted membranes were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and polypeptides resolved by SDS-PAGE. A, corresponding autoradiographs of gels containing the [125I]TID labeling experiments for representative lipid mixtures. The positions of the nAChR subunits and a proteolytic fragment of the γ-subunit (γ') are indicated on the left. B, for each [125I]TID labeling experiment shown above, individual nAChR subunit bands were excised from the dried gel and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. Shown are bar graphs of the amount of 125I cpm incorporated into each nAChR subunit in the absence or presence of Carb (−/+) and presented as the average of triplicate determinations from a single labeling experiment (error bars indicate the standard error). C, Due to radioactive decay (125I) the total cpm levels vary significantly from sample to sample and therefore as a normalization function, for each [125I]TID labeling experiment, the ratio of the amount of [125I]TID labeling in the γ– and α– subunit in the absence of agonist was calculated. Shown is the relationship between the molar percentage of cholesterol in the membrane and the functionality of the nAChR as measured by [125I]TID labeling (γ/α ratio). The γ/α ratio points (●) are means of three different [125I]TID labeling experiments (error bars indicate standard error, see also supporting information). For comparison, the γ/α ratios for nAChRs fully stabilized in the resting and desensitized states are indicated with a dotted line.
RESULTS
Assessing the Cholesterol Requirements
The first set of experiments were designed to quantify the relationship between the cholesterol content of the membrane and nAChR functionality. To achieve this, purified nAChRs were reconstituted into lipid vesicles comprised of DOPC and DOPA and increasing amounts of cholesterol. The molar ratio of each lipid was (DOPC/DOPA/CH, 3:1:[X]) and the overall lipid-protein molar ratio was adjusted to ∼400:1, the approximate lipid-protein ratio in native Torpedo membranes (6). nAChR functionality was assessed using the conformationally sensitive probe [125I]TID (9,14,20-22). Reconstituted nAChRs were incubated with [125I]TID (0.4μM) in the absence and in the presence of 400 μM Carb for 1 h at room temperature under reduced lighting conditions. The samples were then irradiated at 365nm and receptor subunits resolved by SDS-PAGE. The amount of [125I]TID incorporation into each nAChR subunit was determined qualitatively by autoradiography (Fig 1A) and quantatively by γ-counting of excised gel subunit bands (Fig.1B). The extent and pattern of [125I]TID incorporation into nAChR subunits is reflective of the conformation/state in which the receptor is stabilized (9, 14, 20-22). In native Torpedo nAChR-enriched membranes (22) as well as in reconstituted membranes comprised of DOPC/DOPA/CH (3:1:1; Ref. 9) and in the absence of agonist (resting state), [125I]TID photoincorporates into the nAChR γ-subunit at a level four times greater than into the α, β, and δ-subunits on a molar basis (γ/α labeling ratio = 4). In contrast, in the presence of agonist (equilibrium conditions, receptor is in the desensitized state) there is a 2-10-fold reduction in subunit labeling and the γ/α ratio is ∼1 (9, 14, 22). The γ/α ratio thereby provides a quantitative estimate of the proportion of nAChRs stabilized in the resting vs. desensitized state and agonist-induced changes in the γ/α ratio reflect the ability of the receptor to undergo agonist-induced state transitions (i.e functionality). As an assay of receptor functionality, the [125I]TID labeling method has been validated by several different methods (9, 14) and has several distinct advantages including: it is not dependent on the quality (sealed) or morphology of reconstituted lipid vesicles, is independent of the amount of protein loaded onto a given gel, and of the degree of radioactive decay for [125I]TID (i.e 125I).
Shown in Fig. 1A and 1B are representative autoradiographs of SDS-PAGE gels containing [125I]TID labeled nAChRs reconstituted in DOPC/DOPA lipid mixtures containing 0, 10, 15, and 20 mol% cholesterol, and the corresponding amounts 125I cpm ([125I]TID) incorporated into each nAChR subunit. Using the γ/α labeling ratio for nAChRs in the absence of agonist as a measure of functionality (that is ability to undergo agonist-induced state transitions), the relationship between the percentage of the cholesterol in the membrane and nAChR functionality is plotted in Fig.1C. In the absence of cholesterol (DOPC/DOPA alone) a small but significant proportion of nAChRs are stabilized in the resting state (γ/α labeling ratio = 1.83) and undergo an agonist-induced reduction in labeling (Fig. 1A, 1B). As the amount of cholesterol in the membrane increases, the γ/α ratio (nAChR functionality) increases and saturates (γ/α labeling ratio = 4.6) at a cholesterol content of ∼35 mol%, which is the amount of cholesterol present in the native Torpedo membranes. These results establish that cholesteol is not absolutely required for nAChR functionality (23% efficacy in the absence of cholesterol; see also Ref. 9) but robust levels of cholesterol are required to achieve full functionality.
Assessing the Phosphatidic Acid Requirements
Purified nAChRs reconstituted into lipid vesicles comprised of DOPC alone are stabilized in the desensitized (or a desensitized-like) state and are completely nonfunctional (9, 14, 32). As shown in the previous section, receptors reconstituted into vesicles containing both DOPC and DOPA at molar ratio of 3:1 are partially functional (Fig. 1B and 1C). To further quantify the effect of membrane DOPA on nAChR functionality, purified receptors were reconstituted into lipid vesicles containing DOPC, cholesterol, and increasing amounts of DOPA. The molar ratio of each lipid mixture was DOPC/DOPA/CH 3:[X]:1 and the overall lipid-protein molar ratio was adjusted to ∼ 400:1. Fig 2A shows the amount of 125I cpm ([125I]TID) incorporated into each nAChR subunit for receptors reconstituted into lipid mixtures containing 0, 2.5, 5 and 20 mol% DOPA in the absence (−lane) and presence (+ lane) of agonist. The relationship between the percentage of the DOPA in the reconstituted membrane and receptor functionality is shown in Fig. 2B. In the absence of DOPA (DOPC/CH alone) and in the absence of agonist the receptor is stabilized in the nonfunctional desensitized (or desensitized-like) state (γ/α labeling ratio = 1.06), indicating that there is an absolute requirement of DOPA for nAChR functionality (but see Ref. 9). As the amount of DOPA in the membrane increases, the resting γ/α labeling ratio (nAChR functionality) increases and saturates (γ/α ratio = 4.4) at about 12 mol% DOPA.
Figure 2. Effect of DOPA levels in DOPC/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
Affinity-purified nAChRs were reconstituted into membranes comprised of DOPC, cholesterol and increasing amounts of DOPA. The molar ratio of each lipid mixture (DOPC/DOPA/CH) was 3:[X]: 1 respectively. Reconstituted membranes were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and polypeptides resolved by SDS-PAGE. A, for each [125I]TID labeling experiment, individual nAChR subunit bands were excised from the dried gel and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. Shown are bar graphs of the amount of 125I cpm incorporated into each nAChR subunit in the absence or presence of Carb (−/+) and presented as the average of triplicate determinations from a single [125I]TID labeling experiment (error bars indicate the standard error). B, for each [125I]TID labeling experiment, the ratio of the amount of [125I]TID labeling in the γ– and α–subunit in the absence of agonist was calculated. Shown is the relationship between the molar percentage of DOPA in the membrane and the functionality of the nAChR as measured by [125I]TID labeling (γ/α ratio). The γ/α ratio points (●) are means of three different [125I]TID labeling experiments (error bars indicate standard error, see also supporting information). For comparison, the γ/α ratios for nAChRs fully stabilized in the resting and desensitized states are indicated with a dotted line.
Assessing the Phosphatidylserine Requirements
The results in Figure 2A and 2B indicate that there is an absolute requirement of DOPA for nAChR functionality, and to fully support functionality the membrane should contain at least 12 mol% DOPA. This level of DOPA is at least an order of magnitude greater than that found in native Torpedo membranes (∼0.3 mol%; Ref. 6). We hypothesize that these results indicate that other anionic lipids, such as PS and PI, which are present in much greater abundance (∼9 and 3 mol% respectively) in native Torpedo membranes are able to substitute for PA. To test the ability of phosphatidylserine to support nAChR functionality, purified nAChRs were reconstituted into lipid vesicles receptors comprised of DOPC/CH and increasing amounts of phosphatidylserine (DOPS). The molar ratio of each lipid was DOPC/DOPS/CH 3:[X]:1 and the overall lipid-protein molar ratio was adjusted to ∼ 400:1. Fig 3A shows the amount of 125I cpm ([125I]TID) incorporated into each nAChR subunit for nAChRs reconstituted into DOPC/CH lipid mixtures containing 0, 5, 10 and 20 mol% DOPS, in the absence (−lane) and presence (+ lane) of agonist. The relationship between the percentage of the DOPS in the membrane and receptor functionality is shown in Fig. 3B. Increasing levels of DOPS in DOPC/CH membranes leads to increased receptor functionality, but the effect saturates without achieving maximum functionality (γ/α labeling ratio = 3.12). These results indicate that DOPS can substitute for DOPA in supporting nAChR functionality, but with a reduced efficacy (60%).
Figure 3. Effect of DOPS levels in DOPC/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
Affinity-purified nAChRs were reconstituted into membranes comprised of DOPC, cholesterol and increasing amounts of DOPS. The molar ratio of each lipid mixture (DOPC/DOPS/CH) was 3:[X]: 1 respectively. Reconstituted membranes were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and polypeptides resolved by SDS-PAGE. A, for each [125I]TID labeling experiment, individual nAChR subunit bands were excised from the dried gel and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. Shown are bar graphs of the amount of 125I cpm incorporated into each nAChR subunit in the absence or presence of Carb (−/+) and presented as the average of triplicate determinations (error bars indicate the standard error). B, for each [125I]TID labeling experiment, the ratio of the amount of [125I]TID labeling in the γ– and α–subunit in the absence of agonist was calculated. Shown is the relationship between the molar percentage of DOPS in the membrane and the functionality of the nAChR as measured by [125I]TID labeling (γ/α ratio). The γ/α ratio points (●) are means of three different [125I]TID labeling experiments (error bars indicate standard error, see also supporting information). For comparison, the γ/α ratios for nAChRs fully stabilized in the resting and desensitized states are indicated with a dotted line.
Assessing the Phosphatidylinositol Requirements
Purified nAChRs were reconstituted into lipid vesicles containing DOPC/CH and increasing levels of phosphatidylinositol (PLPI). The molar ratio of each lipid was DOPC/PLPI/CH 3:[X]:1 at a lipid-protein molar ratio of ∼400:1. Fig 4A shows the amount of 125I cpm ([125I]TID) incorporated into each nAChR subunit for nAChRs reconstituted into DOPC/CH lipid mixtures containing 0, 5, 10 and 20 mol% PLPI, in the absence (−lane) and presence (+ lane) of agonist. The relationship between the percentage of PLPI in the membrane and receptor functionality is shown in Fig. 4B. At the highest level of PLPI in the membrane (20 mol%), receptor functionality was approximately 50% of the maximum (γ/α labeling ratio = 2.65). These results indicate that PI like PS can support nAChR functionality, but with reduced efficacy compared to PA.
Figure 4. Effect of PLPI levels in DOPC/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
Affinity-purified nAChRs were reconstituted into membranes comprised of DOPC, cholesterol and increasing amounts of PLPI. The molar ratio of each lipid mixture (DOPC/PLPI/CH) was 3:[X]: 1 respectively. Reconstituted membranes were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and polypeptides resolved by SDS-PAGE. A, for each [125I]TID labeling experiment, individual nAChR subunit bands were excised from the dried gel and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. Shown are bar graphs of the amount of 125I cpm incorporated into each nAChR subunit in the absence or presence of Carb (−/+) and presented as the average of triplicate determinations (error bars indicate the standard error). B, for each [125I]TID labeling experiment, the ratio of the amount of [125I]TID labeling in the γ– and α–subunit in the absence of agonist was calculated. Shown is the relationship between the molar percentage of PLPI in the membrane and the functionality of the nAChR as measured by [125I]TID labeling (γ/α ratio). The γ/α ratio points (●) are means of three different [125I]TID labeling experiments (error bars indicate standard error, see also supporting information). For comparison, the γ/α ratios for nAChRs fully stabilized in the resting and desensitized states are indicated with a dotted line.
Synergistic Action of Anionic Phospholipids
Given that DOPA (in the presence of cholesterol) can only fully support nAChR functionality at a level that is at least 10 times higher than that found in native Torpedo membranes, and that neither PS nor PI alone (that is PC/PS/CH or PC/PI/CH) can fully support receptor functionality, we next tested the possibility that the combined presence of these anionic lipids may exert a synergistic effect on nAChR functionality. Purified nAChRs were reconstituted into lipid vesicles comprised of DOPC/DOPS/PLPI/DOPA/CH 1.5:0.3:0.15:0.05:1 (50% PC/ 10% PS/ 5% PI/ 1.6% PA/ 33% CH), reflecting levels of anionic lipids and cholesterol found in native Torpedo membranes. As shown in Fig 5A and 5B, the pattern of [125I]TID photoincorporation into nAChR subunits in the absence of agonist (− lane) is reflective of the receptor stabilized in the resting state and addition of agonist (+ lane) significantly reduces the extent of [125I]TID incorporation into each subunit. The calculated γ/α labeling ratio in the absence of agonist was 4.2, indicating that the receptor is fully stabilized in the resting state, while in the presence of agonist a ratio of approximately 1 is consistent with stabilization of the desensitized (or desensitized-like) state. The combined levels of DOPS and PLPI (15 mol%) present in this reconstituted lipid mixture did not fully support receptor functionality when present alone at this same level (15 mol%; Figs 3B, 4B ). Further, the amount of PA present in this lipid mixture (1.6 mol%) did not stabilize a significant proportion of nAChRs in the resting state (γ/α ratio ∼1.3) when present alone (Fig. 2C). These results suggest that in combination (PA, PS, PI and cholesterol) work together in an independent and synergistic fashion to fully support receptor functionality. If DOPA is left out of the anionic lipid mixture, that is (DOPC/DOPS/PLPI/CH; 1.5:0.3:0.15:1 or molar percentages of 51/10/5/34), there is a small but significant reduction in the γ/α ratio (3.6) suggesting that even small levels of PA contribute to receptor functionality (data not shown).
Figure 5. The synergistic effect of anionic phospholipids in DOPC/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
Affinity-purified nAChRs were reconstituted into lipid vesicles comprised of DOPC/DOPS/DOPI/DOPA/CH at a molar ratio of 1.5:0.3:0.15:0.05:1 (that is 50% PC/ 10% PS/ 5% PI/ 1.6% PA/ 33% CH). The reconstituted membranes were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and the polypeptides resolved by SDS-PAGE. A, corresponding autoradiograph of SDS-PAGE gel containing [125I]TID labeled nAChRs reconstituted into DOPC/DOPS/DOPI/DOPA/CH membranes. The positions of the nAChR subunits are indicated on the left. B, Labeled nAChR subunit bands were excised and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min of counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. Shown are bar graphs of the amount of 125I cpm incorporated into each nAChR subunit in the absence or presence of carbamylcholine (−/+) and presented as the average of triplicate determinations (error bars indicate the standard error). In the absence of agonist, the ratio of [125I]TID labeling in the γ– and α–subunit was calculated (γ/α ratio = 4.6).
Assessing the Phosphatidylcholine Requirements
Phosphatidylcholine (PC) is a zwitterionic phospholipid that is present in high abundance in native Torpedo membranes (∼26 mol% of total membrane lipid; Ref. 6). While nAChRs reconstituted into vesicles containing DOPC alone are stabilized in the nonfunctional desensitized (or desensitized-like) state (9,14), it remains possible that PC has a role in supporting nAChR functionality. To test this possibility, purified nAChRs were reconstituted into lipid vesicles comprised of DOPA/CH and increasing levels of DOPC. The molar ratio of each lipid was (DOPC/DOPA/CH, [X]:1:1) at a lipid-protein molar ratio of ∼400:1. The extent and pattern of [125I]TID labeling was identical for each concentration of DOPC tested (0, 20, 33, and 50 mol%) with the autoradiograph and 125I cpm incorporated into each subunit for 0 mol% DOPC (that is DOPA/CH alone) shown in Fig. 6A and 6B. In DOPA/CH alone, the nAChR is fully functional, the calculated γ/α labeling ratio is 4.6 in the absence of agonist and there is an ∼7-10 fold reduction in subunit labeling in the presence of agonist (γ/α = 1.1). These results indicate that DOPC is not required for stabilization of the nAChR in the resting state or for agonist-induced state transitions.
Figure 6. Effect of the absence of DOPC in DOPA/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
Affinity-purified nAChRs were reconstituted into lipid vesicles comprised of DOPA/CH at a molar ratio of 1:1 (no DOPC is present in lipid mixture). The reconstituted membranes were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and the polypeptides resolved by SDS-PAGE. A, corresponding autoradiograph of SDS-PAGE gel containing [125I]TID labeled nAChRs reconstituted into DOPA/CH membranes. The positions of the nAChR subunits are indicated on the left. B, Labeled nAChR subunit bands were excised and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min of counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. Shown are bar graphs of the amount of 125I cpm incorporated into each nAChR subunit in the absence or presence of carbamylcholine (−/+) and presented as the average of triplicate determinations (error bars indicate the standard error). In the absence of agonist, the ratio of [125I]TID labeling in the γ– and α–subunit was calculated (γ/α ratio = 4.2).
Assessing the Minimum Lipid Requirements
The effects of specific membrane lipids on nAChR functionality have been ascribed to both bulk lipid bilayer properties (10) and/or direct effects on the nAChR protein (7,13; the present study). The seminal report by Jones et al. (13) established that a minimum of ∼45 lipids are required to retain nAChR functionality, consistent with a functional requirement for a single shell of lipids surrounding the protein. However, in these studies the lipid-protein ratio was adjusted by progressive delipidation using ever increasing concentrations (>20%) of detergent (cholate). While the authors reported that there was negligible association of cholate with the nAChR, given the high concentrations involved it remains possible that cholate directly interacts with the protein leading to receptor inactivation (23), rather than solely by removing lipid. To avoid the use of high detergent concentrations, we reconstituted purified nAChRs into lipid vesicles containing DOPA/CH (1:1) at low lipid-protein ratios by adjusting the amount of protein and lipid present during the purification and reconstitution steps. The functionality of the nAChR at each lipid-protein ratio was then assessed using [125I]TID labeling. As shown in Fig. 7 the receptor remains fully stabilized in the resting state (γ/α ratio ∼4.6) and is able to undergo agonist-induced state transitions (data not shown) as the lipid-protein is reduced from 400:1 to ∼65:1. However below ∼65:1 there is a rapid decrease in the proportion of nAChRs stabilized in resting state and below ∼30:1 the receptor is completely stabilized in the desensitized (or desensitized-like) state. These results are consistent with those of Jones et al. (13) and indicate that nAChR functionality can be maintained at a relatively low lipid-protein ratio (∼ 65:1), with receptor functionality rapidly lost only when the number of lipids falls below that needed to completely surround the transmembrane domain of the protein.
Figure 7. Effect of reducing the lipid-protein molar ratio for DOPA/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
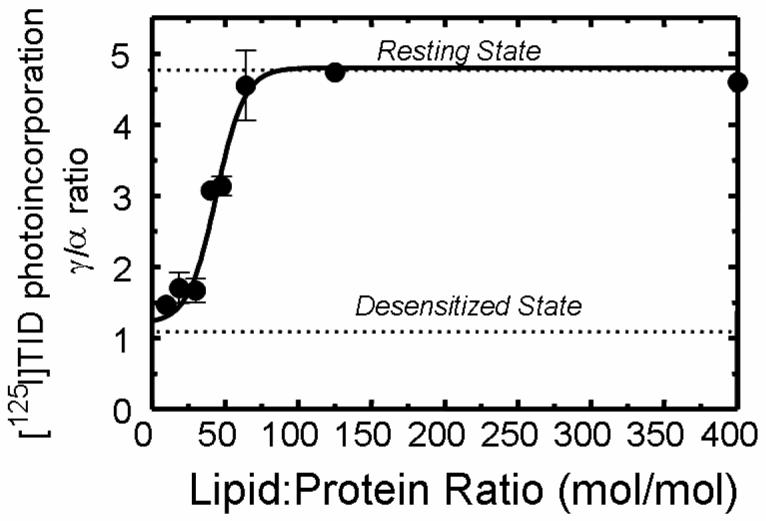
Affinity-purified nAChRs were reconstituted into membranes comprised of DOPA/CH (1:1) in which the overall lipid-protein molar ratio was adjusted to 400:1, 125:1, 65:1, 48:1, 45:1, 30:1, 18:1, and 9:1 respectively. The functionality of the nAChR was determined using [125I]TID labeling as described in Experimental Procedures. Briefly, samples were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and the polypeptides resolved by SDS-PAGE. Labeled nAChR subunit bands were excised from the dried gel and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min of counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. The ratio of [125I]TID labeling in the γ– and α–subunit in the absence of agonist was calculated. Shown is the relationship between the lipid-protein molar ratio and the functionality of nAChR as indicated by the γ/α labeling ratio (error bars indicate the standard error). The γ/α ratio points (●) are means of three different [125I]TID labeling experiments (error bars indicate standard error, see also supporting information). For comparison, the γ/α ratios for nAChRs fully stabilized in the resting and desensitized states are indicated with a dotted line
DISCUSSION
The main goal of this work was to further assess the lipid requirements for nAChR functionality (as defined by the ability of the nAChR to undergo agonist-induced state transitions) as an important step in understanding the underlying molecular mechanisms. In particular, we wished to test two central hypotheses: 1) that anionic lipids other than PA, such as PS and PI, will support receptor functionality; 2) that a single shell of lipids surrounding the nAChR protein is sufficient to fully support receptor functionality.
Cholesterol Requirements
Consistent with earlier studies (9,14) we found that there is no absolute cholesterol requirement for nAChR functionality. When purified nAChRs are reconstituted into PC/PA membranes, small but significant proportions of receptors are stabilized in the resting state and undergo agonist-induced state transitions (23% efficacy; Fig. 1C). However, receptor functionality increases as the amount of cholesterol in DOPC/DOPA membranes is increased, the effect saturating at ∼35 mol% (see also Ref. 24). The amount of cholesterol (35 mol%) required for maximum receptor functionality mirrors the amount found in native Torpedo membranes (6). Previous studies have demonstrated that: 1) lipids surrounding the nAChR protein (annular lipid) and bulk lipids exchange rapidly (2); 2) that the composition of annular lipids is the same as bulk lipid (12,13); and 3) the number of annular lipids is ∼45 (13). Given these results, a requirement for 35 mol% cholesterol extrapolates to ∼15 sterol molecules in the nAChR lipid annulus or approximately three sterol molecules per receptor subunit. Further analysis of the lipid composition surrounding a single receptor subunit indicates that in each leaflet of the bilayer there are ∼4-5 lipids with about 1-2 sterol molecules contributing to the total. These results in combination with structural information regarding the nAChR lipid-protein interface (25,26) are likely to be useful to molecular dynamic studies aimed at gaining insight into the nature of sterol-protein interactions. At the present time, the underlying molecular mechanisms responsible for cholesterol effects on nAChR functionality (1) are by no means well understood. Structural features of the cholesterol molecule known to be important in modulating lipid bilayer properties are not required for support of nAChR functionality, but also indicate that putative sterol binding sites on the receptor protein must possess very lax structural requirements (27). While spin-labeled and photoreactive cholesterol analogs have been used to demonstrate interaction with the nAChR lipid-protein interface (28-30), the presence or absence of cholesterol has no detectable effect on the overall secondary structure of the receptor (31, 32).
Zwitterionic and Anionic Phospholilipid Requirements
Purified nAChRs reconstituted into vesicles comprised of PA/CH (1:1) alone are fully functional, they are stabilized in the resting state (γ/α ratio = 4.6) and undergo agonist-induced state transitions (Fig. 6). These results indicate that the presence of zwitterionic lipids is not required for nAChR functionality, at least by this measure of receptor functionality. Zwitterionic lipids (e.g. PC, PE), which represent ∼42 mol% of native Torpedo membranes, do however interact with the nAChR protein. This is indicated by studies using both spin-labeled and photoreactive analogs lipid analogs (28, 33) and by studies which indicate that the composition of the lipid annulus is the same as that of the bulk lipid bilayer (12, 13). We interpret these results as indicating that zwitterionic lipids exert a neutral, as opposed to a negative, effect on receptor functionality. For example, nAChRs reconstituted into PA/CH (1:1) lipid vesicles are functionally indistinguishable from receptors in PC/PA/CH (3:1:1). This presupposes that cholesterol and the anionic lipid (PA) exert a required positive, rather than neutral, effect on receptor functionality. For example, nAChRs in DOPC alone are nonfunctional but inclusion of either PA or cholesterol serves to stabilize increasing proportions of receptors in the resting state.
Purified nAChRs reconstituted into lipid vesicles comprised of PC/CH alone are nonfunctional; receptors are stabilized in the desensitized state and are unable to undergo agonist-induced state transitions (Figs. 2-4). These results indicate that there is an absolute requirement for the anionic lipid PA. Receptor functionality increases to its maximum level as the amount of PA in DOPC/CH membranes is increased, the effect saturating at ∼12 mol% (Fig 2). This extrapolates to ∼5 PA molecules present in the lipid annulus or about 1 per receptor subunit. On the other hand, a requirement of 12 mol% PA for maximum receptor functionality contrasts with levels of PA found in native Torpedo membranes that are at least an order of magnitude lower (∼0.3 mol%; Ref. 6). We tested the hypothesis that other anionic lipids, such as PS and PI which are relatively abundant in native Torpedo membranes (∼9, 4 mol% respectively), are able to substitute for PA in supporting receptor functionality. Our results indicate that PS and PI are able to substitute for PA, at least partially. Purified nAChRs reconstituted into PC/CH membranes containing either PS (Fig. 3) or PI (Fig. 4) at levels up to 20 mol%, stabilize significant proportions of receptors in the resting state that are capable of undergoing agonist-induced state transitions, but with reduced efficacy (50-60%, respectively) compared to PA. That PS or PI at levels as great as 20 mol% are unable to support nAChR functionality to its maximum level suggests that if in combination these lipid are able to fully support receptor functionality they must do so through an independent and synergistic mechanism rather than by a simple additive effect. We tested this by reconstituting purified nAChRs in membranes containing DOPC/DOPS/PLPI/DOPA/CH at molar levels that reflect those found in native Torpedo membranes (50%/10%/5%/1.6%/33%; Fig. 6). That these receptors are fully functional (γ/α ratio = 4.2) cannot be explained by the additive effect of a single anionic lipid effect on receptor functionality. The combined levels of DOPS and PLPI (15 mol%) present in this reconstituted lipid mixture did not fully support receptor functionality when present alone at this same level (15 mol%; Figs 3B, 4B ) and the amount of PA present in this lipid mixture (1.6 mol%) did not stabilize a significant proportion of nAChRs in the resting state (γ/α ratio ∼1.3) when present alone (Fig. 2C). These results can be explained by each anionic lipid having an independent and synergistic effect. The same conclusion was reached regarding the effects of cholesterol and PA on receptor functionality (9; this report). The proposition that different anionic lipids exert independent effects on nAChR functionality is underscored by results that show that PA and PS interact with the receptor in distinct fashions (11); by studies which demonstrate that charge-interactions do not explain the association of anionic lipids with the nAChR protein (reviewed in Ref. 3); and by differing selectivity's of anionic lipids for the receptor (6,28,34). The presence or absence of anionic lipids has no detectable effect on the overall secondary structure composition of the nAChR protein (31,32), therefore, anionic lipids must exert more subtle structural alterations on the receptor to affect functionality.
Membrane Lipids Affect nAChR Functionality through Direct Interactions
Consistent with the findings of Jones et al. (13) we find that nAChR functionality is fully maintained by a single shell of lipids surrounding the receptor protein. Purified nAChRs reconstituted into PA/CH membranes at lower and lower lipid-protein molar ratios retain full functionality (γ/α ratio >4) until the lipid-protein ratio is reduced below ∼65:1 and are completely nonfunctional below ∼30:1 (Fig. 7). A variety of different methods have shown the lipid annulus surrounding the nAChR protein is comprised of ∼45 lipid molecules (6,13). The implication of these results is that if a single shell of lipids surrounding the nAChR protein is sufficient to fully support functionality, at least as measured by the ability of the receptor to undergo agonist-induced state transitions, then direct protein-lipid interactions are what fundamentally underlie the specific lipid requirements of the nAChR. Undoubtedly properties of the bulk lipid bilayer (e.g. fluidity, microdomains, etc) influence the functionality of the nAChR, but they likely do so by affecting the properties of the lipid annulus. Determining the domains and individual amino acid side-chains of the nAChR that interact with specific structural features of cholesterol and anionic lipids will likely provide critical insight into the underlying molecular mechanisms. However, the nature of the molecular interactions of cholesterol and anionic lipids with the nAChR lipid-protein interface are likely to be far more complicated than perhaps initially thought. As examples of this: as stated earlier electrostatic interactions do not explain the association anionic lipids with the receptor; sterol binding sites at the lipid-protein interface must possess very lax structural requirements; the nature of the interactions between phospholipid fatty acid chains and the nAChR protein contribute to the functional requirements of the nAChR, this is illustrated by the fact that saturated mixtures of PC/PA do not support receptor functionality while unsaturated mixtures do (35).
Supplementary Material
1 Abbreviations
- nAChR
nicotinic acetylcholine receptor
- Carb
carbamylcholine
- MOPS
4-morpholinopropanesulfonic acid
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- VDB
vesicle dialysis buffer
- [125I]TID
3-trifluoromethyl-3-(m-[125I]iodophenyl) diazirine
- DOPA
dioleoyl phosphatidic acid
- DOPS
dioleoyl phosphatidylserine
- PLPI
1-palmitoyl-2-linoleoyl phosphatidylinositol
- DOPC
dioleoyl phosphatidylcholine
- CH
cholesterol
Footnotes
This research was supported in part by National Institutes of Health Grant NS43438 (T.K.M.) and by an Intramural Grant from Texas Tech University Health Sciences Center School of Medicine (M.P.B.).
REFERENCES
- 1.Burger K, Gimpl G, Fahrenholz F. Regulation of receptor function by cholesterol. Cellular and Molecular Life Sciences. 2000;57:1577–1592. doi: 10.1007/PL00000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrantes FJ. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Research Reviews. 2004;47:71–95. doi: 10.1016/j.brainresrev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Tillman T, Cascio M. Effect of membrane lipids on ion channel structure and function. Cell Biochemistry and Biophysics. 2003;38:161–190. doi: 10.1385/CBB:38:2:161. [DOI] [PubMed] [Google Scholar]
- 4.Corringer PJ, Le Novere N, Changeux J-P. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 5.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nature Rev. Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 6.Mantipragada SB, Horvath LI, Arias HR, Schwarzmann G, Sandhoff K, Barrantes FJ, Marsh D. Lipid-protein interactions and effect of local anesthetics in acetylcholine receptor-rich membranes from Torpedo marmorata electric organ. Biochemistry. 2003;42:9167–9175. doi: 10.1021/bi034485q. [DOI] [PubMed] [Google Scholar]
- 7.Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986;25:830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 8.Criado M, Eibl H, Barrantes FJ. Effects of lipid on acetylcholine receptor: essential need of cholesterol for maintenance of agonist-induced state transitions in lipid vesicles. Biochemistry. 1982;21:3622–3629. doi: 10.1021/bi00258a015. [DOI] [PubMed] [Google Scholar]
- 9.daCosta CJB, Ogrel AA, McCardy EA, Blanton MP, Baenziger JE. Lipid-protein interactions at the nicotinic acetylcholine receptor. J. Biol. Chem. 2002;277:201–208. doi: 10.1074/jbc.M108341200. [DOI] [PubMed] [Google Scholar]
- 10.Baenziger JE, Morris M-L, Darsuat TE, Ryan EE. Effect of membrane lipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J. Biol. Chem. 2000;275:777–784. doi: 10.1074/jbc.275.2.777. [DOI] [PubMed] [Google Scholar]
- 11.daCosta CJB, Wagg ID, McKay ME, Baenziger JE. Phosphatidic acid and phosphatidylserine have distinct structural and functional interactions with the nicotinic acetylcholine receptor. J. Biol. Chem. 2004;279:14967–14974. doi: 10.1074/jbc.M310037200. [DOI] [PubMed] [Google Scholar]
- 12.Chang HW, Bock E. Structural stabilization of isolated acetylcholine receptor: specific interaction with phospholipids. Biochemistry. 1979;18:172–179. doi: 10.1021/bi00568a026. [DOI] [PubMed] [Google Scholar]
- 13.Jones OT, Eubanks JH, Earnest JP, McNamee MG. A minimum number of lipids are required to support the functional properties of the nicotinic acetylcholine receptor. Biochemistry. 1988;27:3733–3742. doi: 10.1021/bi00410a032. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy MP, Moore MA. Effect of lipids and detergents on the conformation of the nicotinic acetylcholine receptor from Torpedo californica. J. Biol. Chem. 1992;267:7655–7663. [PubMed] [Google Scholar]
- 15.Pedersen SE, Dreyer EB, Cohen JB. Location of ligand-binding sites on the nicotinic acetylcholine receptor alpha-subunit. J. Biol. Chem. 1986;261:13735–13743. [PubMed] [Google Scholar]
- 16.Bushan A, McNamee MG. Differential scanning calorimetry and fourier transform infrared analysis of lipid-protein interactions involving the nicotinic acetylcholine receptor. Biochim. Biophys. Acta. 1990;1027:93–101. doi: 10.1016/0005-2736(90)90053-q. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr L, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Rousser G, Fleisher S, Yamamoto A. Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.White BH, Howard S, Cohen JB. The hydrophobic photoreagent 3-(trifluoromethyl)-3-m-([125I]iodophenyl) diazirine is a novel noncompetitive antagonist of the nicotinic acetylcholine receptor. J. Biol. Chem. 1991;266:21595–21607. [PubMed] [Google Scholar]
- 21.Chiara DC, Kloczewiak MA, Addona GH, Yu J-A, Cohen JB, Miller KW. Site of resting state inhibition of the nicotinic acetylcholine receptor by a hydrophobic inhibitor. Biochemistry. 2001;40:296–304. doi: 10.1021/bi0021481. [DOI] [PubMed] [Google Scholar]
- 22.White BH, Cohen JB. Photolabeling of membrane bound Torpedo nicotinic acetylcholine receptor with the hydrophobic probe 3-trifluoromethyl-3-(m-[125I]iodophenyl) diazirine. Biochemistry. 1988;27:8741–8751. doi: 10.1021/bi00424a009. [DOI] [PubMed] [Google Scholar]
- 23.Heidmann T, Sobel A, Changeux J-P. Recovery of allosteric interactions between a fluorescent cholinergic agonist and local anesthetics after removal of the detergent from cholate-solubilized membrane fragments rich in acetylcholine receptor. FEBS Lett. 1979;94:397–404. doi: 10.1016/0014-5793(78)80986-7. [DOI] [PubMed] [Google Scholar]
- 24.Rankin SE, Addona GH, Kloczewiak MA, Bugge B, Miller KW. The cholesterol dependence of activation and fast desensitization of the nicotinic acetylcholine receptor. Biophys. J. 1997;73:2446–2455. doi: 10.1016/S0006-3495(97)78273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 26.Blanton MP, Cohen JB. Identifying the lipid-protein interface of the Torpedo nicotinic acetylcholine receptor: secondary structure implications. Biochemistry. 1994;33:2859–2872. doi: 10.1021/bi00176a016. [DOI] [PubMed] [Google Scholar]
- 27.Addona GH, Sandermann H, Kloczewiak MA, Miller KW. Low chemical specificity of the nicotinic acetylcholine receptor sterol activation site. Biochem. Biophys. Acta. 2003;1609:177–182. doi: 10.1016/s0005-2736(02)00685-5. [DOI] [PubMed] [Google Scholar]
- 28.Ellena JF, Blazing MA, McNamee MG. Lipid-protein interaction in reconstituted membrane containing acetylcholine receptor. Biochemistry. 1983;22:5523–5535. doi: 10.1021/bi00293a012. [DOI] [PubMed] [Google Scholar]
- 29.Corbin J, Wang HH, Blanton MP. Identifying the cholesterol binding domain in the nicotinic acetylcholine receptor with [125I]azido-cholesterol. Biochim. Biophys. Acta. 1998;1414:65–74. doi: 10.1016/s0005-2736(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 30.Hamouda AK, Chiara DC, Sauls D, Cohen JB, Blanton MP. Identifying the cholesterol binding domain in the nicotinic acetylcholine receptor: photolabeling studies using [3H]azi-cholesterol. Biophys. J. 2004;86:540a. [Google Scholar]
- 31.Methot N, Demers CN, Baenziger JE. Structure of both the ligand- and lipid-dependent channel-inactive states of the nicotinic acetylcholine receptor probed by FTIR spectroscopy and hydrogen exchange. Biochemistry. 1995;34:15142–15149. doi: 10.1021/bi00046a021. [DOI] [PubMed] [Google Scholar]
- 32.Ryan SE, Demers CN, Chew JP, Baenziger JE. Structural effects of neutral and anionic lipids on the nicotinic acetylcholine receptor. An infrared difference spectroscopy study. J. Biol. Chem. 1996;271:24590–24597. doi: 10.1074/jbc.271.40.24590. [DOI] [PubMed] [Google Scholar]
- 33.Blanton MP, McCardy EA, Huggins A, Parikh D. Probing the structure of the nicotinic acetylcholine receptor with the hydrophobic photoreactive probes [125I]TID-BE and [125I]TIDPC/16. Biochemistry. 1998;37:14545–14555. doi: 10.1021/bi981435q. [DOI] [PubMed] [Google Scholar]
- 34.Dreger M, Krauss M, Hermann A, Hucho F. Interactions of the nicotinic acetylcholine receptor transmembrane segments with the lipid bilayer in native receptor-rich membranes. Biochemistry. 1997;36:839–847. doi: 10.1021/bi960666z. [DOI] [PubMed] [Google Scholar]
- 35.Goodreid M, Baenziger JE. Lipid-protein interactions at the nicotinic acetylcholine receptor. Biophys. J. 2005;88:426a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.