Abstract
A substantial epidemiological literature now supports the existence of a J- or U-shaped association between alcohol consumption and a broad range cardiovascular health outcomes including stroke. Although it is well documented that alcoholics exhibit both global and regional cerebral hypoperfusion in the sober state, little is known regarding the effects of a broader range of alcohol consumption on cerebral blood flow (CBF). The present study employed positron emission tomography with H215O to assess quantitative global and regional CBF in 86 participants (51 men and 35 women; mean age 60.1) as a function of self-reported weekly alcohol consumption (none, <1, 1 to <7, 7 to <15, and >15 drinks per week). Analyses controlling for age, gender, and vascular health (carotid intima-media thickness) revealed that, relative to the weighted population mean, global CBF was greater in the lightest alcohol consumption group (<1 per week) and lower in the heaviest (>15 per week). Effects did not vary across regions of interest. This report is the first to describe an inverted J-shaped relationship between alcohol consumption and CBF in the absence of stroke.
Keywords: alcohol consumption, cerebral blood flow, positron emission tomography
Introduction
A substantial body of literature now supports the existence of a J- or U-shaped association between alcohol consumption and a broad range cardiovascular health outcomes such that light to moderate alcohol consumption (e.g., up to 1 drink daily among women and 1 or 2 drinks daily among men) has been related to health protective effects and increasingly excessive consumption to proportionally worse health outcomes (O'Keefe et al., 2007). Of particular relevance to the present paper are findings that light to moderate alcohol intake is associated with decreased risk of ischemic stroke (Mukamal et al. 2005; Sacco et al. 1999) and heavy drinking with increased risk for hemorrhagic stroke (Klatsky et al., 2002; Thrift et al., 1999). What is not clear is the manner in which alcohol consumption might impact cerebral blood flow (CBF) in the absence of stroke. Numerous studies have illustrated that alcoholics exhibit cerebral hypoperfusion in the sober state both globally and regionally in the frontal, temporal, parietal, and occipital cortices as well as in the thalamus (Erbas et al., 1992; Kuruoglu et al., 1996; Melgaard et al., 1990; Mochizuki et al., 2001; Nicolás et al., 1993; Oishi et al., 1997; Oishi et al., 1999). The reduced CBF observed in alcoholism appears to be largely transitory and tends to normalize following sustained abstinence (Ishikawa et al., 1986), though the reported duration of abstinence associated with a return to normal perfusion has varied from as little as two months (Nicolás et al., 1993) to as long as four years (Gansler et al., 2000).
Whereas a number of studies have examined the effects of acute alcohol consumption on CBF in non-alcoholics (e.g., Sano et al., 1993; Volkow et al., 1998), to our knowledge, only a single study has investigated the effect of routine alcohol intake on CBF in a sober state and demonstrated an inverse relationship between alcohol consumption and gray matter blood flow (Rogers et al., 1983). One potential methodological shortcoming of the Rogers et al. study is the use of the [133Xe] Xenon inhalation method (Obrist et al., 1975; Obrist and Wilkinson, 1990) which is limited by low spatial resolution and less reliable quantitative measures compared to other imaging methods (Cherry and Phelps, 2002). The goal of the present paper is to further examine the relationship between CBF and alcohol consumption in an existing sample of older adults. Positron emission tomography (PET) imaging using H215O was employed to assess both global and regional CBF.
Methods
Participants
Participants were taken from a larger study investigating the relationship between hypertension, CBF, and cognitive function (for further information see Jennings, 2003; Jennings et al., 1998, 2005). The study was approved by the Institutional Review Board at the University of Pittsburgh and procedures conform to the ethical guidelines outlined in the Helsinki Declaration of 1975, as revised in 1983. The sample consisted of 137 adults with unmedicated hypertensive (>140/90 mmHg) or normotensive blood pressure (<135/85 mmHg), with ages ranging from 50−70 years, and were recruited from the general population via posters or mailings. Exclusion criteria included: 1) secondary hypertension; 2) cerebrovascular accident, multiple sclerosis, Alzheimer's dementia, Parkinson's disease, or any other neuropathy or significant traumatic brain injury; 3) endarectomy or other surgery involving the cerebral or peripheral vasculature; 4) current congestive heart failure, atrial fibrillation (or pacemaker), angina, or valvular disease; 5) chronic kidney failure (serum creatinine >= 2.0 md/dl, hepatitis, or cirrhosis); 6) pulmonary disease requiring daily medication; 7) type I diabetes with current insulin use or type II diabetes with complications of renal failure, neuropathy, amputation, or severe retinopathy; 8) rheumatoid arthritis if not ambulatory or if finger dexterity was significantly impaired, lupus with greater than skin involvement, or any rheumatoid condition requiring gluccocorticoids; 9) schizophrenia, manic depression, or any other psychiatric disorder requiring regular psychotropic medications; 10) regular illicit drug use; 11) inability to refrain within 24 hours of lab visit from taking sedating antihistamines, narcotic pain medication, aspirin, sleeping pills, diet aids, cold remedies; 12) body weight or size inconsistent with MRI investigation, 13) less than 8th grade reading skills; and 14) postmenopausal status in female participants.
Thirty-three participants were excluded from further analysis due to incomplete data resulting from: a) technical problems; b) participant problems, e.g., premature termination of PET session due to postural pain/bladder fullness; c) data issues, e.g., insufficient overall radiation count for accurate quantitative CBF assessment; and/or d) failure to place or maintain an arterial catheter. For the purposes of the current investigation, full data were available for 86 participants (51 men and 35 women) with a mean age of 60.1 years (sd: 4.9). Participants were given a small monetary compensation for their participation.
Experimental Procedure
Participants underwent five sessions: initial screening (2 hours), neuropsychological assessment (4 hours), carotid artery ultrasound (30 min), MRI scan (1 hour), and PET session (2.5 hours). Sessions were typically conducted on separate days with the PET scan as the final session. .
Screening session
Written informed consent was obtained from each research participant followed by a medical history questionnaire (Jennings et al., 1998). Brachial blood pressure (BP; mmHg) was measured from the left arm three times using a conventional manual mercury sphygmomanometer (Baumanometer, W.A.Baum Co. Inc, NY) with each measurement separated by a two-minute rest period.
Neuropsychological Testing Session
As part of a larger study, a four hour neuropsychological battery was administered which focused on executive and memory function (for greater detail see Jennings, 2003). These data are not presented here. Preceding neuropsychological assessment, blood pressure was again measured three times.
Carotid Ultrasound Session
Ultrasonographic measurement of intima-media thickness (i.e., combined thickness of the two innermost layers of the arterial wall; IMT) of large superficial arteries, particularly the carotid, is a widely used non-invasive index of atherosclerotic progression and is highly correlated with pathohistologic measurements (Poredos, 2004). Real-time B-mode ultrasonography (SSA-270A, Toshiba) was used to estimate mean IMT from bilateral measurements of the common carotid, carotid bifurcation, and first centimeter of the internal carotid artery (for greater detail see Gianaros et al., 2002).
Magnetic Resonance Imaging (MRI) Session
. Participants underwent an MRI scan using a GE Signa 1.5 Tesla scanner prior to the PET session to provide anatomical reference for region of interest (ROI) identification and screen for cerebrovascular abnormalities. Following a brief scout T1-weighted image, multiple axial series oriented to the plane of the anterior and posterior commissures (AC-PC line) were obtained: fast spin-echo T2-weighted (effective TE/TR = 102/2500, 1 NEX), proton density weighted (effective TE/TR = 17/2000, 1 NEX), and fast fluid-attenuated inversion recovery (FLAIR) [effective TE/TR = 56/9002; with a time to inversion (TI) = 2200; 1 NEX] (Bastianello et al., 1997). Section thickness was 5 mm with a 1-mm intersection gap. A field-of-view of 24 cm and image matrix of 256 × 192 pixels was used for all axial MRI series. A volumetric spoiled-gradient recalled sequence with parameters optimized for maximal contrast among gray matter, white matter, and cerebrospinal fluid was acquired in the coronal plane (TE = 5, TR = 25, flip angle = 40 degrees, NEX = 1, slice thickness = 1.5mm/0mm inter-slice) to guide ROI placement.
Positron Emission Tomography Session
PET scans were acquired using a Siemens/CTI ECAT HR+ PET scanner (CTI Medical Systems, Knoxville, TN) in 3D imaging mode. The tomograph acquired 63 axial planes (2.4-mm thick) and had an in-plane resolution of 4.1 mm full-width at half-maximum. Spoiled gradient recalled volumetric sequence parameters MRI data were registered with PET data on a Sun SPARC station using automated image registration software (Woods et al., 1993). The PET data were corrected for radioactive decay, photon attenuation, and scatter (Watson et al., 1997). Reconstructed image resolution was 7.1 mm (transverse) and 6.7 mm (axial). Head positioning was ensured using an individually-fitted thermoplastic mask. An intravenous line allowed for injection of H215O and a short 21-gauge radial artery catheter allowed for the collection of blood samples used to derive the arterial input function employed in quantitative CBF estimation. A transmission scan using rotating rods of 68Ge/68Ga was used for attenuation correction. H215O preparation and delivery were performed in accordance with accepted methodology (Bandy, 1992; Bunko et al., 1977) and Food and Drug Administration regulations (21-CFR 361.1). An automated injector system delivered a rapid H215O intravenous bolus within +/−10% of an 11 mCi target dose. With onset of tracer injection, blood was continuously sampled and a Siemens Liquid Activity Monitoring System (Siemens Medical Systems, Knoxville, TN) withdrew blood over a 3.5 min period at a rate of 6 ml/min (dual BGO scintillation crystals) for a total blood loss of 21 ml. A 180-second emission scan was acquired in 20 sequential frames, followed by a 7-min rest period for a total of 10 min interval between injections permitting for tracer dissipation. Through calibration measurements, both the blood and PET brain time-activity data were converted to μCi/ml. PET images were corrected for all small head movements using Automated Image Registration (Woods et al., 1993). Each participant's electrocardiogram (ECG) was measured continuously during the PET session using reusable surface Ag-AgCl electrodes placed in a modified Lead 2 configuration.
Experimental Tasks
As previously mentioned, the present data were taken from a larger investigation involving hypertension, CBF, and cognitive function (see Jennings, 2003; Jennings et al., 1998, 2005) and, thus, involved participants completing a number of cognitive tasks designed to elicit task-relevant activations (Smith et al., 1996). Participants completed each of the following tasks two times in a counterbalanced fashion: 1) a verbal working memory task during which a series of letters were displayed sequentially on a computer monitor for 400 ms, with a 2 s inter-trial interval, and participants were asked to indicate with one button press if the current letter displayed had been presented two trials earlier and with a separate button press if it had not; 2) a spatial working memory task during which the same stimuli were presented, but at differing locations on the screen and participants were asked to indicate via button press if the current letter displayed had been presented in the same position two trials earlier and with a separate button press if it had not; and 3) a control task with minimal memory and attention demands that required participants only to indicate via button press whether a displayed letter appeared on the right or left side of a computer monitor. Each task consisted of 120 individual trials, lasted 4 min 48 s, and was initiated 2 min prior to H215O injection. Although six tasks were presented during the PET session, only three assessed CBF quantitatively due to the ethical limitations imposed on blood withdrawal. Tasks that did not include a quantitative measure of CBF are not included in the current paper and so the present data include only the tasks described above.
Data Reduction
Weekly Alcohol Consumption
Two items in the medical history questionnaire aimed to quantify the frequency and quantity of alcohol intake. Participants were asked: Over the past year, on average, how many occasions did you drink alcoholic beverages? To which participants responded by selecting one of the following categories: never drank; 1−2 drinking occasions/year; less than 1 drinking occasions/week; 1−2 drinking occasions/week; 3−4 drinking occasions/week; drink nearly daily; and drink daily. Participants who reported drinking over the prior year were then asked: Over the past year, on average, how many standard drinks (12 fl oz beer, 5 fl oz wine, 1.5 fl oz spirits; Zernig et al., 2000) would you usually drink on each occasion? Participants were categorized into the following weekly alcohol consumption categories based on their responses to the quantity and frequency items: none, <1 drink weekly, 1 to <7 drinks weekly, 7 to <15 drinks weekly, and >15 drinks weekly (Mukamal et al., 2005).
Quantitative CBF
The quantitative H215O data was analyzed using a conventional 1-tissue compartment model which describes the extraction of tracer across the blood-brain barrier based upon a rigid cylinder model of the capillary (Huang and Phelps, 1986). The H215O is delivered to brain tissue by blood flow (F: ml/g/min) and extracted from the vasculature across the capillary walls into brain. Model parameters corresponded to clearance of water from blood-to-brain (K1, ml/min/ml), brain-to-blood transfer (k2, min−1), and an arterial input function timing delay (Δt; Iida et al., 1988). Further details of the model parameters are described in the review by Price (2003). Model parameters were simultaneously determined using iterative least squares curve fitting on a regional basis and the K1 parameter was used as a measure of cerebral blood flow. ROI's were manually drawn on spoiled-gradient recalled MRI images in both hemispheres using Imagetool™ software (CTI PET Systems; Knoxville, TN) on the 2.4 mm slices oriented in the axial plane. There were time activity curves for left, right, and grouped hemispheres from which left, right, and grouped K1 values were calculated, though only the grouped (i.e., bilateral) K1 values are used here.
CBF estimates were derived for the following 7 bilateral ROI's: 1) dorsolateral prefrontal cortex [dlPF; medial aspect of Brodmann's Area (BA) 9 superior to lateral ventricles]; 2) posterior parietal cortex (PP; BA 39 & 40 superior to lateral ventricles); 3) occipital cortex (OC; BA 17 & 18 at the level of the splenium and genu of the corpus callosum); 4) anterior and middle cerebral arteries watershed area, the area of terminal arborization and overlap between the anterior and middle cerebral arteries (AMW; at the level of the splenium and genu); 5) middle and posterior cerebral arteries watershed area (MPW; at the level of the splenium and genu); 6) amygdala/hippocampal areas (AH; at the level of temporal horn of the lateral ventricles); 7) thalamus (TL; at the level of the splenium and genu). In addition, whole brain (WB) CBF was estimated using 12 to 18 circular sampling areas of 20.59 mm diameter drawn on the perimeter of each of the three levels noted above (superior to lateral ventricles, at the splenium and genu, and at the temporal horn of the lateral ventricles) to sample the cortex and encompass grey matter areas. See Jennings et al. (2005) for an illustration of these regions of interest.
Analysis
The relationship between alcohol consumption and CBF was assessed within a multiple regression framework for both whole brain and regional CBF at the 7 ROI's identified above. In all cases, CBF was entered into the models as the dependent measure and weekly alcohol consumption as a categorical predictor using the levels described above (none, <1, 1 to <7, 7 to <15, and >15 drinks per week). Because the proportion of group membership across the drinking categories was quite unequal, a weighted effects coding scheme was employed. As a result, interpretation of the regression coefficients for the individual alcohol category codes are in relation to the weighted sample mean, our most accurate approximation of the population mean CBF (Cohen et al., 2003). Candidate covariates were selected based on literature review of variables impacting CBF as well as exploratory analyses. Specifically, previous studies have identified relationships between CBF and age (Kubota et al., 1983; Naritomi et al., 1979), gender (Gur et al., 1982), and intima-media thickness (Claus et al., 1996; Nobili et al., 1993). Systolic and diastolic blood pressure, body mass index (BMI), and smoking status were also examined as potential covariates. Analyses were performed using the R statistical computing environment (version 2.3.1; R Development Core Team, 2006) and an alpha of 0.05 (two-tailed) was used in all significance tests. Externally studentized residuals were examined to insure model fitting was not unduly influenced by data from any individual subject.
Results
The pattern of relationships between alcohol consumption and CBF observed across both ROIs and the individual tasks were essentially identical, suggesting the effects of alcohol consumption were non-specific with regard to both region and task. In keeping with the general principal that global CBF is largely unaffected by transient changes in regional CBF (Raichle, 2002), paired t-tests of the present data indicate global CBF did not significantly differ across tasks (t's < 1.7; p's > 0.05). There is also evidence that, in the absence of meaningful task effects, data aggregated across tasks can provide more reliable estimates of physiological processes such as CBF (e.g., see Kamarck and Lovallo, 2003). Thus, analyses presented here are based solely on whole brain CBF averaged across tasks (i.e., averages of the K1 parameter derived from task-specific time activity curves).
Demographic characteristics of the full sample as well as stratified by alcohol consumption groups are presented in Table 1. Of the variables explored, only gender (0 = female; 1 = male; p = 0.0116) and CBF (p = 0.0015) were statistically associated with weekly alcohol consumption. Separate bivariate regression models were used to examine the first order effects between CBF and age, gender, body mass index, blood pressure, IMT, and smoking status (0 = no; 1 = yes; see Table 2A). Gender (p = 0.0001) was the only variable showing significant bivariate relationship with CBF. Controlling for gender in a multiple regression model revealed that both IMT (p = 0.0319) and age (p = 0.0684) were at least marginally significant predictors of CBF (see Table 2B). Based on these analyses, gender, IMT, and age were included as covariates.
Table 1.
Demographics for whole sample and stratified by alcohol consumption.
| Weekly Number of Drinks | |||||||
|---|---|---|---|---|---|---|---|
| Full Sample | none | <1 | 1 to <7 | 7 to <15 | >15 | p* | |
| N | 86 | 17 | 29 | 23 | 7 | 10 | |
| age, y | 60.2 | 61.1 | 59.0 | 59.9 | 63.4 | 60.4 | 0.2557 |
| Male | 59.3% | 52.9% | 44.8% | 56.5% | 85.7% | 100% | 0.0116 |
| White | 90.7% | 88.2% | 96.6% | 82.6% | 85.7% | 100% | 0.3274 |
| BMI, kg/m^2 | 28.8 | 27.8 | 29.7 | 28.7 | 28.3 | 28.8 | 0.6163 |
| SBP, mmHg | 130.3 | 127.8 | 127.7 | 129.3 | 139.0 | 138.2 | 0.1572 |
| DBP, mmHg | 77.9 | 75.3 | 77.1 | 77.8 | 82.9 | 81.4 | 0.1968 |
| IMT, mm | 0.85 | 0.85 | 0.83 | 0.87 | 0.86 | 0.87 | 0.9424 |
| Smoker | 12.8% | 11.8% | 6.9% | 13% | 14.3% | 30% | 0.4038 |
| Married | 65.1% | 64.7% | 62.1% | 60.9% | 85.7% | 70.0% | 0.8384 |
| education, y | 15.0 | 15.0 | 14.6 | 15.2 | 15.6 | 15.2 | 0.8880 |
| CBF, ml/min/ml | 0.396 | 0.402 | 0.420 | 0.385 | 0.373 | 0.358 | 0.0015 |
p-values derived from Fisher's exact test for categorical variables and ANOVA for continuous variables.
Table 2.
Summary of (A) bivariate regression models of candidate covariates individually predicting CBF and (B) multiple regression of gender, age, and IMT predicting CBF.
| A | |||
|---|---|---|---|
| β | t | p | |
| age | −0.0013 | −1.23 | 0.2220 |
| male | −0.0393 | −3.99 | 0.0001 |
| BMI | −0.0004 | −0.31 | 0.7580 |
| SBP | 0.0002 | 0.45 | 0.6560 |
| DBP | −0.0009 | −1.51 | 0.1350 |
| IMT | 0.0320 | 0.98 | 0.3280 |
| smoker | −0.0014 | −0.09 | 0.9300 |
| B | |||
| β | t | p | |
| male | −0.0401 | −4.14 | <0.0001 |
| age | −0.0020 | −1.85 | 0.0684 |
| IMT | 0.0712 | 2.18 | 0.0319 |
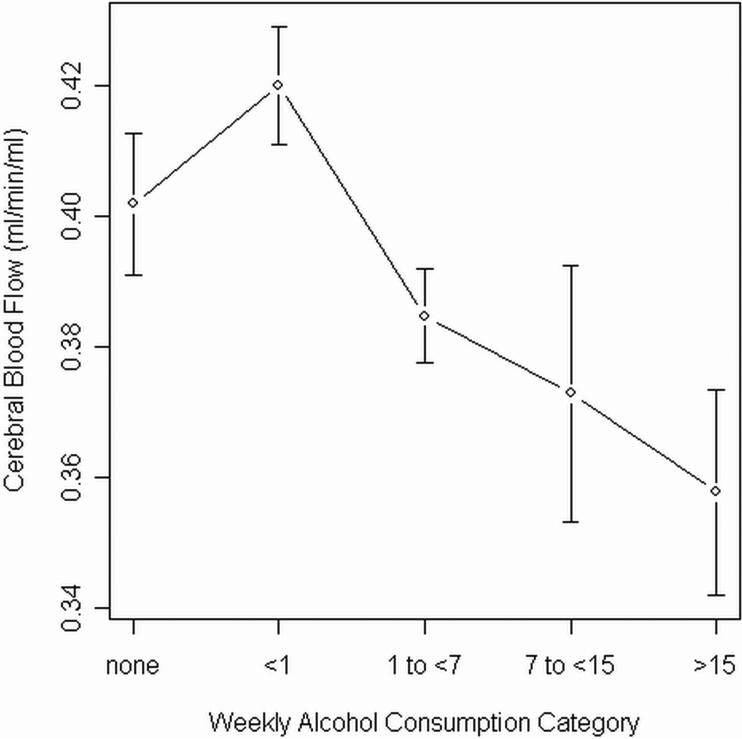
The addition of the weekly alcohol consumption factor to a multiple regression model including the aforementioned covariates resulted in a significant increment in variance explained (ΔR2 = .105; F4,78 = 3.02; p = 0.0227). Individual regression coefficients revealed the lowest drinking category, <1 drink per week, was associated with higher CBF (β= 0.0192; t = 2.93; p = 0.0045) and the highest category, >15 drinks per week, was associated with lower CBF (β= −0.0269; t = −2.06; p = 0.0428). The remaining drinking categories, including nondrinkers, did not statistically differ from the weighted sample mean (see Table 3). Mean CBF and standard errors for each alcohol category are plotted in Figure 1.
Table 3.
Summary of multiple regression of CBF on the weekly alcohol consumption factor controlling for age, gender, and IMT
| β | t | p | |
|---|---|---|---|
| age, y | −0.0018 | −1.65 | 0.1030 |
| male | −0.0305 | −3.07 | 0.0029 |
| IMT | 0.0745 | 2.38 | 0.0198 |
| none | 0.0055 | 0.60 | 0.5531 |
| <1 | 0.0192 | 2.93 | 0.0045 |
| 1 to <7 | −0.0135 | −1.80 | 0.0754 |
| 7 to <15 | −0.0100 | −0.64 | 0.5260 |
| >15 | −0.0269 | −2.06 | 0.0428 |
Figure 1.
Mean whole brain CBF and standard errors by alcohol category.
Discussion
The current findings, that the lower range of alcohol consumption is associated with higher CBF and the upper range with lower CBF, mirror in an inverted fashion the now familiar J-shaped relationship between alcohol consumption and a host of indices of cardiovascular health (O'Keefe et al., 2007). The present results are partially compatible with the only previous study examining the effects of chronic alcohol consumption on CBF (Rogers et al., 1983), which reported an inverse relationship across the range of alcohol consumption, a finding we observed only in our highest alcohol consumption group.
Regarding a possible mechanism, it is plausible the same alcohol effects that convey cardiovascular health benefits may underlie the higher CBF we observed in the lightest drinking group. The enhanced cardiovascular health attributed to light to moderate alcohol consumption is believed to be mediated primarily by three means: increased HDL-cholesterol, enhancement of insulin sensitivity, and reduced fibrinogen levels (Mukumal et al., 2005). Although the effects of HDL-cholesterol and fibrinogen on CBF have not been directly investigated, both low HDL-cholesterol and high plasma fibrinogen levels are powerful risk factors for atherosclerosis and, thus, impaired contractility and elasticity of the small arteries and arterioles of the cerebrum. In fact, fibrinogen has been identified as a primary mediator of age related declines in CBF (Claus et al., 1998). It is less clear how enhanced insulin sensitivity, or more accurately the consequent reductions in hyperglycemia or hypoglycemia, might give rise to our observed increase in CBF. A modest number of human and animal studies have produced mixed results inconsistently relating hyperglycemia and hypoglycemia to both increased and decreased CBF (McCall, 2004). With regard to possible mechanisms explaining the lower CBF observed in the heaviest drinking group, as stated earlier, chronic alcoholism has been consistently related to cerebral hypoperfusion. While alcoholism certainly reduces CBF by a number of pathways, the primary factors appear to be decreased cerebral metabolic rate (Dao-Castellana et al., 1998; Sachs et al., 1987) and alcohol-induced neuronal dysfunction (Erbas et al., 1992; Melgaard et al., 1990). One or both of these factors are plausible explanations for the lower CBF among our heaviest drinkers.
There are a number of limitations in the present study. The mean age of study participants was 61 years and the results may not generalize to substantially younger or older samples. Also, self-report measures of alcohol consumption, although widely used, can underestimate the actual volume of alcohol consumed. This fact may explain why the positive alcohol effects were observed in the drinking category corresponding to <1 drink per week, but not in the 1 to <7 or 7 to <15 drinks per weeks groups, whereas the beneficial cardiovascular effects of alcohol are generally observed at somewhat higher doses (i.e., one or two drinks daily; O'Keefe et al., 2007). In addition, the measures of quantity and frequency of alcohol consumption used in the current study did not differentiate between beer, wine, and spirits. Future studies might include more detailed items aimed at assessing beverage specific quantity-frequency information, which has been shown to be less vulnerable to underreporting (Russell et al., 1991), and may provide insight into beverage specific effects of alcohol on CBF.
Acknowledgments
We gratefully acknowledge funding from National, Heart, Lung, and Blood Institute grants HL57529 (J. R. J.) for the study and HL07560 (University of Pittsburgh) for the training of Dr. Christie. We also thank Mary Assenat for her assistance during data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpert NM, Eriksson L, Chang JY, Bergstrom M, Litton JE, Correia JA, Bohm C, Ackerman RH, Taveras JM. Strategy for the measurement of regional cerebral blood flow using short-lived tracers and emission tomography. J. Cereb. Blood Flow Metab. 1984;4:28–34. doi: 10.1038/jcbfm.1984.4. [DOI] [PubMed] [Google Scholar]
- Bandy DJ. MS Thesis. Arizona State University; 1992. An infusion system to administer short-lived radiopharmaceuticals used in positron emission tomography. [Google Scholar]
- Bastianello S, Bozzao A, Paolillo A, Giugni E, Gasperini C, Koudriavtseva T, Millefiorini E, Horsfield MA, Colonnese C, Toni D, et al. Fast spin-echo and fast fluid-attenuated inversion-recovery versus conventional spin-echo sequences for MR quantification of multiple sclerosis lesions. Am. J. Neuroradiol. 1997;18:699–704. [PMC free article] [PubMed] [Google Scholar]
- Bunko H, Kuwajima A, Kubota A, Hisada K. Development of new radionuclide bolus injector and evaluation of bolus using venous phantom. Radioisotopes. 1977;26:550–553. doi: 10.3769/radioisotopes.26.8_550. [DOI] [PubMed] [Google Scholar]
- Carson R. Tracer kinetic modeling. In: Valk PE, Townsend DW, Bailey DB, Maisey MN, editors. Positron Emission Tomography: Basic Science and Clinical Practice. Springer; New York: 2003. pp. 147–179. [Google Scholar]
- Carson RE, Huang SC, Green MV. Weighted integration method for local cerebral blood flow measurements with positron emission tomography. J. Cereb. Blood Flow Metab. 1986;6:245–258. doi: 10.1038/jcbfm.1986.38. [DOI] [PubMed] [Google Scholar]
- Cherry SR, Phelps ME. Imaging brain function with positron emission tomography. In: Toga AW, Mazziotta JC, editors. Brain Mapping, The Methods. 2nd edn Academic Press; San Diego: 2002. pp. 485–511. [Google Scholar]
- Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, van Swieten JC, van Harskamp F, Hofman A. Vascular risk factors, atherosclerosis, cerebral white matter lesions and cerebral perfusion in a population-based study. Eur. J. Nucl. Med. 1996;23:675–682. doi: 10.1007/BF00834530. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol. Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Erbas B, Bekdik C, Erbengi G, Enunlu T, Aytac S, Kumbasar H, Dogan Y. Regional cerebral blood flow changes in chronic alcoholism using Tc-99m HMPAO SPECT. Clin. Nucl. Med. 1992;17:123–127. doi: 10.1097/00003072-199202000-00012. [DOI] [PubMed] [Google Scholar]
- Frackowiak R, Lenzi G-L, Jones T, Heather J. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: Theory, procedure, and normal values. J. Comput. Assist. Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Harris GJ, Oscar-Berman M, Streeter C, Lewis RF, Ahmed I, Achong D. Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: a pilot SPECT study. J. Stud. Alcohol. 2000;61:32–37. doi: 10.15288/jsa.2000.61.32. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Bleil ME, Muldoon MF, Jennings JR, Sutton-Tyrrell K, McCaffery JM, Manuck SB. Is Cardiovascular Reactivity Associated With Atherosclerosis Among Hypertensives? Hypertension. 2002;40:742–747. doi: 10.1161/01.hyp.0000035707.57492.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–61. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Markham J, Raichle M. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. J. Nucl. Med. 1983;24:782–789. [PubMed] [Google Scholar]
- Huang SC, Carson RE, Phelps ME. Measurement of local blood flow and distribution volume with short-lived isotopes: A general input technique. J. Cereb. Blood Flow Metab. 1982;2:99–108. doi: 10.1038/jcbfm.1982.11. [DOI] [PubMed] [Google Scholar]
- Huang SC, Carson RE, Hoffman EJ, Carson J, MacDonald N, Barrio JR, Phelps ME. Quantitative measurement of local cerebral blood flow in humans by positron computed tomography and 15O-water. J. Cereb. Blood Flow Metab. 1983;3:141–153. doi: 10.1038/jcbfm.1983.21. [DOI] [PubMed] [Google Scholar]
- Huang SC, Phelps ME. In: Positron Emission Tomography and Autoradiography: Principles and Applications for the Brain and Heart. Phelps ME, Mazziotta JC, Schelbert HR, editors. Raven Press; New York: 1986. pp. 347–390. [Google Scholar]
- Iida H, Higano S, Tomura N, Shishido F, Kanno I, Miura S, Murakami M, Takahashi K, Sasaki H, Uemura K. Evaluation of regional differences of tracer appearance time in cerebral tissues using [15O] water and dynamic positron emission tomography. J. Cereb. Blood Flow Metab. 1988;8:285–288. doi: 10.1038/jcbfm.1988.60. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Meyer JS, Tanahashi N, Hata T, Velez M, Fann WE, Kandula P, Mortel KF, Rogers RL. Abstinence improves cerebral perfusion and brain volume in alcoholic neurotoxicity without Wernicke-Korsakoff syndrome. J. Cereb. Blood Flow Metab. 1986;6:86–94. doi: 10.1038/jcbfm.1986.11. [DOI] [PubMed] [Google Scholar]
- Jennings JR. Autoregulation of blood pressure and thought: preliminary results of an application of brain imaging to Psychosomatic Medicine. Psychosom. Med. 2003;65:384–395. doi: 10.1097/01.psy.0000062531.75102.25. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Ryan CM, Mintun MA, Meltzer CC, Townsend DW, Sutton-Tyrrell K, Shapiro AP, Manuck SB. Cerebral blood flow in hypertensive patients: An initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension. 1998;31:1216–1222. doi: 10.1161/01.hyp.31.6.1216. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosom. Med. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol and stroke: An epidemiological labyrinth. Stroke. 2005;36:1835–1836. [PubMed] [Google Scholar]
- Koeppe RA, Holden JE, Ip WR. Performance comparison of parameter estimation techniques for the quantitation of local cerebral blood flow by dynamic positron computed tomography. J. Cereb. Blood Flow Metab. 1985;5:224–34. doi: 10.1038/jcbfm.1985.29. [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamaguchi T, Abe Y, Fujiwara T, Hatazawa J, Matsuzawa T. Effects of smoking on regional cerebral blood flow in neurologically normal subjects. Stroke. 1983;14:720–724. doi: 10.1161/01.str.14.5.720. [DOI] [PubMed] [Google Scholar]
- Kuruoglu AC, Arikan Z, Vural G, Katatas M, Arac M, Isik E. Single positron emission computerised tomography in chronic alcoholism: Antisocial personality disorder may be associated with decreased frontal perfusion. Br. J. Psychiatry. 1996;169:348–354. doi: 10.1192/bjp.169.3.348. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Frackowiak RS, Hoffman JM, Huang SC, Weinberg IN, Dahlbom M, MacDonald NS, Hoffman EJ, Mazziotta JC, Heather JD, et al. The C15O2 build-up technique to measure regional cerebral blood flow and volume of distribution. J. Cereb. Blood Flow Metab. 1989;9:461–470. doi: 10.1038/jcbfm.1989.69. [DOI] [PubMed] [Google Scholar]
- McCall AL. Cerebral glucose metabolism in diabetes mellitus. Eur. J. Pharmacol. 2004;490:147–58. doi: 10.1016/j.ejphar.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta. Neurol. Scand. 1990;82:87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Oishi M, Takasu T. Correlations between P300 components and regional cerebral blood flows. J. Clin. Neurosci. 2001;8:407–410. doi: 10.1054/jocn.2000.0850. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Chung H, Jenny NS, Kuller LH, Longstreth WT, Jr., Mittleman MA, Burke GL, Cushman M, Beauchamp NJ, Jr., Siscovick DS. Alcohol use and risk of ischemic stroke among older adults: The CV Health Study. Stroke. 2005;36:1830–1834. doi: 10.1161/01.STR.0000177587.76846.89. [DOI] [PubMed] [Google Scholar]
- Naritomi H, Meyer JS, Sakai F, Yamaguchi F, Shaw TG. Effects of advancing age on regional cerebral blood flow. Studies in normal subjects and subjects with risk factors for atherothrombotic stroke. Arch. Neurol. 1979;36:410–416. doi: 10.1001/archneur.1979.00500430040005. [DOI] [PubMed] [Google Scholar]
- Nicolás JM, Catafau AM, Estruch R, Lomeña FJ, Salamero M, Herranz R, Monforte R, Cardenal C, Urbano-Marquez A. Regional cerebral blood flow - SPECT in chronic alcoholism: Relation to neuropsychological testing. J. Nucl. Med. 1993;34:1452–1459. [PubMed] [Google Scholar]
- Nobili F, Rodriguez G, Marenco S, DeCarli F, Gambaro M, Castello C, Pontremoli R, Rosadini G. Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke. 1993;24:1148–1153. doi: 10.1161/01.str.24.8.1148. [DOI] [PubMed] [Google Scholar]
- Obrist WD, Thompson HK, Wang HS, Wilkinson WE. Regional cerebral blood flow estimated by 133xenon inhalation. Stroke. 1975;6:245–256. doi: 10.1161/01.str.6.3.245. [DOI] [PubMed] [Google Scholar]
- Obrist WD, Wilkinson WE. Regional cerebral blood flow measurement in humans by xenon-133 clearance. Cerebrovasc. Brain Metab. Rev. 1990;2:283–327. [PubMed] [Google Scholar]
- Ohta S, Meyer E, Fujita H, Reutens DC, Evans A, Gjedde A. Cerebral [15O]water clearance in humans determined by PET: I. Theory and normal values. J. Cereb. Blood Flow Metab. 1996;16:765–80. doi: 10.1097/00004647-199609000-00002. [DOI] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Takasu T. Cerebral blood flow and cerebrovascular response to acetazolamide in patients with chronic alcoholism. J. Neurol. Neurosurg. Psychiatry. 1997;63:100–102. doi: 10.1136/jnnp.63.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Shikata E. Corpus callosum atrophy and cerebral blood flow in chronic alcoholics. J. Neurol. Sci. 1999;162:51–55. doi: 10.1016/s0022-510x(98)00279-2. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J. Am. Coll. Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- Poredos P. Intima-media thickness: indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vasc. Med. 2004;9:46–54. doi: 10.1191/1358863x04vm514ra. [DOI] [PubMed] [Google Scholar]
- Price JC. Principles of tracer kinetic analysis. Neuroimaging Clin N Am. 2003;13:689–704. doi: 10.1016/s1052-5149(03)00107-2. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria.: 2006. Web site: http://www.R-project.org. [Google Scholar]
- Raichle ME. Functional brain imaging. In: Edvinsson L, Kraus DN, editors. Cerebral Blood Flow and Metabolism. 2nd ed Lipincott, Williams, & Wilkins; Philadelphia: 2002. pp. 413–419. [Google Scholar]
- Rogers RL, Meyer JS, Shaw TG, Mortel KF. Reductions in regional cerebral blood flow associated with chronic consumption of alcohol. J. Am. Geriatr. Soc. 1983;31:540–543. doi: 10.1111/j.1532-5415.1983.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Russell M, Welte JW, Barnes GM. Quantity-frequency measures of alcohol consumption: Beverage-specific vs. global questions. Br. J. Addict. 1991;86:409–417. doi: 10.1111/j.1360-0443.1991.tb03418.x. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- Sachs H, Russell JA, Christman DR, Cook B. Alteration of regional cerebral glucose metabolic rate in non-Korsakoff chronic alcoholism. Arch. Neurol. 1987;44:1242–1251. doi: 10.1001/archneur.1987.00520240024007. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J. Stud. Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb. Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Thrift AG, Donnan GA, McNeil JJ. Heavy drinking, but not moderate or intermediate drinking, increases the risk of intracerebral hemorrhage. Epidemiology. 1999;10:307–312. [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1998;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Watson CC, Newport D, Casey ME, deKemp RA, Beanlands RS, Schmand M. Evaluation of stimulation-based scatter correction for 3D PET cardiac imaging. IEEE Trans. Nucl. Sci. 1997;44:90–97. [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J. Comput. Assist. Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Zernig G, Saria A, Kurz M, O'Malley SS. Definitions of a “standard drink”. In: Zernig G, Saria A, Kurz M, O'Malley SS, editors. Handbook of Alcoholism. CRC Press; Boca Raton: 2000. p. 429. [Google Scholar]