Abstract
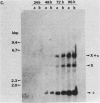
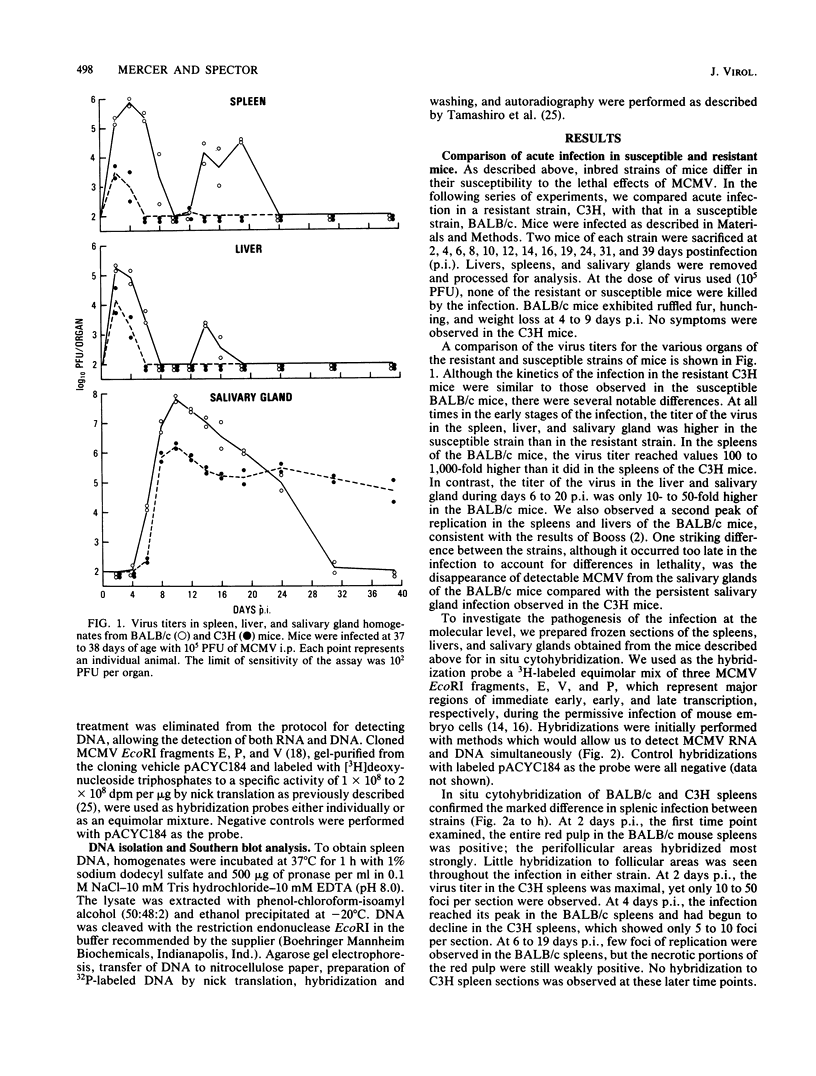
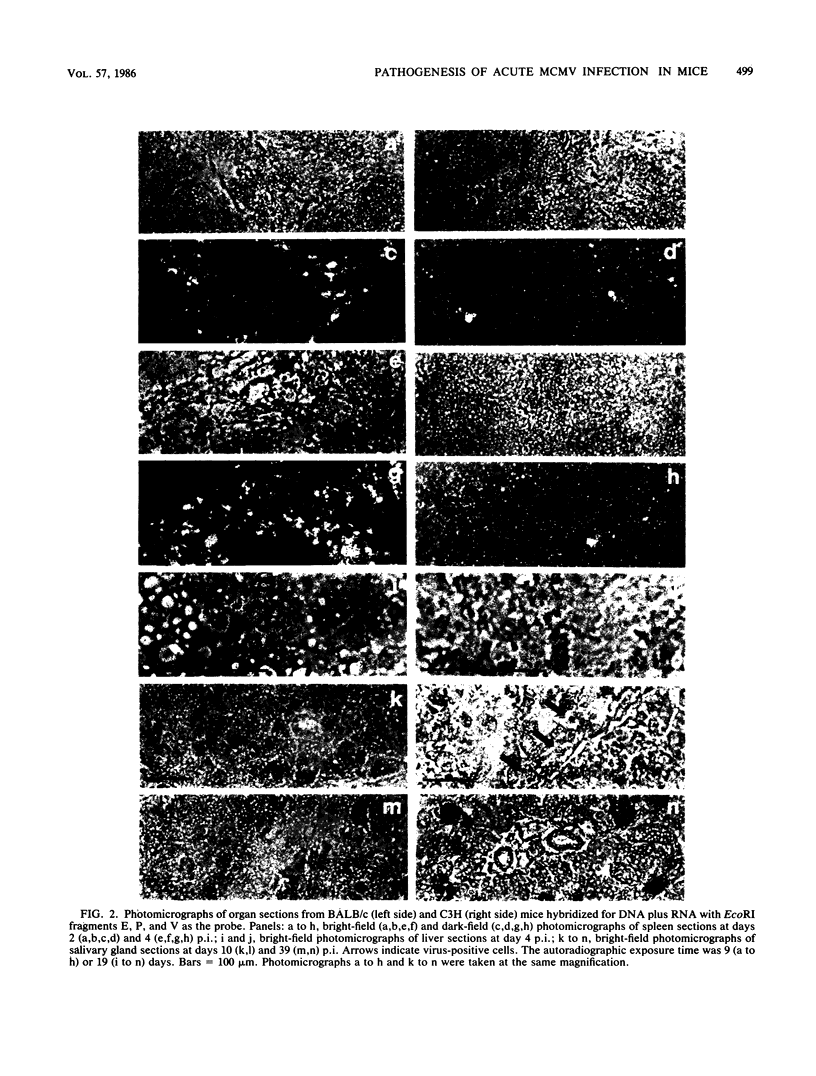
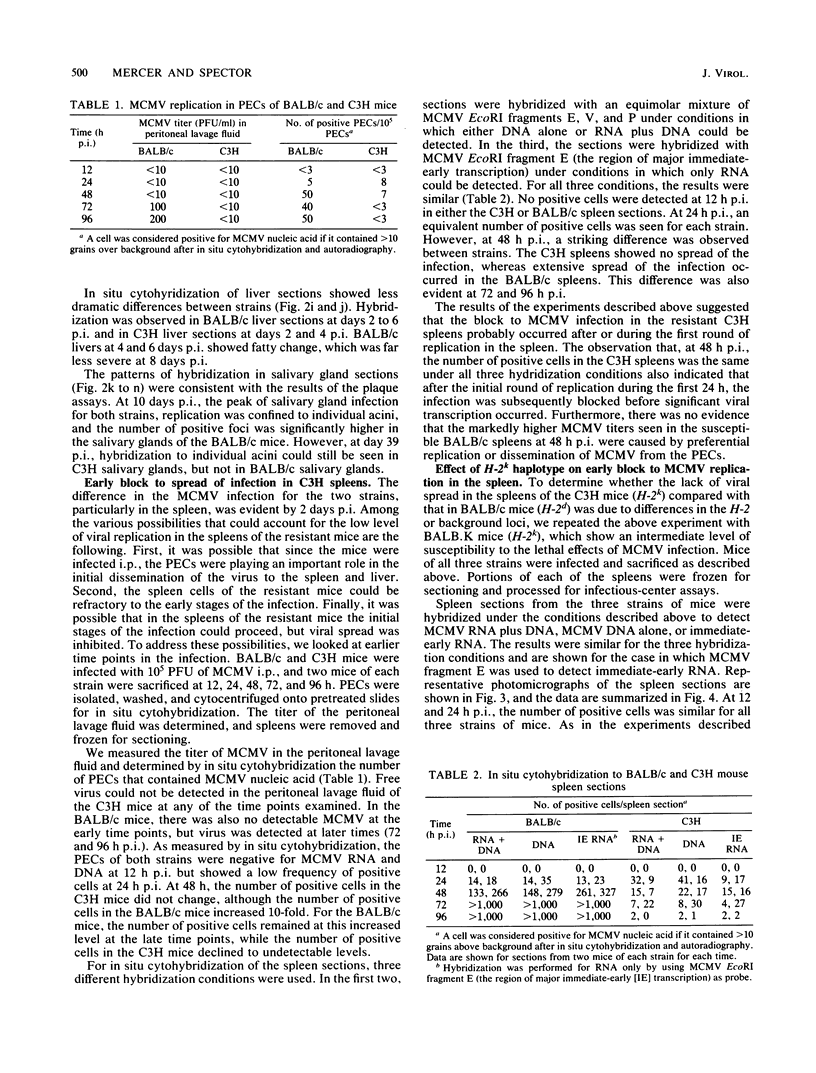
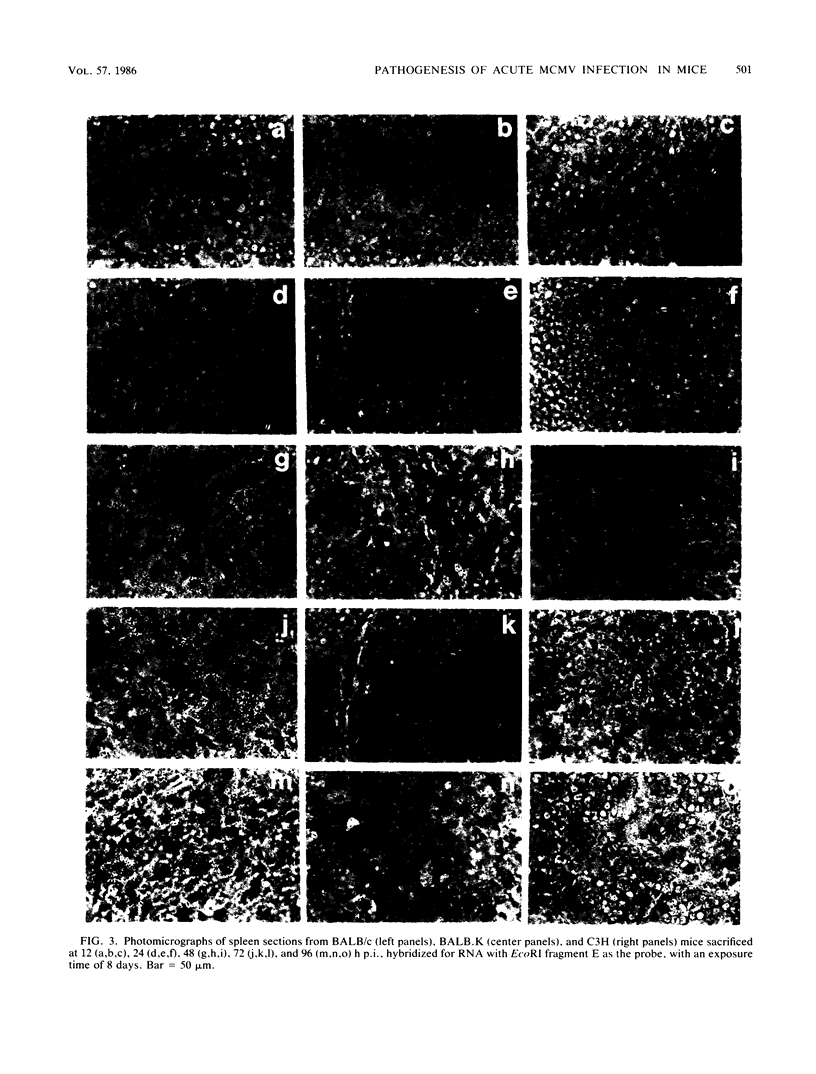
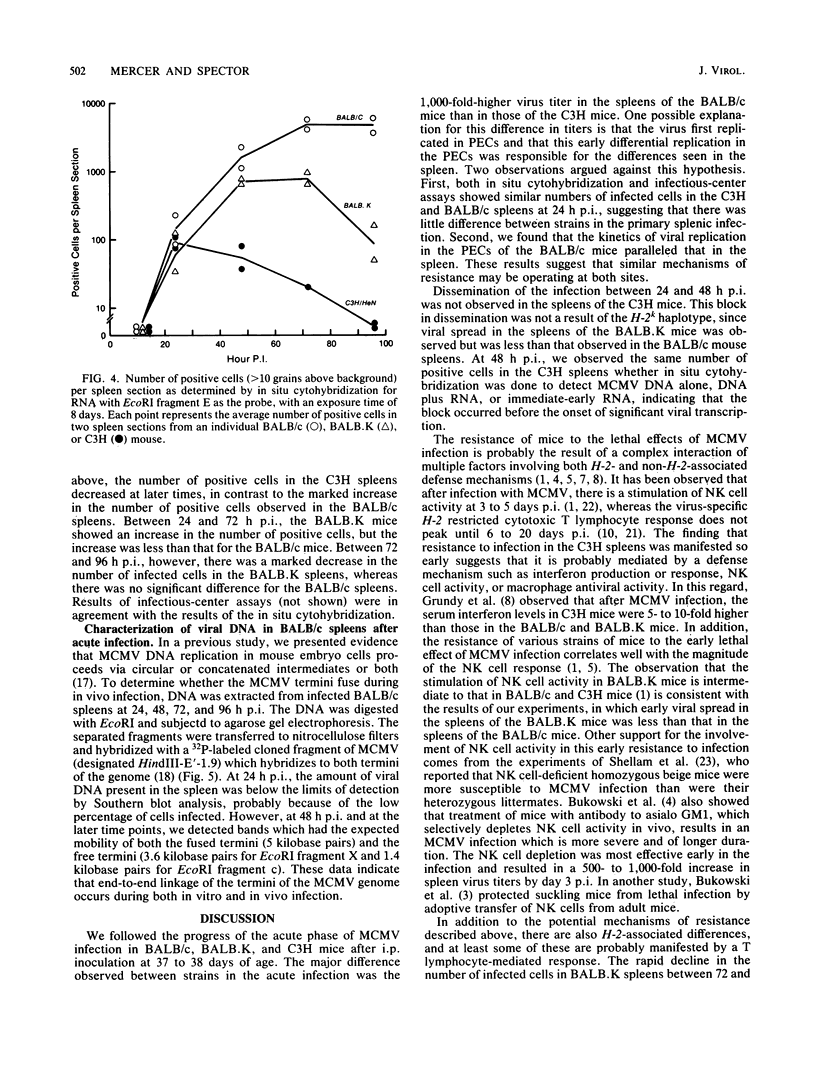
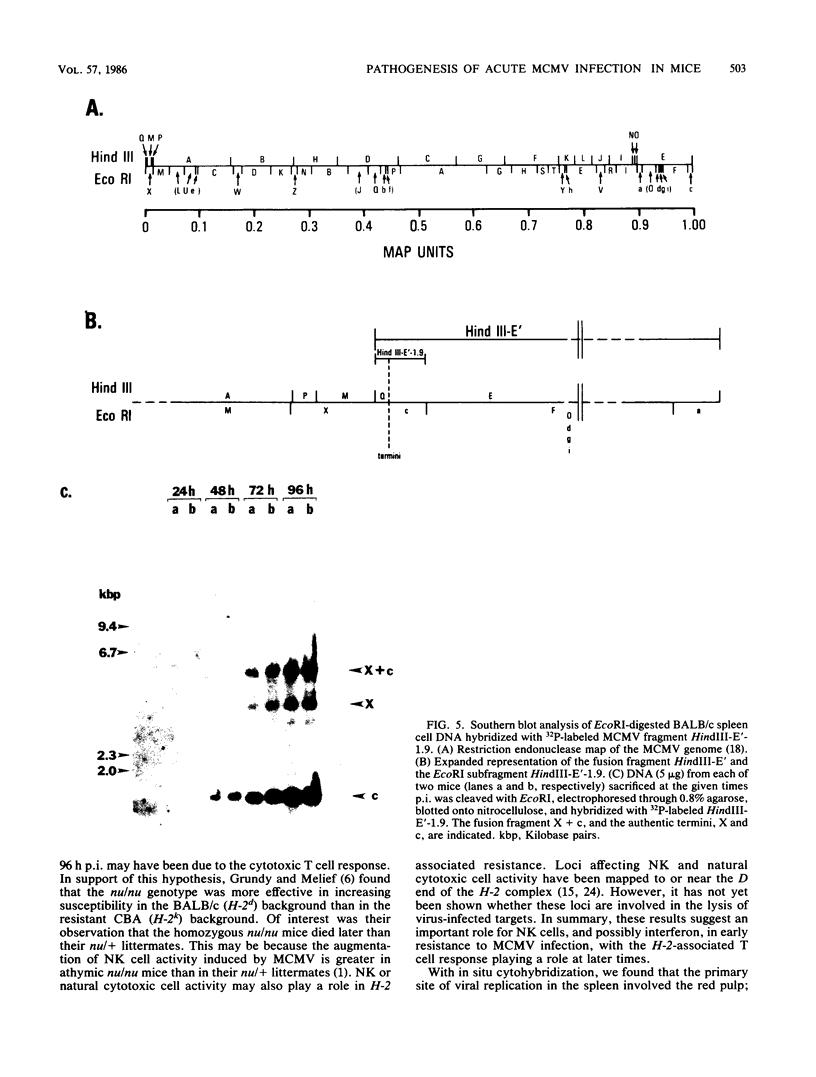
We have characterized the progress of acute murine cytomegalovirus (MCMV) infection in the spleen, liver, and salivary gland of susceptible (BALB/c) and resistant (C3H) strains of mice after intraperitoneal inoculation. Viral replication was analyzed by virus titration, infectious-center assays, and in situ cytohybridization with cloned subgenomic fragments of the MCMV genome. The most striking differences between strains were observed in the spleen. At 24 h postinfection (p.i.), both strains had a similar number of infected spleen cells. At 48 h p.i., BALB/c mice showed marked dissemination of the splenic infection which continued until 96 h p.i. In contrast, the number of infected C3H spleen cells did not increase from the 24-h level but declined later on. This early block in dissemination of MCMV infection in C3H mouse spleens was not a result of the H-2k haplotype, as BALB.K (H-2k) mice, which show an intermediate level of resistance to MCMV infection, exhibited dissemination of the infection between 24 and 48 h p.i., albeit at a reduced level. However, between 72 and 96 h p.i., we observed a decline in the number of infected spleen cells in BALB.K mice similar to that observed in C3H mice. We also demonstrated by Southern blot analysis of DNA from the infected spleen cells that the termini of the MCMV genome fuse after in vivo infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Booss J. Establishment of cytomegaloviral infection in mice: role of a macrophage-enriched subpopulation. J Infect Dis. 1980 Apr;141(4):466–472. doi: 10.1093/infdis/141.4.466. [DOI] [PubMed] [Google Scholar]
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Welsh R. M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984 Oct;52(1):119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Mackenzie J. S., Stanley N. F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981 Apr;32(1):277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., Melief C. J. Effect of Nu/Nu gene on genetically determined resistance to murine cytomegalovirus. J Gen Virol. 1982 Jul;61(Pt 50):133–136. doi: 10.1099/0022-1317-61-1-133. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Trapman J., Allan J. E., Shellam G. R., Melief C. J. Evidence for a protective role of interferon in resistance to murine cytomegalovirus and its control by non-H-2-linked genes. Infect Immun. 1982 Jul;37(1):143–150. doi: 10.1128/iai.37.1.143-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980 Mar;27(3):767–776. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B. The murine cytomegalovirus as a model for the study of viral pathogenesis and persistent infections. Arch Virol. 1979;62(1):1–29. doi: 10.1007/BF01314900. [DOI] [PubMed] [Google Scholar]
- Jordan M. C. Latent infection and the elusive cytomegalovirus. Rev Infect Dis. 1983 Mar-Apr;5(2):205–215. doi: 10.1093/clinids/5.2.205. [DOI] [PubMed] [Google Scholar]
- Katzenstein D. A., Yu G. S., Jordan M. C. Lethal infection with murine cytomegalovirus after early viral replication in the spleen. J Infect Dis. 1983 Sep;148(3):406–411. doi: 10.1093/infdis/148.3.406. [DOI] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. R., Mercer J. A., Spector D. H. Transcription in mouse embryo cells permissively infected by murine cytomegalovirus. Virology. 1983 Nov;131(1):247–254. doi: 10.1016/0042-6822(83)90550-0. [DOI] [PubMed] [Google Scholar]
- Marks J. R., Spector D. H. Fusion of the termini of the murine cytomegalovirus genome after infection. J Virol. 1984 Oct;52(1):24–28. doi: 10.1128/jvi.52.1.24-28.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. A., Marks J. R., Spector D. H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith Strain). Virology. 1983 Aug;129(1):94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Gould J. Splenic necrosis in mice infected with cytomegalovirus. J Infect Dis. 1978 May;137(5):587–591. doi: 10.1093/infdis/137.5.587. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Manischewitz J. F., Kirmani N. Involvement of natural killer cells in the pathogenesis of murine cytomegalovirus interstitial pneumonitis and the immune response to infection. J Gen Virol. 1982 Jan;58(Pt 1):173–180. doi: 10.1099/0022-1317-58-1-173. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E., Ennis F. A. Cytotoxic T lymphocyte response to murine cytomegalovirus infection. Nature. 1978 Jun 15;273(5663):541–543. doi: 10.1038/273541a0. [DOI] [PubMed] [Google Scholar]
- Shellam G. R., Allan J. E., Papadimitriou J. M., Bancroft G. J. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]