Abstract
Background and purpose:
This study investigated whether deletion of the α1A-adrenoceptor gene influences contractile responses of mouse prostate to noradrenaline. Responses of mouse prostate to noradrenaline are known to be mediated by α1L-adrenoceptors, which are thought to be a functional phenotype of α1A-adrenoceptor.
Experimental approach:
Prostate tissues from α1A-adrenoceptor knockout mice which were homozygous (α1A−/−) and heterozygous (α1A+/−) for the disrupted α1A-adrenoceptor gene, as well as wild-type (α1A+/+) littermates were mounted in glass-isolated organ baths. Electrical field stimulation of nerves and exogenous application of noradrenaline were used to investigate the effects of α1A-adrenoceptor disruption on prostate contractility.
Key results:
Frequency–response curves to electrical field stimulation (0.5 ms pulse duration, 60 V, 0.1–20 Hz) yielded frequency-dependent contractions. At frequencies of 10 and 20 Hz, prostates from α1A−/− mice elicited an approximately 30% decreased response compared with prostates from α1A+/+ mice. Prazosin (0.3 μM) attenuated responses to electrical field stimulation in prostates from α1A+/+ and α1A+/− mice but not from α1A−/− mice. Increasing concentrations of exogenously administered noradrenaline (10 nM–1 mM) produced mean concentration–response curves in prostates from α1A+/+ and α1A+/− mice, which were not different. Maximum responses to noradrenaline were decreased by approximately 80% in prostates from α1A−/− mice compared with α1A+/+ mice. Prazosin attenuated responses to noradrenaline in all genotypes.
Conclusions and implications:
α1L-Adrenoceptor-mediated responses in mouse prostate are abolished in α1A−/− mice, demonstrating that the α1A-adrenoceptor gene is essential to the manifestation of the prostatic α1L-adrenoceptor phenotype. This implies that α1L-adrenoceptors are indeed a functional phenotype of α1A-adrenoceptor.
Keywords: noradrenaline, prazosin, tamsulosin, benign prostatic hyperplasia, BPH, smooth muscle, nerve stimulation
Introduction
Benign prostatic hyperplasia (BPH) is a common condition in ageing men, which is characterized by a non-malignant enlargement of the prostate (McNeal, 1978). BPH has both a static component, arising due to hyperplasia of the glandular and stromal tissue, and a dynamic component, mediated through increased noradrenergic activation of α1-adrenoceptors. Both of these components place pressure on the urethra and bladder, and result in a number of troublesome lower urinary tract symptoms.
The dynamic component of BPH is more influential in producing symptoms, as the severity of symptoms is not related to prostate size (Eckhardt et al., 2001a, 2001b, 2001c), and drugs that relax prostatic smooth muscle are faster acting and more effective in relieving symptoms (Rigatti et al., 2003; Hasan et al., 2007).
Benign prostatic hyperplasia is associated with an increased expression of α1-adrenoceptors in hyperplastic tissue compared with normal prostate tissue (Walden et al., 1999; Yamada et al., 2001). Furthermore, α1A-adrenoceptor mRNA has been shown to be nine times more abundant in BPH samples than in non-BPH samples (Nasu et al., 1996). Although studies have focused on α1A-adrenoceptor expression, it is the pharmacologically classified α1L-adrenoceptor that is responsible for mediating the contractile responses in the human (Muramatsu et al., 1994; Israilova et al., 2004), rat (Hiraoka et al., 1999) and guinea-pig prostrates (Pennefather et al., 1999), as well as in the mouse prostate (Gray and Ventura, 2006). Although the α1L-adrenoceptor is yet to be cloned (Ramsay et al., 2004), functional pharmacological studies have shown it to have a high affinity for tamsulosin and a low affinity for prazosin, RS-17053 and WB 4101 compared with the α1A-adrenoceptor (Muramatsu et al., 1994; Ford et al., 1996, 1997; Noble et al., 1997; Hiraoka et al., 1999).
The α1L-adrenoceptor is believed to be a functional phenotype of the α1A-adrenoceptor (Ford et al., 1997), although the exact mechanisms behind the development of the functional α1L-adrenoceptor phenotype are unknown. The α1L-adrenoceptor may result from specific isoforms of the α1A-adrenoceptor; however, cloned α1A-adrenoceptor isoforms have been shown to exhibit functional pharmacology of α1A-adrenoceptors, indicating that no single isoform of the α1A-adrenoceptor is responsible for the generation of the α1L-adrenoceptor (Daniels et al., 1999). The functional profile of the α1A-adrenoceptor is determined by the structure of the C terminus (Suzuki et al., 2000); however, when homo- and hetero-dimers of three C-terminal splice variants of the α1A-adrenoceptor were formed, they did not display the ligand-binding characteristics of the α1L-adrenoceptor (Ramsay et al., 2004).
Evidence to support the notion that the α1L-adrenoceptor may be a functional phenotype of the α1A-adrenoceptor comes from studies using the ‘uroselective' α1A-adrenoceptor antagonist tamsulosin. Tamsulosin was the first α1A-subtype-selective adrenoceptor antagonist and is currently used as the first-line treatment for BPH, as it is effective at selectively relaxing prostatic smooth muscle and relieving the symptoms associated with BPH (Walden et al., 1998; Flannery et al., 2006; Suzuki et al., 2006), without causing the common cardiovascular side effects seen with other α1-adrenoceptor antagonists.
Gene knockout technology provides us with an invaluable tool for elucidating the role of certain gene products in the mediation of physiological responses. Although it has been hypothesized that the α1L-adrenoceptor is a functional phenotype of the α1A-adrenoceptor, no one has yet examined the α1L-adrenoceptor response in a system where the α1A-adrenoceptor gene is absent. Such a study would determine whether the α1A-adrenoceptor gene plays a role in the manifestation of the α1L-adrenoceptor-mediated response. To our knowledge, this is the first report on the effects of genetic disruption of the α1A-adrenoceptor on functional α1L-adrenoceptor-mediated responses.
Methods
Animals
Prior approval for animal experimentation was obtained from the Monash University Standing Committee of Animal Ethics in Animal Experimentation (Ethics number: VCPA 2006/8).
Adult α1A-adrenoceptor knockout mice were purchased from JAX Mice (The Jackson Laboratory, Bar Habor, ME, USA). Heterozygous breeding pairs produced mice that were homozygous (α1A−/−) and heterozygous (α1A+/−) for the gene disruption, as well as age-matched wild-type (α1A+/+) littermate controls. Mice were bred on a C57Bl6J background and routinely genotyped by PCR with primer sequences obtained from Professor Paul Simpson (Rokosh and Simpson, 2002). Mice were housed in a PC2 facility at the Monash Animal Services central animal facility at 22 °C and exposed to a photoperiod of 12 h light/12 h dark. Animals had access to food and water ad libitum. Adult mice (aged between 8–16 weeks) were weighed before being killed by cervical dislocation.
Prostate dissection
A lower abdominal incision was made exposing the male urogenital tract of the mouse. The penile muscles were cut posteriorly to expose the prostate gland. Whole prostate glands were carefully dissected out and placed in a Petri dish containing Krebs–Henseleit solution (mM: NaCl 118.1, KCl 4.69, KH2PO4 1.2, NaHCO3 25.0, glucose 11.7, MgSO4 1.1, CaCl2 2.5; carbogenated to pH 7.4) where excess fat and connective tissue was removed. Prostates were weighed at the conclusion of experiments.
Isolated organ bath studies
The whole prostate tissues were mounted in 10 mL glass, water-jacketed organ baths containing Krebs–Henseleit solution, bubbled with 5% CO2 in O2 and maintained at 37 °C. One end of the prostate was attached to a Perspex tissue holder and the other to a Grass FTO3C transducer (Grass-Telefactor, West Warwick, RI, USA) for recording of isometric contractions of the prostatic smooth muscle. A PowerLab data acquisition system (Chart 3.6) (ADInstruments, Bella Vista, NSW, Australia), run on an HP Compaq dc7100 personal computer, recorded the force developed by each tissue. Preparations were placed under a resting tension of approximately 0.5 g and were equilibrated for 60 min. During the equilibration period, nerve terminals within the prostatic smooth muscle were electrically stimulated via two vertical parallel platinum electrodes incorporated in the tissue holder, connected to a Grass S88 stimulator. Stimulation parameters during equilibration were 0.5 ms pulse duration, 60 V at 0.01 Hz.
Electrical field stimulation and agonist studies
Frequency–response curves (0.5 ms pulse duration, 60 V, 0.5–20 Hz) to electrical field stimulation were constructed using a frequency progression ratio of approximately one-third of a log unit. Trains of pulses were delivered at intervals of 10 min. Each train consisted of 10 pulses at frequencies ⩽1 Hz or was for 10 s duration at frequencies ⩾1 Hz. An initial frequency–response curve was constructed to determine the contractile response of each tissue at each frequency. Following the initial frequency–response curve, tissues were washed with 4–5 times the bath volume and allowed to rest for 60 min. After the 60-min rest period, discrete concentration–response curves to noradrenaline (10 nM–1 mM) were constructed using a dose progression ratio of approximately half a log unit. Once the contractile response to each concentration of noradrenaline had reached a maximum, tissues were washed with 4–5 times the bath volume and allowed 10 min to recover before the next concentration was applied. If no response was observed after 30 s, tissues were washed and allowed 10 min to recover. Following the initial concentration–response curve, tissues were exposed to the α1-adrenoceptor antagonist prazosin (0.3 μM), for 60 min before a second frequency–response curve was constructed using the same frequency–response protocol as above. At the conclusion of the second frequency–response curve, isolated prostates were washed and prazosin was re-added to the baths. Prazosin was left in contact with the tissues for 60 min before a second concentration–response curve to noradrenaline was constructed as described above. Prazosin was replaced after each bath wash. An appropriate time control curve was also constructed concurrently where the tissues were not exposed to prazosin during the second frequency–response or concentration–response curves.
In a subset of experiments, prostates taken from α1A+/+ and α1A−/− mice were incubated in a high K+ Krebs–Henseleit solution in which KCl had been increased to 84.7 mM and to compensate NaCl had been reduced to 38 mM. To assess whether changes to non-receptor muscle responsiveness had occurred between genotypes, prostates were subjected to the high K+ Krebs–Henseleit solution three times at intervals of 1 h.
Analysis of data
All data analyses were carried out using GraphPad Prism (v 4.0). Results are expressed as the mean±s.e.mean, where the value of n represents the number of experimental animals used. In all data analyses, P⩽0.05 was considered significant.
Mouse, prostate and percentage of prostate weights, as well as maximum agonist and high K+-induced responses were analysed by one-way ANOVA with a Bonferroni post-test for multiple comparisons where appropriate. P-values were the probability of a significant difference in mean values across genotype.
The peak force (g) of electrical field stimulation- or agonist-induced contractile responses was measured at each frequency or concentration in each genotype, and a mean frequency–response or concentration–response curve was constructed. In frequency–response and concentration–response graphs, the mean contractile responses in the presence of prazosin were compared with the previously obtained control responses (no drug). Differences in frequency–response and concentration–response curves were analysed by a two-way repeated-measures ANOVA. P-values used to evaluate statistical significance were the probabilities of a significant interaction between frequency or concentration and the presence of antagonist.
To determine if responses differed between genotypes, responses from each genotype to electrical field stimulation were plotted on a single graph and analysed using a two-way repeated-measures ANOVA with a Bonferroni post-test for multiple comparisons. For noradrenaline concentration–response curves, raw data were expressed as a percentage of the maximum response obtained in the control concentration–response curve before being plotted and analysed. P-values were the probabilities of a significant difference between genotype and frequency or concentration.
To assess the affinity of prazosin on the different phenotypes, raw data were normalized as a percentage of the maximum response obtained in the initial control concentration–response curve to noradrenaline. Normalized data were then analysed using nonlinear regression to fit a variable slope sigmoidal dose–response curve. Concentration ratios were determined by comparing the noradrenaline EC50 in the presence of prazosin with the EC50 of the control concentration–response curve in the presence of vehicle. KB values were then calculated by dividing the concentration of prazosin used by the concentration ratio−1. Antagonist affinity estimates were expressed as apparent pKB values (−log of the KB value).
Drugs and vehicle solutions
The following drugs were obtained from Sigma (St Louis, MO, USA): (−)arterenol (noradrenaline) bitartrate and prazosin hydrochloride. Noradrenaline was dissolved and diluted to the required concentrations in a catecholamine diluent (mM: NaCl 154.0, NaH2PO4 1.2 and ascorbic acid 0.2). Prazosin was dissolved and diluted to required concentrations in distilled water. The drug and molecular target nomenclature used in this paper conform with the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Mouse and prostate weights
Mean mouse weights (α1A+/+=28.8±0.7 g, α1A+/−=28.2±1.2 g and α1A−/−=28.7±1.1 g; P=0.898, n=8) and prostate weights (α1A+/+=14.6±0.9 mg, α1A+/−=15.5±1.4 mg and α1A−/−=13.8±1.1 mg; P=0.578, n=8) were not different between genotypes.
Isolated organ bath studies
Responses to electrical field stimulation
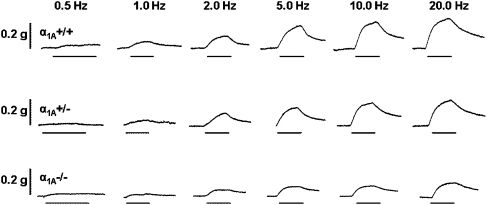
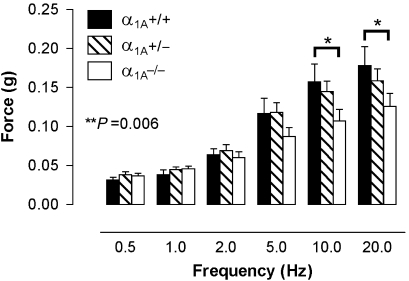
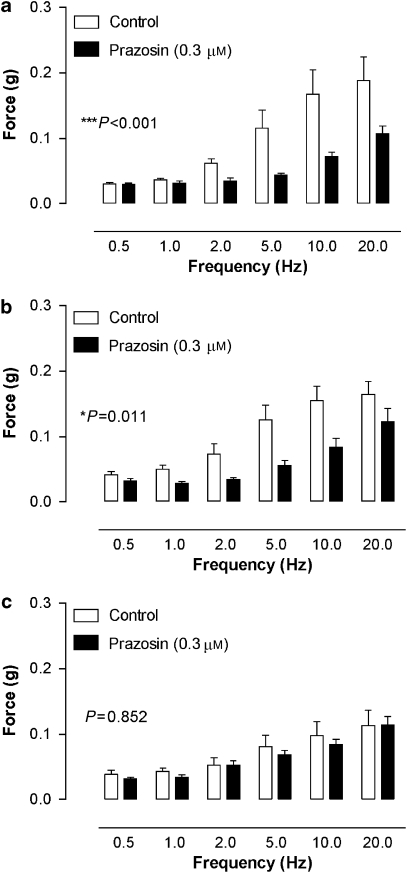
Representative traces of electrical field stimulation-induced responses in prostates from α1A+/+, α1A+/− and α1A−/− mice are shown in Figure 1. Increasing frequency resulted in an increased magnitude of the contractile response, which differed significantly across the genotypes (n=8; Figure 2). At frequencies of 10 and 20 Hz, responses of prostates from α1A−/− mice were approximately 30% smaller than the responses of prostates from α1A+/+ mice and approximately 20% smaller than the responses of prostates from α1A+/− mice. Responses were reproducible over the time course of the experiment regardless of genotype (P⩾0.360, n=4). Prazosin was able to significantly attenuate the electrical field stimulation-induced responses at frequencies greater than 1 Hz in prostates from α1A+/+ (n=4; Figure 3a) and α1A+/− mice (n=4; Figure 3b) but not from α1A−/− mice (n=4; Figure 3c).
Figure 1.
Representative traces of the contractile responses to electrical field stimulation (0.5 ms, 60 V, 0.5–20 Hz, 10 pulses or 10 s) in isolated preparations of prostate from α1A-adrenoceptor wild-type (α1A+/+), heterozygous (α1A+/−) and knockout (α1A−/−) mice. ‘__' indicates period of electrical field stimulation. Traces are representative of eight experiments.
Figure 2.
Mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 0.5–20 Hz, 10 pulses or 10 s) in prostates from α1A-adrenoceptor wild-type (α1A+/+), heterozygous (α1A+/−) and knockout (α1A−/−) mice. Bars represent the mean force obtained from eight experiments. Error bars represent the s.e.mean. P-values shown in the figure represent the probability of a significant interaction between genotype and response (two-way repeated-measures ANOVA), *Indicates significant differences between genotypes (Bonferroni post-tests) **P<0.01 and *P<0.05.
Figure 3.
Mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 0.5–20 Hz, 10 pulses or 10 s) in prostates from α1A-adrenoceptor wild-type (a), heterozygous (b) and knockout (c) mice in the absence or presence of prazosin (0.3 μM). Bars represent the mean force developed in four experiments. Error bars represent the s.e.mean. P-values shown in the figure represent the probability of a significant interaction between treatment and frequency (two-way repeated-measures ANOVA), ***P<0.001 and *P<0.05.
Agonist studies
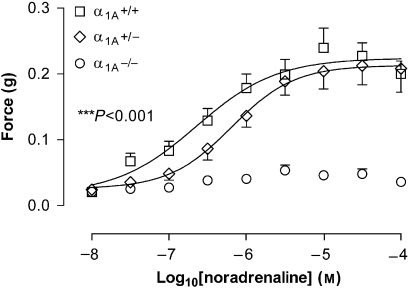
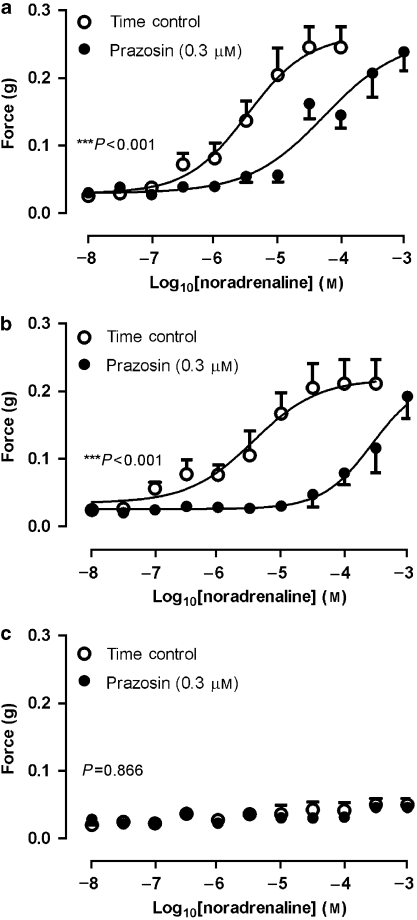
Increasing concentrations of exogenously administered noradrenaline (10 nM–0.1 mM) resulted in increased tonic contractile responses in prostates from α1A+/+ (EC50=0.26 μM(95% confidence limits: 0.10–0.68 μM)) and α1A+/− (EC50=0.79 μM (95% confidence limits: 0.54–1.15 μM)) mice (Figure 4). Responses from α1A+/− prostates were not different from the responses of prostates from α1A+/+ mice (P=0.191, n=8). The contractile responses were reproducible over the time course of the experiment (P⩾0.128, n=8). Exogenously administered noradrenaline elicited small contractile responses in prostates from α1A−/− mice; however, the responses were not dose-dependent (Figure 4). Maximum noradrenaline-induced responses of prostates from α1A+/+ and α1A+/− mice were not different (P=0.643, n=8; Figure 4); however, they were much greater than the maximum response of α1A−/− prostates (P<0.001, n=8; Figure 4). Prazosin was able to significantly alter the responses to noradrenaline in prostates taken from either α1A+/+ or α1A+/− mice (P⩽0.042, n=4; pKB=7.72±0.04 and 8.73±0.19, respectively; Figure 5).
Figure 4.
Mean contractile responses to exogenous administration of noradrenaline in unstimulated preparations of prostates from α1A-adrenoceptor wild-type (α1A+/+; □), heterozygous (α1A+/−; ◊) and knockout (α1A−/−; ○) mice. Points represent the mean force obtained in eight prostates. Error bars represent the s.e.mean. The P-value shown in the figure represents the probability of a significant interaction between genotype and response (two-way repeated-measures ANOVA), ***P<0.001.
Figure 5.
Log concentration–response curves to exogenous administration of noradrenaline in unstimulated prostatic preparations from α1A-adrenoceptor wild-type (a), heterozygous (b) and knockout (c) mice in the absence (open) and presence (closed) of prazosin (0.3 μM). Points represent the mean force obtained in four prostates. Error bars represent the s.e.mean. P-values shown in the figure represent the probability of a significant interaction between treatment and concentration (two-way repeated-measures ANOVA), ***P<0.001.
Responses to high K+
Contractile responses to high K+ were not different in prostates taken from α1A+/+ and α1A−/− mice (0.12±0.01 and 0.11±0.01 g, respectively; P=0.939, n=4). Furthermore, contractile responses did not change over time in prostates taken from mice of either genotype (P=0.838, n=4).
Discussion
Evidence for the sub-classification of α1-adrenoceptors was first described over 20 years ago (Flavahan and Vanhoutte, 1986; Morrow and Creese, 1986). Since then research into adrenoceptor classification and function has continued, resulting in the current nomenclature of α1A-, α1B- and α1D-adrenoceptor subtypes (Hieble et al., 1995; Bylund et al., 1998; Alexander et al., 2008). In addition to the three cloned α1-adrenoceptor subtypes, an additional, pharmacologically distinct subtype has been shown to exist, the α1L-adrenoceptor (Muramatsu et al., 1994; Ford et al., 1997; Daniels et al., 1999; Hiraoka et al., 1999; Pennefather et al., 1999). This α1L-adrenoceptor subtype has been postulated to be a functional phenotype of the α1A-adrenoceptor, as it has been identified in functional studies but has not been defined by molecular cloning.
The presence of the α1L-adrenoceptor in the mouse prostate and the availability of the α1A-adrenoceptor gene knockout mouse allowed us to investigate the recent suggestion that to observe the α1L-adrenoceptor phenotype, the α1A-adrenoceptor gene needs to be present (Nelson and Challiss, 2007).
We examined the contractile response in mouse prostates from α1A-adrenoceptor knockout mice and observed that the responses to electrical field stimulation were reduced by approximately 30% in α1A−/− mice compared with α1A+/+ mice. This reduction in response was not due to any nonspecific changes in muscle responsiveness, as prostates taken from α1A-adrenoceptor knockout mice responded normally to high K+ Krebs solution. We have previously shown that the contractile response of the mouse prostate to these electrical field stimulation parameters is mediated almost entirely by nerves, as they are virtually abolished by tetrodotoxin (Gray and Ventura, 2005). Responses to electrical field stimulation of prostates taken from either wild-type or heterozygous genotypes were attenuated by prazosin. Although we have not conclusively provided evidence that the nerve-mediated response is also mediated by α1L-adrenoceptors, this presumed α1L-adrenoceptor component of the response to nerve stimulation has been lost after disruption of the α1A-adrenoceptor gene. The possibility cannot be ruled out that endogenously released noradrenaline mediates a response through α1A-adrenoceptors, as reports from isolated blood vessel preparations have shown evidence that different adrenoceptor subtypes can be activated by neuronally released and exogenously administered noradrenaline (Yang and Chiba, 2001; Zacharia et al., 2004). Nevertheless, these results are consistent with the adrenergic response induced by nerve stimulation being mediated through α1L-adrenoceptors in both genotypes. This supports the hypothesis that the α1A-adrenoceptor gene is necessary for the manifestation of the α1L-adrenoceptor-mediated response. Furthermore, the inability of prazosin to further reduce the response in α1A−/− mouse prostates indicates that the α1A/α1L-adrenoceptor-mediated component of the response to electrical field stimulation has been completely lost after genetic disruption of the α1A-adrenoceptor. The mediator of the residual response observed in prostates from α1A−/− and α1A+/+ mice in the presence of prazosin remains to be elucidated; however, we have previously shown positive immunohistochemistry for a number of purinoceptors as well as for many non-adrenergic, non-cholinergic transmitters, including calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and/or endothelin, which may be responsible for the response (Gray and Ventura, 2005). Although ATP has been shown to be a co-transmitter with noradrenaline in prostates from rats (Ventura et al., 2003) and guinea-pigs (Buljubasich and Ventura, 2004), this is unlikely in the mouse, as we have shown suramin and α,β-methylene ATP to be without effect on nerve-mediated contractile responses of the mouse prostate (Gray and Ventura, 2005).
In this study, prazosin was found to have a greater potency in prostates taken from α1A+/− mice compared with α1A+/+ mice. This may be a consequence of fewer functional α1-adrenoceptors being present in the prostates taken from α1A+/− mice compared with α1A+/+ mice. Nevertheless, as in our earlier study (Gray and Ventura, 2006), shifts of noradrenaline concentration–response curves by prazosin yielded affinity estimates in both α1A+/+ and α1A+/− mice, which confirmed the presence of α1L-adrenoceptors. The ability of exogenously administered noradrenaline to induce dose-dependent contractile responses in prostate tissue from α1A+/+ and α1A+/− mice, but not from α1A−/− mice is further indication of the requirement of the α1A-adrenoceptor gene to observe the α1L-adrenoceptor phenotype. These findings are completely consistent with the idea that the α1L-adrenoceptor is a functional phenotype of the α1A-adrenoceptor (Ford et al., 1997; Ruffolo and Hieble, 1999; Nelson and Challiss, 2007).
In addition, although noradrenaline was unable to produce dose-dependent responses in α1A−/− mice, very small contractile responses were observed. This observation suggests that a discrete population of α1B- and/or α1D-adrenoceptors could be present in the mouse prostate and is responsible for mediating a very minor portion of the contractile response. Small sub-populations of both the α1B-adrenoceptor and α1D-adrenoceptor have been shown to be present in both the human (Watanabe et al., 1988; Nasu et al., 1996; Walden et al., 1999) and rabbit (Suzuki et al., 1997; Piao et al., 2000) prostate. Despite their presence, it would appear that the α1B- and α1D-adrenoceptor subtypes play only a negligible role, if at all any, in prostate contractility.
The unchanged weight of prostates from α1A−/− mice compared with α1A+/+ mice indicates that the α1A-adrenoceptor gene plays little or no role in the regulation of prostate growth. Interestingly, phenylephrine, acting on α1-adrenoceptors, has been shown to induce ventral prostate hyperplasia in rats due to decreased apoptosis rather than increased proliferation (Marinese et al., 2003). Another study also found that in human prostates, α1-adrenoceptor antagonists induce apoptosis but do not affect cellular proliferation (Kyprianou et al., 2000). These results using α1A-adrenoceptor knockout mice indicate that the α1B/α1D-adrenoceptor subtypes may be involved in prostate growth and development rather than in contraction.
This study is the first, to our knowledge, to investigate the result of genetic disruption of the α1A-adrenoceptor gene on the α1L-adrenoceptor-mediated response. The conclusions of this study are that the α1L-adrenoceptor is indeed a functional phenotype of the α1A-adrenoceptor.
Acknowledgments
This work was supported by grants to S Ventura from the Appel Family Bequest, Arthur and Mary Osborn Estate and the National Health & Medical Research Council (Australia).
Abbreviations
- BPH
benign prostatic hyperplasia
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 (Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buljubasich R, Ventura S. Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol. 2004;499:335–344. doi: 10.1016/j.ejphar.2004.07.080. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Bond RA, Clarke DE, Eikenburg DC, Hieble JP, Langer SZ, et al. Adrenoceptors The IUPHAR Compendium of Receptor Characterization and Classification 1998IUPHAR Media: London; 58–74.In: Girdlestone D (ed) [Google Scholar]
- Daniels DV, Gever JR, Jasper JR, Kava MS, Lesnick JD, Meloy TD, et al. Human cloned α1A-adrenoceptor isoforms display α1L-adrenoceptor pharmacology in functional studies. Eur J Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- Eckhardt MD, van Venrooij GE, Boon TA. Interactions between prostate volume, filling cystometric estimated parameters, and data from pressure-flow studies in 565 men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Neurourol Urodyn. 2001a;20:579–590. doi: 10.1002/nau.1010. [DOI] [PubMed] [Google Scholar]
- Eckhardt MD, van Venrooij GE, Boon TA. Symptoms and quality of life versus age, prostate volume, and urodynamic parameters in 565 strictly selected men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2001b;57:695–700. doi: 10.1016/s0090-4295(00)01101-8. [DOI] [PubMed] [Google Scholar]
- Eckhardt MD, van Venrooij GE, Boon TA. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology. 2001c;58:966–971. doi: 10.1016/s0090-4295(01)01413-3. [DOI] [PubMed] [Google Scholar]
- Flannery MT, Ramsdell J, Ranhosky A, Davidai G, Ruoff G. Efficacy and safety of tamsulosin for benign prostatic hyperplasia: clinical experience in the primary care setting. Curr Med Res Opin. 2006;22:721–730. doi: 10.1185/030079906X96443. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Vanhoutte PM. Alpha-1 and alpha-2 adrenoceptor: response coupling in canine saphenous and femoral veins. J Pharmacol Exp Ther. 1986;238:131–138. [PubMed] [Google Scholar]
- Ford AP, Arredondo NF, Blue DR, Jr, Bonhaus DW, Jasper J, Kava MS, et al. RS-17053 (N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α, α-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KT, Ventura S. Evaluation of the mouse prostate as a suitable model for the study of human prostate function. J Pharmacol Toxicol Methods. 2005;51:41–50. doi: 10.1016/j.vascn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gray KT, Ventura S. Alpha1L-adrenoceptors mediate contractions of the isolated mouse prostate. Eur J Pharmacol. 2006;540:155–161. doi: 10.1016/j.ejphar.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Hasan M, Parveen F, Shamsuzzaman AK, Kibria MD. Comparison of efficacy between tamsulosin and finasteride on symptomatic benign prostatic hyperplasia. Mymensingh Med J. 2007;16:154–159. [PubMed] [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Hiraoka Y, Ohmura T, Oshita M, Watanabe Y, Morikawa K, Nagata O, et al. Binding and functional characterization of alpha1-adrenoceptor subtypes in the rat prostate. Eur J Pharmacol. 1999;366:119–126. doi: 10.1016/s0014-2999(98)00895-4. [DOI] [PubMed] [Google Scholar]
- Israilova M, Tanaka T, Suzuki F, Morishima S, Muramatsu I. Pharmacological characterization and cross talk of alpha1a- and alpha1b-adrenoceptors coexpressed in human embryonic kidney 293 cells. J Pharmacol Exp Ther. 2004;309:259–266. doi: 10.1124/jpet.103.061796. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Chon J, Benning CM. Effects of alpha(1)-adrenoceptor (alpha(1)-AR) antagonists on cell proliferation and apoptosis in the prostate: therapeutic implications in prostatic disease. Prostate Suppl. 2000;9:42–46. doi: 10.1002/1097-0045(2000)45:9+<42::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Marinese D, Patel R, Walden PD. Mechanistic investigation of the adrenergic induction of ventral prostate hyperplasia in mice. Prostate. 2003;54:230–237. doi: 10.1002/pros.10170. [DOI] [PubMed] [Google Scholar]
- McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- Morrow AL, Creese I. Characterization of alpha 1-adrenergic receptor subtypes in rat brain: a reevaluation of [3H]WB4104 and [3H]prazosin binding. Mol Pharmacol. 1986;29:321–330. [PubMed] [Google Scholar]
- Muramatsu I, Oshita M, Ohmura T, Kigoshi S, Akino H, Gobara M, et al. Pharmacological characterization of alpha 1-adrenoceptor subtypes in the human prostate: functional and binding studies. Br J Urol. 1994;74:572–578. doi: 10.1111/j.1464-410x.1994.tb09186.x. [DOI] [PubMed] [Google Scholar]
- Nasu K, Moriyama N, Kawabe K, Tsujimoto G, Murai M, Tanaka T, et al. Quantification and distribution of alpha 1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br J Pharmacol. 1996;119:797–803. doi: 10.1111/j.1476-5381.1996.tb15742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CP, Challiss RA. ‘Phenotypic' pharmacology: the influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem Pharmacol. 2007;73:737–751. doi: 10.1016/j.bcp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Noble AJ, Chess-Williams R, Couldwell C, Furukawa K, Uchyiuma T, Korstanje C, et al. The effects of tamsulosin, a high affinity antagonist at functional α1A- and α1D-adrenoceptor subtypes. Br J Pharmacol. 1997;120:231–238. doi: 10.1038/sj.bjp.0700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather JN, Lau WA, Chin C, Story ME, Ventura S. α1L-Adrenoceptors mediate noradrenaline-induced contractions of the guinea-pig prostate stroma. Eur J Pharmacol. 1999;384:25–30. doi: 10.1016/s0014-2999(99)00667-6. [DOI] [PubMed] [Google Scholar]
- Piao H, Taniguchi T, Nakamura S, Zhu J, Suzuki F, Mikami D, et al. Cloning of rabbit alpha(1b)-adrenoceptor and pharmacological comparison of alpha(1a)-, alpha(1b)- and alpha(1d)-adrenoceptors in rabbit. Eur J Pharmacol. 2000;396:9–17. doi: 10.1016/s0014-2999(00)00171-0. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Carr IC, Pediani J, Lopez-Gimenez JF, Thurlow R, Fidock M, et al. High-affinity interactions between human α1A-adrenoceptor C-terminal splice variants produce homo- and heterodimers but do not generate the α1L-adrenoceptor. Mol Pharmacol. 2004;66:228–239. doi: 10.1124/mol.66.2.228. [DOI] [PubMed] [Google Scholar]
- Rigatti P, Brausi M, Scarpa RM, Porru D, Schumacher H, Rizzi CA. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2003;6:315–323. doi: 10.1038/sj.pcan.4500680. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Hieble JP. Adrenoceptor pharmacology: urogenital applications. Eur Urol. 1999;36 (Suppl 1):17–22. doi: 10.1159/000052313. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Miyamoto S, Takita M, Oshita M, Watanabe Y, Kakizuka A, et al. Cloning, functional expression and tissue distribution of rabbit α1d-adrenoceptor. Biochim Biophys Acta—Biomembr. 1997;1323:6–11. doi: 10.1016/s0005-2736(96)00229-5. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Taniguchi T, Takauji R, Murata S, Muramatsu I. Splice isoforms of α1a-adrenoceptor in rabbit. Br J Pharmacol. 2000;129:1569–1576. doi: 10.1038/sj.bjp.0703242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Yano M, Awa Y, Nakatsu H, Egoshi K, Mikami K, et al. Clinical impact of tamsulosin on generic and symptom-specific quality of life for benign prostatic hyperplasia patients: using international prostate symptom score and Rand Medical Outcomes Study 36-item Health Survey. Int J Urol. 2006;13:1202–1206. doi: 10.1111/j.1442-2042.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Ventura S, Dewalagama RK, Lau LC. Adenosine 5′-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland. Br J Pharmacol. 2003;138:1277–1284. doi: 10.1038/sj.bjp.0705167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden PD, Gerardi C, Lepor H. Localization and expression of the α1A−1, α1B and α1D-adrenoceptors in hyperplastic and non-hyperplastic human prostate. J Urol. 1999;161:635–640. [PubMed] [Google Scholar]
- Walden PD, Ittmann M, Monaco ME, Lepor H. Endothelin-1 production and agonist activities in cultured prostate-derived cells: implications for regulation of endothelin bioactivity and bioavailability in prostatic hyperplasia. Prostate. 1998;34:241–250. doi: 10.1002/(sici)1097-0045(19980301)34:4<241::aid-pros1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Shima M, Kojima M, Ohe H. Dynamic study of nervous control on prostatic contraction and fluid excretion in the dog. J Urol. 1988;140:1567–1570. doi: 10.1016/s0022-5347(17)42128-8. [DOI] [PubMed] [Google Scholar]
- Yamada S, Okura T, Kimura R. In vivo demonstration of alpha(1A)-adrenoceptor subtype selectivity of KMD-3213 in rat tissues. J Pharmacol Exp Ther. 2001;296:160–167. [PubMed] [Google Scholar]
- Yang XP, Chiba S. Existence of different alpha(1)-adrenoceptor subtypes in junctional and extrajunctional neurovascular regions in canine splenic arteries. Br J Pharmacol. 2001;132:1852–1858. doi: 10.1038/sj.bjp.0704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharia J, Hillier C, Macdonald A. Pharmacological characterization of alpha1-adrenoceptors in mouse isolated femoral small arteries. Eur J Pharmacol. 2004;503:155–163. doi: 10.1016/j.ejphar.2004.09.046. [DOI] [PubMed] [Google Scholar]