Abstract
Grafting of [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] on a silica partially dehydroxylated at 700°C (SiO2- (700)) generates the corresponding monosiloxy complex [(≡SiO)W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)] as the major species (≈90%) along with [(≡SiO)W(≡NAr)(CH2tBu)(2,5-Me2NC4H2)2], according to mass balance analysis, IR, and NMR studies. This heterogeneous catalyst displays good activity and stability in the metathesis of propene. Very importantly, solid state NMR spectroscopy allows observation of the propagating alkylidene as well as stable metallacyclobutane intermediates. These species have the same reactivity as the initial surface complex [(≡SiO)W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)], which shows that they are the key intermediates of alkene metathesis.
Keywords: alkylidene, heterogeneous catalysis, metallacyclobutanes, solid state NMR
Alkene metathesis, a key reaction in industry, is used for the synthesis of both basic and fine chemicals as well as polymers (1–3). Because of an increasing world demand for propene, one of the most important processes involving metathesis is now the synthesis of propene through cross-metathesis of ethene and 2-butene. This process relies on heterogeneous catalysis because of its many technical and economical advantages. However, the development of more efficient systems in terms of activity, selectivity, and stability is still required because of the need to optimize the use of resources within the context of a more sustainable development. Although several breakthroughs in the preparation and characterization of single-site heterogeneous catalysts have already appeared (4–7), including those in the field of alkene metathesis catalysts (8–15), understanding deactivation phenomena is still a challenge today. In this respect, the observation and determination of the fate of reaction intermediates would help tackle this problem and thereby lead to improved catalyst performances. In the specific case of alkene metathesis, it has been proposed that carbenes and metallacyclobutanes are key reaction intermediates (16). Although they have been unambiguously prepared and observed for homogeneous catalysts (17–29), observing them as heterogeneous catalysts has proved to be a formidable challenge (30–32). Note that because the surface can play such a crucial role, one should never assume that the intermediates are identical in heterogeneous and homogeneous systems (31, 32). Currently, whereas well defined highly active silica supported metallocarbene precursors having neopentylidene and neophylidene ligands can be prepared and characterized at a molecular level, observation of propagating alkylidene or metallacyclobutanes have remained elusive even for these well defined systems (33).
Here, we describe the preparation of a well defined silica supported W alkylidene species [(≡SiO)W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)] (compound 1), report its activity in alkene metathesis, observe the methylidene and metallacyclobutane species, and show they are key reaction intermediates in alkene metathesis.
Results and Discussion
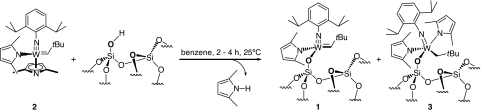
Grafting [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (compound 2) (34) on a silica that has been partially dehydroxylated at 700°C (SiO2- (700)), [2/SiO2- (700)] gives the corresponding monosiloxy complex [(≡SiO)W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)] (compound 1) as the major species (≈90%) along with [(≡SiO)W(≡NAr)(CH2tBu)(2,5-Me2NC4H2)2] (compound 3), according to mass balance analysis, IR, and NMR studies as previously observed in the case of the Mo homolog (Scheme 1) (14). Monitoring the grafting step by IR spectroscopy shows that most surface silanols are consumed and that aromatic and alkyl ligands appear as evidenced from the appearance of signals in the 3,100–2,700 (νC-H) and 1,600–1,350 (δC-H) cm−1 regions [supporting information (SI) Fig. S1]. In addition, 0.9 eq of 2,5-dimethylpyrrole is present after grafting, and elemental analyses of the resulting solid (W = 3.73 ± 0.05% wt, C = 5.70 ± 0.1% wt, and N = 0.71 ± 0.1%wt) corresponds to 0.21 mmol/g W, 24 ± 2 carbons, and 2.5 ± 0.5 nitrogen molecules per grafted W, which also is consistent with consumption of most of the surface silanols (0.21 mmol/g W for 0.26 mmol/g SiOH in SiO2- (700)) and formation of 1 as the major surface species, for which 23 C/Mo and 2 N/Mo are expected. Furthermore, the 1H magic angle spinning (MAS) solid state NMR spectrum, and more convincingly, the constant time (CT) (35, 36) 1H MAS NMR spectrum (Fig. S2), display the following resonances: 9.0 ([W] = CHtBu), 7.0 (Csp2-H), 6.7 (Csp2-H), 5.5 (pyrrolyl-Csp2-H), 3.2 (CHMe2), and 0.9 ([W] = CHCMe3 + pyrrolyl-Csp3-H + CHMe2) ppm as expected for 1, with the signal at 1.9 ppm being associated with the methylene protons in the neopentyl ligand, [W]-CH2tBu, of 3 (see Scheme 1). Conversely, the 13C cross-polorization (CP) MAS spectrum (Fig. S3 and Table S1) displays the expected signals for 1, with the exception of the carbenic carbon [W]=CHtBu, which is not detected, as well as an extra signal at 69 ppm, which can be associated with the methylene carbon [W]CH2tBu of the neopentyl ligand in 3. These assignments are confirmed by performing the 2D 1H-13C heteronuclear correlation spectroscopy (HETCOR) solid state NMR on the 13C labeled species [(≡SiO)W(≡NAr)(=*CHtBu)(2,5-Me2NC4H2)] (compound 1*), 33% 13C labeled on the α-carbon to W, which clearly shows a correlation associated with the neopentyl carbon [W]-*CH2tBu in 3 at 70 ppm and its proton at 1.9 ppm, but which also, and more importantly, displays the neopentylidene carbon signal [W]=*CHtBu at 260 ppm and its correlation with the corresponding proton [W]=CHtBu at 9.0 ppm, associated with 1 (Fig. S4). All of these data are consistent with the formation of 1 as the major surface species (90%) via cleavage of the W N bond by the surface silanol, and the presence of a minor amount of 3, formed through the competing reaction of addition of a surface silanol with the W=C double bond of 2.
N bond by the surface silanol, and the presence of a minor amount of 3, formed through the competing reaction of addition of a surface silanol with the W=C double bond of 2.
Scheme. 1.
Formation of [(≡SiO)W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)] (compound 1, 90%) and [(≡SiO)W(=NAr)(CH2tBu)(2,5-Me2NC4H2)2] (compound 3, 10%) by grafting of [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (compound 2) with the isolated silanols of SiO2- (700).
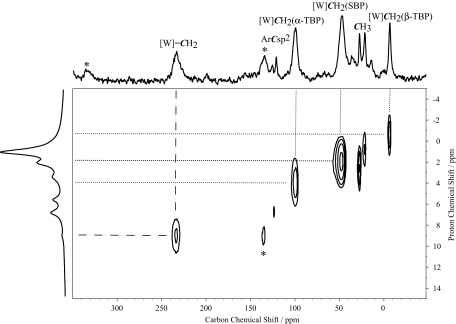
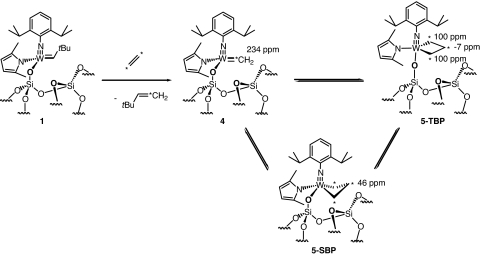
Contacting [2/SiO2- (700)] with propene in a flow reactor (≈82 ml/min−1; ≈420 mol of propene per mol of W per minute) selectively yields ethene and 2-butenes with an initial activity of 0.41 s−1 (Fig. S5). Deactivation is slow, and the activity after 1,500 min is still ≈0.1 s−1, so that 25,000 turnovers can be achieved within this time. Although not especially active, 2/SiO2- (700) displays good stability compared with Mo- and Re-based catalysts, which typically deactivate rapidly (loss of 75% of the activity within 100–400 min). Stability of this catalyst led us to investigate the possibility of observing the NMR spectroscopic signatures of alkene metathesis reaction intermediates, i.e., the propagating carbenes and metallacyclobutane intermediates (10, 37). Therefore, 13C dilabeled ethene was contacted at −196°C with [2/SiO2- (700)]. Upon warming the reaction mixture to room temperature, the yellow powder turned red. After 4 h at 25°C, the gas phase contained ≈0.4 eq of 95% 13C monolabeled tBuCH=*CH2. The corresponding solid was then analyzed by solid state NMR. From 1H, 13C CP and 1H-13C HETCOR MAS experiments (Fig. 1), it is clear that intense new peaks have appeared that can be readily assigned as follows (Scheme 2): Correlation of the 1H and 13C signals at (1H: 9.0/13C: 234 ppm) is fully consistent with a methylidene ligand [W]=CH2 in [(≡SiO)W(≡NAr)(=CH2)(2,5-Me2NC4H2)] (compound 4) (38) resulting from the cross metathesis of the neopentylidene and the 13C di-labeled ethene.
Fig. 1.
2D 1H-13C HETCOR solid state NMR spectrum of [2/SiO2- (700)] contacted with 13C dilabeled ethene. This spectrum was acquired under eDUMBO-112.5 (44) homonuclear decoupling (F1) and SPINAL-64 (45) heteronuclear decoupling (F2) at ν1H = 100 kHz. The contact time for CP and the recycle delay were 500 μs and 2 s, respectively. A total of 48 t1 points with 512 scans each were collected. A scaling factor (λexp) of 0.54 was used in F1 for eDUMBO-112.5 homonuclear decoupling. Asterisks indicate spinning side bands of the alkylidene resonance [W] = CH2. The 13C CP MAS spectrum (512 scans) recorded with a CP step of 500 μs and a recycle delay of 2 s is indicated above the 2D plot. To the left of the 2D plot is shown the 1H single pulse spectrum (eight scans). Dashed and dotted correlations correspond to the methylidene [W]=CH2 and metallacyclobutane [W](CH2CH2CH2) moieties in 4 and 5, respectively.
Scheme. 2.
Formation and NMR spectroscopic signatures of methylidene (compound 4) and tungstacyclobutane (compound 5) species by of [(≡SiO)W(≡NAr)(= CHtBu)(2,5-Me2NC4H2)] (1) with 13C dilabeled ethene.
Resonances appearing at (1H: −0.9/13C: −7 ppm) and (1H: 4.0/13C: 100 ppm) correspond to β- and α-carbons of the unsubstituted metallacyclobutane [(≡SiO)W(≡NAr)(CH2CH2CH2)(2,5-Me2NC4H2)] with a trigonal bipyramid geometry (5-TBP). Proton and carbon resonances at 2.0 and 46 ppm, respectively, can be attributed to the protons and carbons of a metallacyclobutane with a square based pyramid geometry (5-SBP).
Observation of stable metallacyclobutanes in two types of geometries is fully consistent with previous experimental (in solution) (18–25) and theoretical studies (39–43) for d0 metal complexes. It is also noteworthy that in solution, the SBP/TBP isomer ratio and the reactivity in metathesis are correlated, for a series of isoelectronic metallacyclobutane intermediates of the general formula [(RO)2W(≡NAr)(CH2CH2CH2)], to the electronic properties of the RO ligand as follows: (i) for RO = (CF3)2(CH3)CO (the most electron-withdrawing ligand), only the TBP isomer is observed, and the complex is very reactive; (ii) for RO = (CF3)(CH3)2CO, a mixture of TBP (a few %) and SBP isomers is observed, and they display intermediate reactivity; and (iii) for RO = (CH3)3CO, only the SBP isomer is observed, with very low reactivity because of the high stability of the metallacyclobutane (21, 22). In the case of the active silica supported system reported here, for which the two RO ligands have been replaced by one large siloxy surface ligand and one 2,5-dimethylpyrrolyl ligand, both isomers are observed in roughly equal amounts, and they show good activity (see above). Modulating the ligands in the future by probing the ratio of metallacyclobutane isomers and comparing reactivity opens up a way to access structure-reactivity relationships for heterogeneous alkene metathesis catalysts directly from reaction intermediates.
Moreover, contacting this sample with propene in a flow reactor under the same reaction conditions as those described for [2/SiO2- (700)] display activities (0.51 s−1) similar to that observed for the original catalyst (0.41 s−1 for [2/SiO2- (700)]) (Fig. S6), albeit with a slightly lower stability. The fact that the methylidene and metallacyclobutane species display similar activity with the well defined silica supported catalyst precursor 1 bearing a neopentylidene ligand clearly suggests that these species are indeed the key reaction intermediates in the alkene metathesis pathway.
Conclusions
We have shown that (i) a well defined silica supported W imido alkylidene surface complex [(≡SiO)W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)] (compound 1), prepared and characterized at a molecular level, displays a good activity, selectivity, and stability in the metathesis of propene; (ii) contacting 1 with 13C labeled ethene yields stable methylidene and metallacyclobutane intermediates, resulting from cross metathesis and [2 + 2]-cycloaddition (16, 41, 42); and (iii) these species are indeed the key intermediates of the catalytic cycle of alkene metathesis. Overall, these results serve as further evidence that well defined silica-supported systems do behave in a manner similar to homogeneous systems. Direct observation by NMR of these intermediates and their associated spectroscopic signatures is the first step toward a better molecular understanding of heterogeneous olefin metathesis catalysts, and further closes the gap between homogeneous and heterogeneous catalysis. Finally, the design of more efficient catalytic systems may be possible as more information of catalytic intermediates and their stability becomes available (2, 3).
Methods
General Procedures.
All experiments were carried out under dry and oxygen-free Ar using either standard Schlenk or glove box techniques for the organometallic synthesis. For the synthesis and treatment of surface species, reactions were carried out using high vacuum lines (1.34 Pa) and glove box techniques. [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (Ar = 2,6-iPr2C6H3) (compound 2) was prepared as described previously (34). [(1-13C 33%) W(≡NAr)(=*CHtBu)(2,5-Me2NC4H2)2] (compound 2*) was synthesized as described previously (9) using [(1-13C 33%) tBu13CH2MgCl] as an alkylating agent. [(1-13C 33%) tBu13CH2MgCl] was prepared by mixing (1-13C 99%) tBu13CH2Cl and natural abundance tBuCH2Cl followed by the Grignard reaction. Silica (200 m2/g; Aerosil Degussa) was compacted with distilled water, calcined at 500°C under air for 2 h and treated under vacuum (1.34 Pa) at 500°C for 12 h and then at 700°C for 4 h (support referred to as SiO2- (700)). Benzene and C6D6 (SDS) were distilled from sodium benzophenone ketyl. Propene (99.95%; Scott) and 13C di-labeled ethene (99% 13C; CIL) were purified over R3–11 BASF catalyst/MS 4 Å before use. Cp2Fe (98%; Aldrich) was used as received. Elemental analyses were performed at the Mikroanalythisches Labor Pascher. Infrared spectra were recorded on a Nicolet 550-FT by using an infrared cell equipped with CaF2 windows, allowing in situ studies. Typically, 16 scans were accumulated for each spectrum (resolution, 2 cm−1). Products were identified by gas chromatography (GC)/MS. Gas phase analyses were performed on a Hewlett Packard 5890 series II GC apparatus equipped with a flame ionization detector and a KCl/Al2O2 column (50 m × 0.32 mm).
NMR Spectroscopy.
Liquid state NMR spectroscopy.
Liquid state NMR spectra were recorded in C6D6 using a Bruker DRX 500 spectrometer and referenced to the residual protonated solvent peaks (δH = 7.15 ppm). The amount of 2,5-dimethylpyrrole released during grafting was monitored by quantitative 1H NMR with spin presaturation using Cp2Fe as an internal standard.
Solid state NMR spectroscopy.
All solid state NMR spectra were recorded under MAS on a Bruker Avance or Avance II 500 MHz spectrometer with a conventional double-resonance, 4-mm CP MAS probe. The MAS frequency was set to 12.5 kHz for all of the experiments reported here. The proton radiofrequency (RF) field strength was set to 83 kHz for pulses and 100 kHz during decoupling. For the CP step, the RF field was set to 70 kHz, and for carbon, a ramped RF field was applied on protons and matched to obtain optimal signals. All other experimental details are given in the figure legends. 1H delayed acquisition spectra including 1H constant-time spectra were recorded without homonuclear decoupling schemes (36). SPINAL64-1H (45) heteronuclear decoupling was used in all 13C experiments. An exponential line broadening of 80 Hz was applied before Fourier transform of all 13C experiments. eDUMBO-112.5 (44) homonuclear dipolar decoupling was used for the HETCOR experiment presented in Fig. 1. The experimental scaling factor (λexp) of 0.54 was obtained by comparing the proton spectra of l-alanine recorded under fast MAS conditions (30 kHz) and under eDUMBO-112.5 decoupling as described previously (44). The samples were filled in a 4-mm zirconia rotor in a glove box and closed with tightly fitting caps. Proton and carbon chemical shifts are reported in ppm downfield from liquid SiMe4 (0 ppm).
Grafting of [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (compound 2) Monitored by in Situ IR Spectroscopy.
Silica (55 mg) was pressed into a 18-mm self-supporting disk and put into a sealed glass high vacuum reactor equipped with CaF2 windows. After calcination at 500°C under air for 2 h, the silica disk was treated under vacuum (1.34 Pa) at 500°C for 12 h and then at 700°C for 4 h. The silica support thus obtained (SiO2- (700), ≈15 μmol SiOH) was then immersed into a benzene (10 ml) solution of [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (2) (28 mg, 45 μmol, 3.1 eq) at 25°C for 12 h. After washing the pellet three times with benzene (10 ml) and drying under vacuum (1.34 Pa) at 25°C for 2 h, an IR spectrum of the yellow disk was recorded: 3,066, 3,031, 2,966, 2,936, 2,906, 2,873, 1,613, 1,589, 1,578, 1,473, 1,463, 1,433, 1,387, 1,363 and 1,351 cm−1 (Fig. S1). All of the wash solutions were collected and analyzed by 1H NMR spectroscopy (in C6D6), which showed that 2,5-dimethylpyrrole was formed during grafting.
Grafting of [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (2) by Impregnation.
A mixture of [W(≡NAr)(=CHtBu)(2,5-Me2NC4H2)2] (2) (61 mg, 0.10 mmol) and SiO2- (700) (421 mg, 0.11 mmol SiOH) in benzene (12 ml) was stirred at 25°C for 3 h. After filtration, the volatiles were distilled off, and benzene (≈8 ml) was added. The yellow–orange solid was then washed, filtered and the volatiles distilled off. This was repeated three times, and then the resulting yellow powder was dried under vacuum (1.34 Pa) at 25°C for 2 h to yield [2/SiO2- (700)]. All of the filtrate solutions were collected and analyzed by quantitative 1H NMR spectroscopy (in C6D6) using Cp2Fe (13.3 mg, 71.5 μmol, 1 H) as an internal standard, and 27.4 μmol of 2,5-dimethylpyrrole was formed during grafting (0.9 eq of 2,5-dimethylpyrrole/Wsurf). Elemental analysis: 3.73%wt W, 5.70%wt C, 0.71%wt N, 24 ± 2 C/Mo, 2.5 ± 0.5 N/Mo.
Grafting of [W(≡NAr)(=*CHtBu)(2,5-Me2NC4H2)2] (compound 2*) by Impregnation.
This reaction was carried out as described above by using [W(≡NAr)(=*CHtBu)(2,5-Me2NC4H2)2] (compound 2*) (33 mg, 0.05 mmol), SiO2- (700) (232 mg, 0.06 mmol SiOH) and benzene (7 ml), thus yielding a yellow powder, and 44 μmol of 2,5-dimethylpyrrole was formed during grafting (0.9 eq of 2,5-dimethylpyrrole/Wsurf).
Reaction of 13C Dilabeled Ethene with [2/SiO2- (700)].
To [2/SiO2- (700)] (52 mg, 10.6 μmol W) was added 13C dilabeled ethene (65 Torr, 140 μmol, 13 eq), while maintaining the sample at liquid nitrogen temperature. The sample was then brought to room temperature, and its color changed from yellow to red. After 4 h, analysis by gas chromatography indicated the formation of 3.9 μmol of 3,3-dimethylbutene (0.37 eq) with the following isotopomeric composition: 3,3-dimethylbutene (5 ± 5%), 3,3-dimethylbutene-13C1 (95 ± 5%), 3,3-dimethylbutene-13C2 (<1%) and 3,3-dimethylbutene-13C3 (< 1%). The red powder was then dried under vacuum (1.34 Pa) at 25°C for 30 min to yield an orange solid as a mixture of [(≡SiO)W(≡NAr)(=*CH2)(2,5-Me2NC4H2)] (compound 4) and [(≡SiO)W(≡NAr)(*CH2*CH2*CH2)(2,5-Me2NC4H2)2] (compound 5).
Propene Metathesis in a Flow Reactor.
The solid catalyst (40 mg, 8.1 μmol) was loaded in a flow reactor in the glove box, the isolated reaction chamber was then connected to the propene line, the propene pressure was set to 1 bar, and the tubes were flushed with propene for 2 h. The flow rate was set to 82 ml/min (6.6 mol of propene per mol of Mo per second), the temperature was set to 30°C and the opening of the valve corresponds to the beginning of the catalysis. The reaction was monitored by GC using an autosampler.
Supplementary Material
Acknowledgments.
This work was supported by a Ministère de la Recherche et de l'Education graduate fellowship (to F.B. and R.B.), Centre National de la Recherche Scientifique and École Supérieure Chimie Physique Électronique de Lyon (F.B. and C.C.), French Agence Nationale del la Recherche Young Investigator Fellowship ANR JC05_46372 (to C.C.), and National Science Foundation Grant CHE-0138495 (to R.R.S.). NMR spectra were recorded at the Rhône-Alpes Large Scale Facility for NMR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802147105/DCSupplemental.
References
- 1.Mol JC. Industrial applications of olefin metathesis. J Mol Cat. 2004;213:39–45. [Google Scholar]
- 2.Grubbs RH. Olefin-metathesis catalysts for the preparation of molecules and materials. Angew Chem Int Ed. 2006;45:3760–3765. doi: 10.1002/anie.200600680. [DOI] [PubMed] [Google Scholar]
- 3.Schrock RR. Multiple metal-carbon bonds for catalytic metathesis reactions. Angew Chem Int Ed. 2006;45:3748–3759. doi: 10.1002/anie.200600085. [DOI] [PubMed] [Google Scholar]
- 4.Marks TJ. Surface-bound metal hydrocarbyls—Organometallic connections between heterogeneous and homogeneous catalysis. Acc Chem Res. 1992;25:57–65. [Google Scholar]
- 5.Copéret C, Chabanas M, Petroff Saint-Arroman R, Basset J-M. Homogeneous and heterogeneous catalysis: Bridging the gap through surface organometallic chemistry. Angew Chem Int Ed. 2003;42:156–181. doi: 10.1002/anie.200390072. [DOI] [PubMed] [Google Scholar]
- 6.Corma A. Attempts to fill the gap between enzymatic, homogeneous, and heterogeneous catalysis. Catal Rev Sci Eng. 2004;46:369–417. [Google Scholar]
- 7.Thomas JM, Raja R. The advantages and future potential of single-site heterogeneous catalysts. Top Catal. 2006;40:3–17. [Google Scholar]
- 8.Chabanas M, Baudouin A, Copéret C, Basset J-M. A highly active well-defined rhenium heterogeneous catalyst for olefin metathesis prepared via surface organometallic chemistry. J Am Chem Soc. 2001;123:2062–2063. doi: 10.1021/ja000900f. [DOI] [PubMed] [Google Scholar]
- 9.Chabanas M, et al. Perhydrocarbyl ReVII complexes: Comparison of molecular and surface complexes. J Am Chem Soc. 2003;125:492–504. doi: 10.1021/ja020136s. [DOI] [PubMed] [Google Scholar]
- 10.Copéret C. Molecular design of heterogeneous catalysts: The case of olefin metathesis. New J Chem. 2004;28:1–10. [Google Scholar]
- 11.Blanc F, et al. Surface versus molecular siloxy ligands in well-defined olefin metathesis catalysts: [{(RO)3SiO}Mo(=NAr)(=CHtBu)(CH2tBu)] Angew Chem Int Ed. 2006;45:1216–1220. doi: 10.1002/anie.200503205. [DOI] [PubMed] [Google Scholar]
- 12.Rhers B, et al. A well-defined, silica-supported tungsten imido alkylidene olefin metathesis catalyst. Organometallics. 2006;25:3554–3557. [Google Scholar]
- 13.Blanc F, et al. Highly active, stable, and selective well-defined silica supported Mo imido olefin metathesis catalysts. J Am Chem Soc. 2007;129:1044–1045. doi: 10.1021/ja068249p. [DOI] [PubMed] [Google Scholar]
- 14.Blanc F, et al. Dramatic improvements of well-defined silica supported Mo-based olefin metathesis catalysts by tuning the N-containing ligands. J Am Chem Soc. 2007;129:8434–8435. doi: 10.1021/ja073095e. [DOI] [PubMed] [Google Scholar]
- 15.Copéret C. Design and understanding of heterogeneous alkene metathesis catalysts. Dalton Trans. 2007;47:5498–5504. doi: 10.1039/b713314f. [DOI] [PubMed] [Google Scholar]
- 16.Herisson JL, Chauvin Y. Transformation catalysis of olefins by tungsten complexes 2. Telomerization of cyclic olefins in presence of acyclic olefins. Makromol Chem. 1971;141:161–176. [Google Scholar]
- 17.Kress J, Osborn JA. Proof of the carbene-olefin intermediate in tungsten-containing metathesis catalysts with the metal in a higher oxidation state. Angew Chem Int Ed. 1992;31:1585–1587. [Google Scholar]
- 18.Schrock RR, et al. Preparation and reactivity of several alkylidene complexes of the type W(CHR′)(N-2,6-C6H3-iso-Pr2)(OR)2 and related tungstacyclobutane complexes. Controlling metathesis activity through the choice of alkoxide ligand. J Am Chem Soc. 1988;110:1423–1435. [Google Scholar]
- 19.Feldman J, Murdzek JS, Davis WM, Schrock RR. Reaction of neopentylidene complexes of the type M(CH-t-Bu)(N-2,6-C6H3-i-Pr2)(OR)2 (M = W, Mo) with methyl acrylate and N,N-dimethylacrylamide to give metallacyclobutane complexes. Organometallics. 1989;8:2260–2265. [Google Scholar]
- 20.Feldman J, Davis WM, Schrock RR. Trigonal-bipyramidal and square-pyramidal tungstacyclobutane intermediates are both present in systems in which olefins are metathesized by complexes of the type W(CHR')(N-2,6-C6H3-iso-Pr2)(OR)2. Organometallics. 1989;8:2266–2268. [Google Scholar]
- 21.Feldman J, Davis WM, Thomas JK, Schrock RR. Preparation and reactivity of tungsten(VI) metallacyclobutane complexes. Square pyramids versus trigonal bipyramids. Organometallics. 1990;9:2535–2548. [Google Scholar]
- 22.Feldman J, Schrock RR. Recent advances in the chemistry of d0 alkylidene and metallacycobutane complexes. Prog Inorg Chem. 1991;39:1–74. [Google Scholar]
- 23.Tsang WCP, Schrock RR, Hoveyda AH. Evaluation of enantiomerically pure binaphthol-based molybdenum catalysts for asymmetric olefin metathesis reactions that contain 3,3′-diphenyl- or 3,3′-dimesityl-substituted binaphtholate ligands. Generation and decomposition of unsubstituted molybdacyclobutane complexes. Organometallics. 2001;20:5658–5669. [Google Scholar]
- 24.Tsang WCP, et al. Alkylidene and metalacyclic complexes of tungsten that contain a chiral biphenoxide ligand. Synthesis, asymmetric ring-closing metathesis, and mechanistic investigations. J Am Chem Soc. 2003;125:2652–2666. doi: 10.1021/ja0210603. [DOI] [PubMed] [Google Scholar]
- 25.Tsang WCP, et al. Investigations of reactions between chiral molybdenum imido alkylidene complexes and ethylene: Observation of unsolvated base-free methylene complexes, metalacyclobutane and metalacyclopentane complexes, and molybdenum(IV) olefin complexes. Organometallics. 2004;23:1997–2007. [Google Scholar]
- 26.Romero PE, Piers WE. Direct observation of a 14-electron ruthenacyclobutane relevant to olefin metathesis. J Am Chem Soc. 2005;127:5032–5033. doi: 10.1021/ja042259d. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DR, Hickstein DD, O'Leary DJ, Grubbs RH. Model compounds of ruthenium-alkene intermediates in olefin metathesis reactions. J Am Chem Soc. 2006;128:8386–8387. doi: 10.1021/ja0618090. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel AG, Grubbs RH. Ruthenium metallacycles derived from 14-electron complexes. New insights into olefin metathesis intermediates. J Am Chem Soc. 2006;128:16048–16049. doi: 10.1021/ja0666598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero PE, Piers WE. Mechanistic studies on 14-electron ruthenacyclobutanes: Degenerate exchange with free ethylene. J Am Chem Soc. 2007;129:1698–1704. doi: 10.1021/ja0675245. [DOI] [PubMed] [Google Scholar]
- 30.Siaj M, et al. Dissociation of acetaldehyde on β-Mo2C to yield ethylidene and oxo surface groups: A possible pathway for active site formation in heterogeneous olefin metathesis. J Am Chem Soc. 2004;126:9514–9515. doi: 10.1021/ja048640f. [DOI] [PubMed] [Google Scholar]
- 31.Salameh A, et al. CH3ReO3 on γ-Al2O3: Understanding its structure, initiation, and reactivity in olefin metathesis. Angew Chem Int Ed. 2007;46:3870–3873. doi: 10.1002/anie.200700211. [DOI] [PubMed] [Google Scholar]
- 32.Salameh A, et al. CH3ReO3 on γ-Al2O3: Activity, selectivity, active site and deactivation in olefin metathesis. J Catal. 2008;253:180–190. [Google Scholar]
- 33.Chabanas M. Lyon, France: Université Lyon; 2001. pp. 1–167. PhD thesis. [Google Scholar]
- 34.Kreickmann T, Arndt S, Schrock RR, Mueller P. Imido alkylidene bispyrrolyl complexes of tungsten. Organometallics. 2007;26:5702–5711. [Google Scholar]
- 35.Lesage A, Duma L, Sakellariou D, Emsley L. Improved resolution in proton NMR spectroscopy of powdered solids. J Am Chem Soc. 2001;123:5747–5752. doi: 10.1021/ja0039740. [DOI] [PubMed] [Google Scholar]
- 36.Blanc F, et al. Better characterization of surface organometallic catalysts through resolution enhancement in proton solid state NMR spectra. Inorg Chem. 2006;45:9587–9592. doi: 10.1021/ic061222m. [DOI] [PubMed] [Google Scholar]
- 37.Blanc F, Copéret C, Lesage A, Emsley L. High resolution solid state NMR spectroscopy in surface organometallic chemistry: Access to molecular understanding of active sites of well-defined heterogeneous catalysts. Chem Soc Rev. 2008;37:518–526. doi: 10.1039/b612793m. [DOI] [PubMed] [Google Scholar]
- 38.Arndt S, Schrock RR, Mueller P. Synthesis and reactions of tungsten alkylidene complexes that contain the 2,6-dichlorophenylimido ligand. Organometallics. 2007;26:1279–1290. [Google Scholar]
- 39.Goumans TPM, Ehlers AW, Lammertsma K. The asymmetric Schrock olefin metathesis catalyst. A computational study. Organometallics. 2005;24:3200–3206. [Google Scholar]
- 40.Poater A, et al. DFT calculations of d(0) M(NR)(CHtBu)(X)(Y) (M = Mo, W; R = CPh3, 2,6-iPr-C6H3; X and Y = CH2tBu, OtBu, OSi(OtBu)3) olefin metathesis catalysts: Structural, spectroscopic and electronic properties. Dalton Trans. 2006;25:3077–3087. doi: 10.1039/b604481f. [DOI] [PubMed] [Google Scholar]
- 41.Solans-Monfort X, Clot E, Copéret C, Eisenstein O. d(0) Re-based olefin metathesis catalysts, Re(=CR)(=CHR)(X)(Y): The key role of X and Y ligands for efficient active sites. J Am Chem Soc. 2005;127:14015–14025. doi: 10.1021/ja053528i. [DOI] [PubMed] [Google Scholar]
- 42.Poater A, et al. Understanding d(0)-olefin metathesis catalysts: Which metal, which ligands. J Am Chem Soc. 2007;129:8207–8216. doi: 10.1021/ja070625y. [DOI] [PubMed] [Google Scholar]
- 43.Handzlik J. Theoretical investigations of isolated Mo(VI) and Mo(IV) centers of a molybdena-silica catalyst for olefin metathesis. J Phys Chem C. 2007;111:9337–9348. [Google Scholar]
- 44.Elena B, de Paepe G, Emsley L. Direct spectral optimisation of proton-proton homonuclear dipolar decoupling in solid-state NMR. Chem Phys Lett. 2004;398:532–538. [Google Scholar]
- 45.Fung BM, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.