Abstract
HDL protects against vascular disease by accepting free cholesterol from macrophage foam cells in the artery wall. This pathway is critically dependent on lecithin:cholesterol acyltransferase (LCAT), which rapidly converts cholesterol to cholesteryl ester. The physiological activator of LCAT is apolipoprotein A-I (apoA-I), the major HDL protein. However, cholesterol removal is compromised if apoA-I is exposed to reactive intermediates. In humans with established cardiovascular disease, myeloperoxidase (MPO) oxidizes HDL, and oxidation by MPO impairs apoA-I's ability to activate LCAT in vitro. Because a single methionine residue in apoA-I, Met-148, resides near the center of the protein's LCAT activation domain, we determined whether its oxidation by MPO could account for the loss of LCAT activity. Mass spectrometric analysis demonstrated that oxidation of Met-148 to methionine sulfoxide associated quantitatively with loss of LCAT activity in both discoidal HDL and HDL3, the enzyme's physiological substrates. Reversing oxidation with methionine sulfoxide reductase restored HDL's ability to activate LCAT. Discoidal HDL prepared with apoA-I containing a Met-148→Leu mutation was significantly resistant to inactivation by MPO. Based on structural data in the literature, we propose that oxidation of Met-148 disrupts apoA-I's central loop, which overlaps the LCAT activation domain. These observations implicate oxidation of a single Met in apoA-I in impaired LCAT activation, a critical early step in reverse cholesterol transport.
Keywords: atherosclerosis, dysfunctional HDL, hypochlorous acid, inflammation, methionine sulfoxide reductase
Genetic, epidemiological, and clinical studies indicate that HDL is cardioprotective (1). A critical mechanism is reverse cholesterol transport (2), in which HDL accepts cholesterol from macrophage foam cells in the artery wall and transports it back to the liver for excretion (3). Strong evidence for this process in humans was provided by the identification of the molecular defects in Tangier disease as mutations in ABCA1. This membrane transporter exports free cholesterol to poorly lipidated apoA-I, the major HDL protein (3). Lecithin:cholesterol acyltransferase (LCAT), which is bound to HDL, then converts free cholesterol to cholesteryl ester, making it sufficiently hydrophobic to be sequestered in the core of the HDL particle (2, 4).
It has been proposed that HDL's cardioprotective effects also depend on the types of particles generated in vivo (5, 6), and that HDL in humans with established cardiovascular disease is dysfunctional (7). Indeed, animal studies convincingly demonstrate that genetically engineered changes in HDL metabolism can promote atherosclerosis that is independent of plasma levels of HDL cholesterol (7). The failure of recent clinical trials of an HDL-elevating therapy suggests that these observations may be relevant to human vascular disease (8).
One important pathway for generating dysfunctional HDL may involve oxidative damage by myeloperoxidase (MPO), a heme protein expressed at high levels by macrophages in human atherosclerotic tissue (9). The enzyme uses hydrogen peroxide (H2O2) and chloride ion to produce hypochlorous acid (HOCl), which converts Tyr to 3-chlorotyrosine (10, 11), a stable fingerprint of MPO activity in vivo (12). Oxidation of Tyr and Met residues in apoA-I dramatically impairs the protein's ability to promote cholesterol efflux by the ABCA1 pathway (13–15). Levels of 3-chlorotyrosine are markedly higher in circulating HDL isolated from patients with cardiovascular disease than in HDL isolated from controls (13, 16, 17), indicating that MPO targets HDL for oxidation in humans.
Met residues may serve as protein-bound antioxidants that sense oxidative stress and regulate cellular behavior (18, 19). For example, methionine sulfoxide [Met(O)], has been detected in circulating HDL of humans (20), and reactive intermediates are implicated in impairment of LCAT activity (21–25). Moreover, it was recently shown that the Met(O) content of apoA-I is elevated in human type 1 diabetes, a disorder strongly associated with increased levels of oxidative stress (26). Because a single Met residue, Met-148, lies in the LCAT activation domain of apoA-I (27), we investigated the role of Met oxidation in the loss of LCAT activity that occurs when MPO oxidizes apoA-I. Our observations indicate that oxidation of Met-148 impairs apoA-I's ability to activate LCAT, suggesting that Met(O) impairs reverse cholesterol transport in the artery wall.
Results
HDL3 Oxidized by MPO Loses Its Ability to Activate LCAT.
We initially centered our studies in HDL3, the dense subfraction of HDL. HDL3 exposed to increasing concentrations of HOCl or H2O2 in the complete MPO-H2O2-chloride system progressively lost the ability to activate LCAT (Fig. 1A). In both cases, HDL3 lost ≈45% of its LCAT activity when it was exposed to a 10-fold molar ratio of oxidant (mol/mol, oxidant/apoA-I).
Fig. 1.
Loss of LCAT activation after HDL3 is oxidized by MPO is associated with oxidation of Met-148 and Trp-72 of apoA-I. HDL3 was incubated with the indicated molar ratios (oxidant:apoA-I) of HOCl or H2O2 in the complete MPO system. Reactions were initiated by adding oxidants and terminated by adding Met. LCAT activation by oxidized HDL3 was monitored as the conversion of [3H]cholesterol to [3H]cholesteryl ester. Generation of oxidized peptides containing the indicated residues was quantified by MS and reconstructed ion chromatograms of proteolytic digests of apoA-I. Results are from three independent experiments.
MPO Oxidizes Specific Residues of ApoA-I.
The side chains in the three Met, four Trp, and seven Tyr residues in apoA-I are potentially susceptible to oxidation (28, 29). To determine whether MPO can oxidize these amino acids, we exposed HDL3 to HOCl or the MPO-H2O2-chloride system, digested apoA-I with trypsin or Glu-C, and used liquid chromatography-electrospray ionization-MS/MS to analyze the resulting peptide mixture. Together, peptides from tryptic and Glu-C digests span the entire sequence of apoA-I.
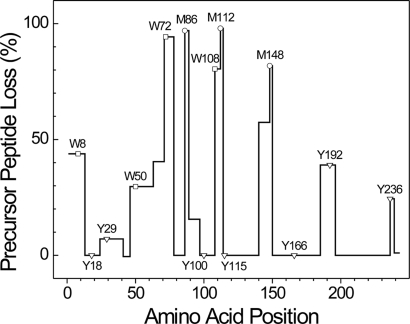
We used the loss of precursor peptides to determine which amino acids were targets of HOCl or the MPO system. Peptide loss was quantified by isotope dilution by using 15N-labeled apoA-I as an internal standard. We first focused on Met residues because of their high reactivity with HOCl (28, 29) and proposed role in activating the cholesterol export activity of ABCA1 (15). When HDL3 was exposed to a 20-fold molar ratio of oxidant to apoA-I protein, isotope dilution revealed that all three peptides containing a Met residue were lost quantitatively (95%, 100%, and 80% for Met-86, Met-112, and Met-148, respectively) (Fig. 2), suggesting that the amino acid's thiol ether side chain was a major target for MPO. In contrast, there was no evidence of peptide loss in several regions of the protein composed of oxidation-resistant residues (amino acids 41–45, 78–85, and 196–235) (Fig. 2).
Fig. 2.
Loss of precursor peptides of apoA-I in HDL3 exposed to the complete MPO system. HDL3 was exposed to a 20-fold molar ratio of H2O2 in the MPO system. A mixture of 7 μg oxidized HDL3 or control HDL3 and 2.5 μg of 15N-labeled apoA-I was digested with trypsin or Glu-C. After liquid chromatography-electrospray ionization-MS/MS (LC-ESI-MS/MS) analysis, the ratio of precursor peptides derived from apoA-I in oxidized HDL3 or control HDL3 relative to 15N-labeled peptides derived from 15N-labeled apoA-I was calculated from extracted ion chromatograms. Results are from two independent experiments.
We used the same approach to monitor the loss of peptides containing Trp or Tyr from HDL3. All four peptides containing Trp were lost in significant yield (90%, 80%, 40%, and 30% for Trp-72, Trp-108, Trp-8, and Trp-50, respectively) (Fig. 2). In contrast, only two of the five peptides containing Tyr (Tyr-192 and Tyr-236) showed significant losses (40% and 25%, respectively) (Fig. 2). Because a 20-fold molar ratio of HOCl caused HDL3 to lose ≈65% of its ability to activate LCAT (Fig. 1A), these observations suggest that oxidation of one or more of the three Met residues and/or four Trp residues might impair reverse cholesterol transport by apoA-I. It is noteworthy that three peptides (amino acids 63–70, 89–96, and 140–147) that lack oxidation-sensitive amino acids were also significantly lost (40%, 15%, and 55%, respectively) (Fig. 2). One possible explanation is that oxidation of Trp-72, Met-86, and Met-148 might induce local conformational changes that decrease apoA-I's susceptibility to proteolytic degradation.
MPO Quantitatively Converts Met Residues to Met(O) and Tyr Residues to 3-Chlorotyrosine.
We used MS/MS analysis and reconstructed chromatograms of digests of HDL3 exposed to HOCl or the MPO system (14, 15, 30) to confirm that Met(O), hydroxy- and dihydroxyTrp (Trp[O] and Trp[O2]), and 3-chlorotyrosine were the major oxidation products in apoA-I. The yields of these oxidized amino acids were virtually identical when HDL3 was exposed to either HOCl or the MPO system. At a 20-fold molar ratio of oxidant, almost 100% of Met-86 and Met-112, and 70% of Met-148 was converted to Met(O) [supporting information (SI) Fig. S1A]. A single Tyr residue, Tyr-192, was the major chlorination site (≈25% product yield), whereas the yield of chlorinated Tyr-236 and Tyr-115 was only ≈5%. We observed a lower level (<5%) of chlorination at Tyr-166 and Tyr-29 and none at Tyr-18 and Tyr-100 (Fig. S1C).
Product yields of Met(O) and 3-chlorotyrosine were in excellent quantitative agreement with those obtained by monitoring the loss of precursor peptides by isotope dilution, except that 3-chlorotyrosine accounted for only 20% of the loss of the peptide containing Tyr-236. In contrast, only Trp-72 of apoA-I was converted in high yield to detectable Trp[O] and Trp[O2] isomers (Fig. S1B). This likely reflects difficulties in using MS to detect all potential Trp oxidation products (31). Taken together, these observations indicate that exposing apoA-I to MPO converts Met residues and Trp-72 in near-quantitative yield to Met(O) and oxygenated Trp, respectively. The other three Trp residues are also susceptible to oxidation by MPO (30–70% loss of precursor peptide), but the major products have yet to be identified.
Oxidation of Met-148 and Trp-72 Associates Strongly with Loss of LCAT Activity.
Oxidation of peptides containing Met-148 or Trp-72 from HDL3 exposed to the MPO-H2O2-Cl− system associated strongly with the loss of LCAT activity (Fig. 1B). Importantly, the ratio of LCAT inhibition to oxidation of these two amino acids was ≈1:1, which suggests that oxidation of those two residues might impair apoA-I's ability to activate LCAT. In contrast, there was little correlation between LCAT activity and the loss of peptides containing other Met, Trp, or Tyr residues (Fig. 1B). We obtained similar results when we exposed HDL3 to HOCl (data not shown).
Peroxynitrite Poorly Oxidizes Met-148 and Is a Weak Inhibitor of LCAT Activation by HDL3.
We next compared the ability of peroxynitrite (ONOO−), a powerful oxidant derived from NO (14, 50), to inhibit HDL3's LCAT activity with those of HOCl and the MPO-H2O2-chloride system. At a 25-fold molar ratio of oxidant, HOCl reduced LCAT activity by ≈85%, whereas ONOO− decreased it by only ≈20% (Fig. S2A). MS/MS analysis of tryptic and Glu-C digests of oxidized HDL3 revealed that ONOO− had oxidized ≈60% of Met-86, ≈90% of Met-112, and ≈20% of Met-148, whereas HOCl had oxidized ≈70% of Met-148 and almost 100% of Met-86 and Met-112 (Fig. S2B). In HOCl-oxidized HDL3, Tyr-192 was the single major site of chlorination (≈20% product yield) (Fig. S2C). In contrast, all seven Tyr residues were nitrated to similar extents by ONOO− (15–30% product yield) (Fig. S2D), and Trp oxidation products were undetectable at this molar ratio of ONOO−. These results indicate that ONOO− is relatively inefficient at oxidizing Met residues in apoA-I under our experimental conditions, and that oxidation of Met-148 associates with the loss of LCAT activation. In contrast, Trp oxidation and Tyr nitration did not associate with the loss of LCAT activation.
Oxidation of ApoA-I Decreases the Protein's Apparent Km and Vmax in the LCAT Reaction.
To investigate the kinetic parameters for cholesteryl ester formation by LCAT, we constructed Lineweaver–Burk plots (32) by using HDL3 exposed to a 25-fold molar ratio of oxidant (HOCl, MPO, or ONOO−) (Fig. S3). The overall ability of HOCl- or MPO-oxidized HDL3 to activate LCAT (the apparent Vmax/Km values) was reduced ≈3-fold. In contrast, the overall LCAT activity (the apparent Vmax/Km value) of ONOO−-oxidized HDL3 was comparable to that of control HDL3 (Table S1).
Enzymatic Reduction of Met(O) to Met Restores the LCAT Activity of HDL3 Exposed to HOCl or MPO.
PilB, a bacterial methionine sulfoxide reductase, reduces both the S and R epimers of Met(O) back to Met (45). To assess the contribution of Met oxidation to the loss of LCAT activity, we incubated HOCl- or MPO-oxidized HDL3 with PilB and 15 mM DTT. We then assessed the lipoprotein's ability to activate LCAT and used MS/MS to analyze its Met(O) and Met content. Although PilB completely converts all three Met sulfoxide residues of lipid-free apoA-I back to Met (15), MS/MS analysis revealed that it reduced only ≈50% of the oxidized Met-112 in lipid-associated apoA-I. In contrast, PilB almost completely reduced oxidized Met-148 and Met-86 (Fig. S4). At a 25-fold molar ratio of oxidant, HDL3 exposed to HOCl or H2O2 in the MPO-chloride system lost ≈80% of its LCAT activity (Fig. 3, open bars). Incubation of HOCl- or MPO-exposed HDL3 with PilB caused a 3.5-fold increase in the lipoprotein's ability to activate LCAT (Fig. 3, filled bars). This observation strongly implicates Met oxidation in the loss of LCAT activity that is seen after HDL3 is exposed to HOCl or the MPO-H2O2-chloride system.
Fig. 3.
Methionine sulfoxide reductase restores the LCAT activity of oxidized HDL. LCAT activity was determined in HDL3 exposed to a 25-fold molar ratio of the indicated oxidant or the same oxidized HDL preparation after incubation with PilB, a bacterial methionine sulfoxide reductase with activity on both epimers of Met(O). Results are from two independent experiments (means ± SDs).
HOCl Targets Met and Trp Residues of ApoA-I for Oxidation in Recombinant HDL (rHDL).
Discoidal rHDL is thought to mimic the nascent, poorly lipidated apoA-I particles that are the earliest substrates of LCAT in reverse cholesterol transport (3, 33, 34). We therefore used 9.6 nm rHDL particles (prepared from apoA-I, free cholesterol, and 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine by cholate dialysis) to determine whether HOCl or MPO can alter apoA-I's ability to activate LCAT. rHDL particles exposed to increasing concentrations of HOCl or peroxide in the complete MPO system progressively lost their ability to activate LCAT (data not shown). In each case, ≈50% of the LCAT activity was lost at a 20-fold molar ratio of oxidant.
Loss of precursor peptides in rHDL was quantified by isotope dilution by using 15N-labeled apoA-I as an internal standard. Using both the tryptic and Glu-C digests, we plotted the loss of precursor peptide against the amino acid position in apoA-I (Fig. S5A). At a 20-fold molar ratio of HOCl, the precursor peptide containing Met-112 almost completely disappeared. Approximately 90% of the precursor peptides containing Trp-108 or Met-86 was also lost, as was ≈80% of the precursor containing Trp-72 and 65% of the precursor containing Met-148. Peptides containing Trp-8, Trp-50, Tyr-192, or Tyr 236 were also oxidized significantly (30–50%). We observed similar results when we used the MPO-H2O2-chloride system (data not shown).
We then used reconstructed ion chromatograms to monitor the formation of oxidized amino acids in the discoidal particles. There was a strong linear relationship between oxidation of Trp-72 and Met-148 and the loss of LCAT activity of apoA-I in rHDL. In contrast, oxidation of other Trp and Met residues and chlorination of Tyr-192 or Tyr-166 did not associate quantitatively with decreased LCAT activation (Fig. S5B). These observations are remarkably similar to those we observed when we exposed HDL3 to HOCl or MPO, and they strongly suggest that oxidation of Met-148 and/or Trp-72 impairs apoA-I's LCAT activity.
rHDL Prepared with ApoA-I Lacking Trp Residues Is Not Protected from Oxidative Inactivation by HOCl.
Oxidation of Trp-72 in either HDL3 or rHDL associated strongly with the loss of LCAT activity. We therefore engineered a mutant apoA-I protein by deleting all four Trp residues (ΔW mutant). The sequence of this ΔW apoA-I mutant was confirmed by dideoxy DNA sequencing of the expression vector and MS analysis of the protein. The abilities of control or mutant rHDL particles to activate LCAT were comparable (Fig. 4A) over a range of rHDL concentrations (data not shown), indicating that deleting apoA-I's Trp residues has little effect on the protein's secondary or tertiary structure in these particles. Importantly, exposing control and ΔW apoA-I rHDL particles to HOCl impaired their ability to activate LCAT to a similar extent (Fig. 4B). The overall patterns of peptide loss and amino acid oxidation were similar in HOCl-oxidized control and ΔW apoA-I rHDL (data not shown). Our observations strongly suggest that oxidation of Trp residues in apoA-I does not greatly impair HDL's ability to activate LCAT.
Fig. 4.
Mutation of Met-148 to Leu protects apoA-I from the oxidative loss of LCAT activity. rHDL was prepared with control or mutated apoA-I. (A) LCAT activity of native rHDLs. (B) LCAT activity of wild-type (WT) and ΔW apoA-I exposed to the indicated concentrations of HOCl. (C) LCAT activity of Met-mutated rHDLs exposed to a 30-fold molar ratio of HOCl. (D) LCAT activity of Met-, Trp-, and Tyr-mutated rHDLs exposed to a 40-fold molar ratio of HOCl. Results are from two independent experiments. DeltaW, deletion all four Trp residues of apoA-I. 3M/L, Met86Leu/Met112Leu/Met148Leu mutant apoA-I.
rHDL Prepared with ApoA-I Lacking Met-148 Is Protected from Oxidative Inactivation by HOCl.
To determine whether oxidizing Met residues in apoA-I with HOCl or MPO impairs LCAT activity, we generated four mutant apoA-I proteins by replacing Met with Leu (Met86Leu, Met112Leu, Met148Leu, and Met86Leu/Met112Leu/Met148Leu). The LCAT activity of Met→Leu apoA-I was comparable to that of apoA-I prepared from control rHDL (Fig. 4A) over a range of rHDL concentrations (data not shown). MS/MS analysis confirmed that the Met residues had been replaced by Leu, and that HOCl or the MPO system failed to oxidize Leu-86, Leu-112, or Leu-148.
We next compared the effects of oxidation on the ability of control and Met-substituted rHDL to activate LCAT. Both the Met148Leu and Met86Leu/Met112Leu/Met148Leu mutant rHDLs retained significant ability to activate LCAT after oxidation (Fig. 4C). The Met86Leu mutant rHDL also appeared modestly resistant to oxidative inactivation (Fig. 4C). As assessed by isotope dilution and reconstructed ion chromatograms, the overall patterns of peptide loss and amino acid oxidation were similar in control apoA-I and Met→Leu apoA-I after oxidation with HOCl (data not shown). Our observations strongly suggest that converting Met-148 to Met(O) significantly impairs HDL's ability to activate LCAT.
To determine whether oxidation of Tyr-166 or Tyr-192 impairs LCAT activity, we generated two additional apoA-I mutants: Tyr166Phe and Tyr192Phe/Met86Leu/Met112Leu/Met148Leu (quadruple mutant). rHDLs prepared with either of these mutated apoA-I proteins showed a slight decrease (≈20%) in LCAT activation compared with WT rHDL (Fig. 4A). We then compared the effects of oxidation on the ability of control and Met- or Tyr-substituted apoA-I rHDL to activate LCAT. Both the Met86Leu/Met112Leu/Met148Leu and the quadruple mutant rHDLs retained significant ability to activate LCAT after oxidation (Fig. 4D). However, the quadruple Tyr192Phe/3Met→3Leu substitution did not show additional protection compared with the triple Met substitution (Fig. 4D), indicating that oxidation of Tyr-192 did not contribute to the loss of activity. Similarly, the Tyr166Phe substitution failed to protect apoA-I from inactivation by HOCl (Fig. 4D). Our observations suggest that oxidation of Tyr-166 or Tyr-192 of apoA-I does not make a major contribution to the impaired LCAT activity that occurs when HDL is oxidized by MPO.
Discussion
In the current studies, we demonstrated that oxidation of Met-148 by MPO impairs apoA-I's ability to activate LCAT, an enzyme that plays a central role in HDL maturation and reverse cholesterol transport (2, 4, 27, 33–35). Moreover, we previously showed that oxidation of Met residues and Tyr-192 of apoA-I by MPO impairs cholesterol efflux by the ABCA1 pathway (15). Taken together, our results indicate that Met oxidation inhibits two critical early events in reverse cholesterol transport: sterol export by ABCA1 and activation of LCAT.
Importantly, HDL isolated from subjects with established cardiovascular disease shows evidence of oxidation by MPO (13, 16, 17). Similarly, HDL isolated from human atherosclerotic lesions contains ≈0.2–4 mmol of 3-chlorotyrosine per mol Tyr (13, 16, 17), and this abnormal amino acid signifies damage by MPO (12). Because HOCl, a major product of MPO, reacts with the thioether group of Met 800,000 times more rapidly than with the phenol group of Tyr (28), Met residues of apoA-I might be oxidized in near quantitative yield in the pericellular environment of the macrophage. Therefore, oxidation of Met in apoA-I may serve as a switch for generating dysfunctional HDL in humans.
We used two strategies to estimate the contribution of Met-148 oxidation to the loss of apoA-I's ability to activate LCAT. The first centered on methionine sulfoxide reductase, whose only known activity is to convert Met(O) to Met (36). Treating oxidized HDL3 with methionine sulfoxide reductase increased its LCAT activity from 20% to 70%, suggesting a major role for Met(O) in the loss of activity. MS and MS/MS analysis of the treated protein strongly supported the proposal that the enzyme made only one change in oxidized apoA-I: reduction of Met(O) to Met.
The second strategy was to mutate various residues of apoA-I to oxidation-resistant forms. The advantage of this approach is the ability to alter individual amino acids. A limitation is the possibility that the substituted amino acid(s) affects other properties of the protein. Indeed in our hands, the Tyr166Phe mutant lost 20% of its ability to activate LCAT. Using the mutation strategy, we showed that the Met148Leu mutant protein exposed to a relatively high concentration of HOCl retained twice as much activity as the WT protein (≈50% vs. ≈25%). Altering all three Met residues offered no additional protection. The difference in the levels of protection provided by methionine sulfoxide reductase and the Met148Leu substitution may reflect differences in the mutant protein's intrinsic properties. Collectively, our observations provide strong evidence for the role of Met-148 in the oxidative inactivation of LCAT.
Levine et al. (18) suggested that Met functions as a protein-bound antioxidant. The observation that Met residues in apoA-I scavenge lipid hydroperoxides and block Tyr chlorination by HOCl is consistent with this suggestion (15, 37). Importantly, Met is very hydrophobic, and oxidation of its thiol ether moiety markedly increases its hydrophilicity (19, 21, 38). Therefore, Met oxidation clearly has the potential to alter HDL function because apoA-I's α-helical structure depends critically on hydrophobic amino acids, which form its lipid- and self-associating faces (33, 34, 38, 39).
A key question is why oxidation of Met-148 destroys apoA-I's LCAT activity. Studies have shown that residues 143–164, located in repeat 6 of apoA-I, are crucial for LCAT activation (27). Based on structural studies of discoidal rHDL, we recently proposed that a loop centered at position 139 and bordered by residues 133 and 146 partially overlaps the LCAT activation domain (40). It is noteworthy that Met-148 lies next to residue 146 of this central loop.
We propose that oxidation of Met-148 diminishes apoA-I's ability to activate LCAT because it disrupts the central loop and the conformation of repeat 6. In this model, oxidation shifts Met-148 from the protein's hydrophobic face to its hydrophilic face, rotating the residue's orientation to the solvent. This relocation unwinds the α-helix between residues 145 and 148, shifting those lipid-associated residues into the central loop and thereby perturbing the LCAT activation site. This hypothesis assumes that the central loop is more dynamic (and hence more prone to structural perturbation) than the helical remainder of the apoA-I molecule, which is stabilized by salt bridges to a second molecule of apoA-I (33, 34) and by its association with phospholipids. Our observations may be of widespread importance because oxidation of Met residues in proteins could be a general mechanism for disrupting loop domains and the hydrophobic helix–lipid and helix–helix interactions that are key features of proteins.
Other structural or conformational changes that result from oxidizing apoA-I must also contribute to LCAT inactivation because reduction of Met(O) back to Met restored only ≈70% of the activity of MPO-oxidized HDL3. One potential mechanism involves irreversible unfolding of apoA-I (41). Consistent with the proposal that Met(O) exerts a localized effect on the conformation of apoA-I, analysis of HOCl- or MPO-oxidized HDL3 by circular dichroism revealed that its apolipoproteins had lost <8% of their α-helical content (ref. 42; unpublished data).
Recently, Wu et al. (25) reported that Tyr-166 of apoA-I contributes to LCAT activation. In their study, rHDL prepared with a Tyr166Phe mutant of apoA-I lost 70% of its ability to activate LCAT. Wu et al. also reported an association between Tyr chlorination and LCAT inactivation. Based on these observations, they proposed that Tyr-166 chlorination accounted for oxidized apoA-I's inability to activate LCAT.
The conclusion that Tyr-166 is a quantitatively important target for oxidation was apparently based on the 70% loss of LCAT activity in Tyr166Phe apoA-I (25). In contrast, the Tyr166Phe mutant protein we prepared lost only 20% of its activity. We used MS on the intact protein and MS/MS on tryptic and Glu-C digests of the Tyr166Phe mutant of apoA-I to verify the protein sequence. The discrepancy in LCAT activity of the mutant proteins in the two studies may reflect differences in the methods used to generate rHDL or other experimental conditions.
Importantly, Wu et al. (25) found that only ≈1.8% of Tyr-166 was chlorinated when LCAT activity was inhibited by ≈50%. This result is very similar to our finding that <5% of Tyr-166 was chlorinated even when HDL was exposed to a high concentration of HOCl or H2O2 in the MPO system. Thus, we found that chlorination of Tyr-166 could not account quantitatively for the loss of LCAT activity, and Wu et al. reported very similar results.
Our observations suggest the following model for oxidative regulation of reverse cholesterol transport by Met oxidation. Activated macrophages use a membrane-associated NADPH oxidase to produce locally high concentrations of H2O2. MPO converts H2O2 to HOCl, which modifies specific Met and Tyr residues in pericellular apoA-I. Oxidized apoA-I lacks the ability to activate ABCA1 and LCAT, two key early events in cholesterol efflux from macrophages. Thus, oxidation of Met residues may play a critical role in impaired reverse cholesterol transport by apoA-I, promoting foam cell formation and atherogenesis.
In future studies, it will be of interest to determine whether oxidation also impairs cholesterol efflux by the ABCG1 and scavenger receptor class B1 (SR-B1) pathways, which are also implicated in macrophage cholesterol homeostasis.
Experimental Procedures
Isolation of HDL3, ApoA-I, MPO, and PilB.
HDL3 (density 1.125–1.210 g/ml) was isolated from EDTA plasma of fasting adults by sequential ultracentrifugation (43). ApoA-I, MPO (EC 1.11.1.7), and truncated PilB of Neisseria gonorrhoeae were isolated from HDL (43), human neutrophils (44), and Escherichia coli (45), respectively.
Bacterial Expression of ApoA-I.
Control and mutated apoA-I were expressed in E. coli (46). Individual substitution mutations within the human apoA-I cDNA were introduced by primer-directed PCR mutagenesis or mega-primer PCR. All mutations were verified by dideoxy automated fluorescent sequencing of cDNA and confirmed by MS analysis of the protein. 15N-Labeled apoA-I was prepared by growing bacteria in minimal medium supplemented with [15N]nitrite.
Preparation of rHDL.
rHDL was prepared by sodium deoxycholate dialysis with a molar ratio of 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (Avanti Polar Lipids):free cholesterol:apoA-I of 80:4:1 (47). After dialysis, rHDL was isolated by discontinuous gradient density ultracentrifugation. Then 9.6-nm diameter rHDL particles (47, 48) were isolated by high resolution size-exclusion chromatography. Electron microscopy and nondenaturing gel electrophoresis confirmed that rHDL was discoidal and exhibited a single subclass of particles with an average diameter of ≈9.6 nm (47).
Oxidation Reactions.
Reactions were carried out at 37°C for 1 h in 10 mM sodium phosphate buffer (pH 7.4) containing 100 μM diethylenetriaminepentaacetic acid (49). For the MPO-H2O2-chloride system, the reaction mixture was supplemented with 50 nM MPO and 100 mM NaCl. ONOO− was synthesized from nitrite and H2O2 (50). Concentrations of ONOO−, HOCl, and H2O2 were determined spectrophotometrically (ε302 = 1,670 M−1 cm−1, ε292 = 350 M−1 cm−1, and ε240 = 39.4 M−1 cm−1, respectively) (50–52). Oxidized HDL was incubated with PilB (4:1, HDL:enzyme, wt/wt) for 2 h at 37°C in 25 mM Tris·HCl (pH 7.4) containing 15 mM DTT (15).
LCAT Activity.
LCAT purified from human plasma was provided by Dr. John Parks (Wake Forest University, NC). LCAT activation by HDL3 or rHDL labeled with [1,2-3H (N)]cholesterol was quantified by monitoring the conversion of free cholesterol to cholesteryl ester as described in ref. 47.
Liquid Chromatography-Electrospray Ionization-MS.
HDL was digested with sequencing grade modified trypsin (Promega) or sequencing grade endoproteinase Glu-C (Roche Applied Science) (14, 53, 54). MS and MS/MS analyses were performed in the positive ion mode (mass range 200–2,000 Da) with a Thermo-Finnigan LCQ Deca XP Plus instrument (14, 53, 54).
Quantifying ApoA-I Oxidation.
Peptide ion currents and two complementary MS methods were used to quantify oxidation of apoA-I. First, the loss of precursor peptides was quantified by isotope dilution, by using 15N-labeled apoA-I as an internal standard added before digestion (55). The loss of precursor peptide was calculated from the ratio of peak area of precursor peptide of apoA-I from HDL to the corresponding 15N-labeled peptide of control 15N-labeled apoA-I. Second, product yield of oxidized peptides was determined with reconstructed ion chromatograms of product and precursor peptides, calculated as: product yield (%) = [(product ion peak area)/(precursor ion peak area + product ion peak area)] × 100. This method assumes that all precursor peptide is converted into known oxidation products, and that the MS response characteristics of the product ions are similar to those of the precursor ion (54).
Statistical Analysis.
Results are reported as means and standard deviations. Student's two-tailed, unpaired t test was used for statistical analyses, and P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Dr. John Parks for providing LCAT. Mass spectrometry experiments were performed in the Mass Spectrometry Resource, Department of Medicine, University of Washington. This research was supported by Tobacco-Related Disease Research Program of California Grants 16FT-0054 and 16RT-0163, National Institutes of Health Grants HL086798, HL77268, P30ES07083, PO1HL030086, and K99HL091055, and Diabetes Education and Research Center Grant P30DK017047, University of Washington. B.S. is the recipient of a Fellowship Award from Philip Morris External Research Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802025105/DCSupplemental.
References
- 1.Gordon DJ, Rifkind BM. High-density lipoprotein-the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Glomset JA. The plasma lecithin:Cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 3.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 4.Jonas A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim Biophys Acta. 1991;1084:205–220. doi: 10.1016/0005-2760(91)90062-m. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtiss LK, Valenta DT, Hime NJ, Rye KA. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler Thromb Vasc Biol. 2006;26:12–19. doi: 10.1161/01.ATV.0000194291.94269.5a. [DOI] [PubMed] [Google Scholar]
- 7.Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 8.Rader DJ. Illuminating HDL-is it still a viable therapeutic target? N Engl J Med. 2007;357:2180–2183. doi: 10.1056/NEJMe0707210. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst JK, Barrette WC., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- 12.Gaut JP, et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci USA. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergt C, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao B, et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 15.Shao B, et al. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 16.Pennathur S, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao CC, Ma YS, Stadtman ER. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc Natl Acad Sci USA. 1997;94:2969–2974. doi: 10.1073/pnas.94.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankhurst G, et al. Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J Lipid Res. 2003;44:349–355. doi: 10.1194/jlr.M200256-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Anantharamaiah GM, et al. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988;29:309–318. [PubMed] [Google Scholar]
- 22.Bielicki JK, Forte TM. Evidence that lipid hydroperoxides inhibit plasma lecithin:Cholesterol acyltransferase activity. J Lipid Res. 1999;40:948–954. [PubMed] [Google Scholar]
- 23.Wang K, Subbaiah PV. Importance of the free sulfhydryl groups of lecithin-cholesterol acyltransferase for its sensitivity to oxidative inactivation. Biochim Biophys Acta. 2000;1488:268–277. doi: 10.1016/s1388-1981(00)00130-x. [DOI] [PubMed] [Google Scholar]
- 24.McCall MR, Carr AC, Forte TM, Frei B. LDL modified by hypochlorous acid is a potent inhibitor of lecithin-cholesterol acyltransferase activity. Arterioscler Thromb Vasc Biol. 2001;21:1040–1045. doi: 10.1161/01.atv.21.6.1040. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 26.Brock JW, et al. Increased methionine sulfoxide content of apoA-I in type 1 diabetes. J Lipid Res. 2008;49:847–855. doi: 10.1194/jlr.M800015-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Sorci-Thomas MG, Thomas MJ. The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc Med. 2002;12:121–128. doi: 10.1016/s1050-1738(01)00163-3. [DOI] [PubMed] [Google Scholar]
- 28.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 29.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 30.Bergt C, et al. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes HDL. J Biol Chem. 2004;279:7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 31.Finley EL, Dillon J, Crouch RK, Schey KL. Identification of tryptophan oxidation products in bovine alpha-crystallin. Protein Sci. 1998;7:2391–2397. doi: 10.1002/pro.5560071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. [Google Scholar]
- 33.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MJ, Bhat S, Sorci-Thomas MG. The use of chemical cross-linking and mass spectrometry to elucidate the tertiary conformation of lipid-bound apolipoprotein A-I. Curr Opin Lipidol. 2006;17:214–220. doi: 10.1097/01.mol.0000226111.05060.f4. [DOI] [PubMed] [Google Scholar]
- 35.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 36.Weissbach H, et al. Peptide methionine sulfoxide reductase: Structure, mechanism of action, and biological function. Arch Biochem Biophys. 2002;397:172–178. doi: 10.1006/abbi.2001.2664. [DOI] [PubMed] [Google Scholar]
- 37.Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273:6088–6095. doi: 10.1074/jbc.273.11.6088. [DOI] [PubMed] [Google Scholar]
- 38.Panzenbock U, Kritharides L, Raftery M, Rye KA, Stocker R. Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J Biol Chem. 2000;275:19536–19544. doi: 10.1074/jbc.M000458200. [DOI] [PubMed] [Google Scholar]
- 39.Segrest JP, et al. The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 40.Martin DD, Budamagunta MS, Ryan RO, Voss JC, Oda MN. Apolipoprotein A-I assumes a “looped belt” conformation on reconstituted high density lipoprotein. J Biol Chem. 2006;281:20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- 41.Mehta R, Gantz DL, Gursky O. Human plasma high-density lipoproteins are stabilized by kinetic factors. J Mol Biol. 2003;328:183–192. doi: 10.1016/s0022-2836(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 42.Bergt C, et al. Reagent or myeloperoxidase-generated hypochlorite affects discrete regions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J 346 Pt. 2000;2:345–354. [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez AJ, Oram JF, Bierman EL. Protein kinase C as a mediator of high density lipoprotein receptor-dependent efflux of intracellular cholesterol. J Biol Chem. 1991;266:10104–10111. [PubMed] [Google Scholar]
- 44.Heinecke JW, Li W, Daehnke HLD, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993;268:4069–4077. [PubMed] [Google Scholar]
- 45.Lowther WT, Brot N, Weissbach H, Honek JF, Matthews BW. Thiol-disulfide exchange is involved in the catalytic mechanism of peptide methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2000;97:6463–6468. doi: 10.1073/pnas.97.12.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 47.Cavigiolio G, et al. The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry. 2008;47:4770–4779. doi: 10.1021/bi7023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maiorano JN, Jandacek RJ, Horace EM, Davidson WS. Identification and structural ramifications of a hinge domain in apolipoprotein A-I discoidal high-density lipoproteins of different size. Biochemistry. 2004;43:11717–11726. doi: 10.1021/bi0496642. [DOI] [PubMed] [Google Scholar]
- 49.Heinecke JW, Baker L, Rosen H, Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986;77:757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 51.Morris JC. Acid ionization constant of HOCl from 5 to 35 degrees. J Phys Chem. 1966;70:3798–3805. [Google Scholar]
- 52.Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV) Anal Biochem. 1972;49:474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- 53.Shao B, et al. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem. 2005;280:36386–36396. doi: 10.1074/jbc.M508169200. [DOI] [PubMed] [Google Scholar]
- 54.Shao B, Heinecke JW. Using tandem mass spectrometry to quantify site-specific chlorination and nitration of proteins: Model system studies with high-density lipoprotein oxidized by myeloperoxidase. Methods Enzymol. 2008;440:33–63. doi: 10.1016/S0076-6879(07)00803-8. [DOI] [PubMed] [Google Scholar]
- 55.Heinecke JW, et al. Detecting oxidative modification of biomolecules with isotope dilution mass spectrometry: Sensitive and quantitative assays for oxidized amino acids in proteins and tissues. Methods Enzymol. 1999;300:124–144. doi: 10.1016/s0076-6879(99)00121-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.