Abstract
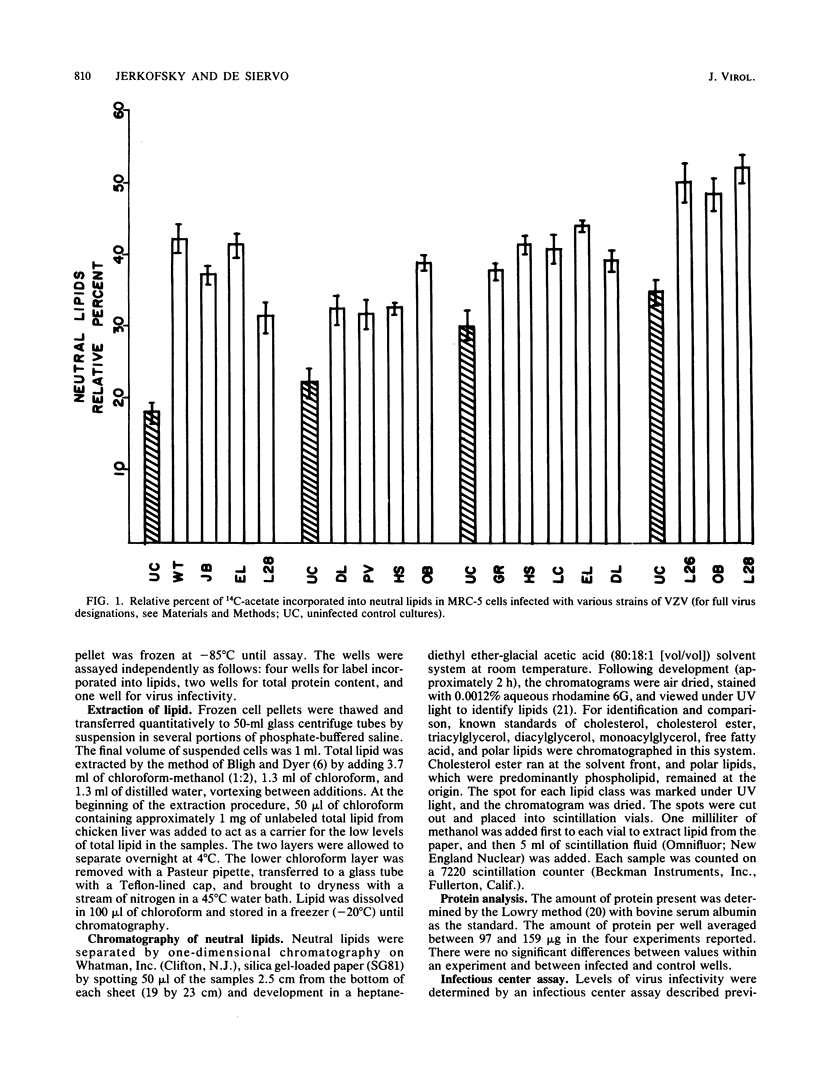
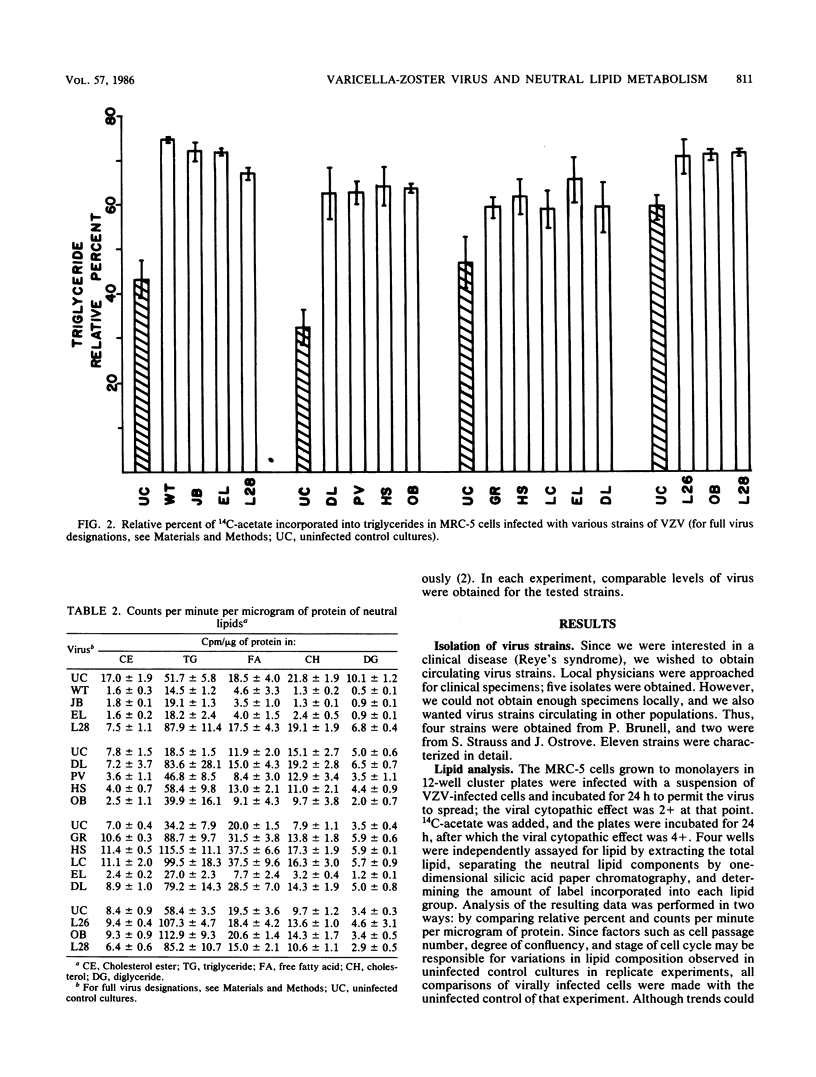
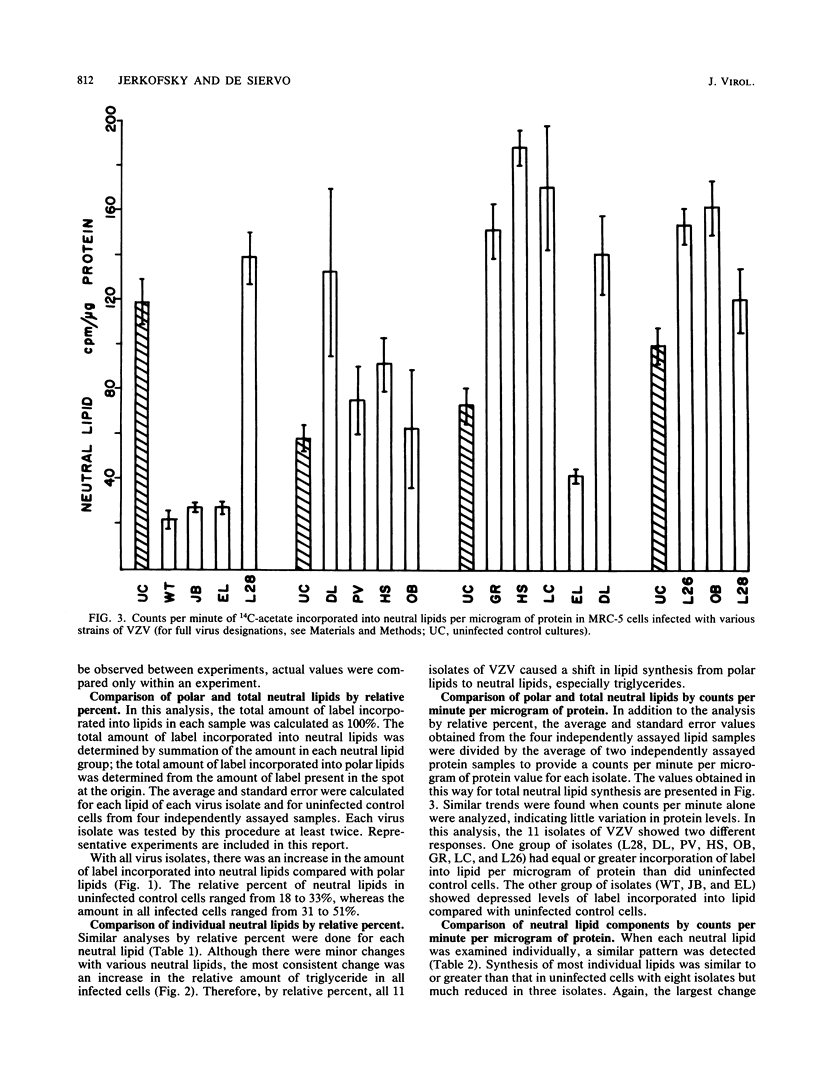
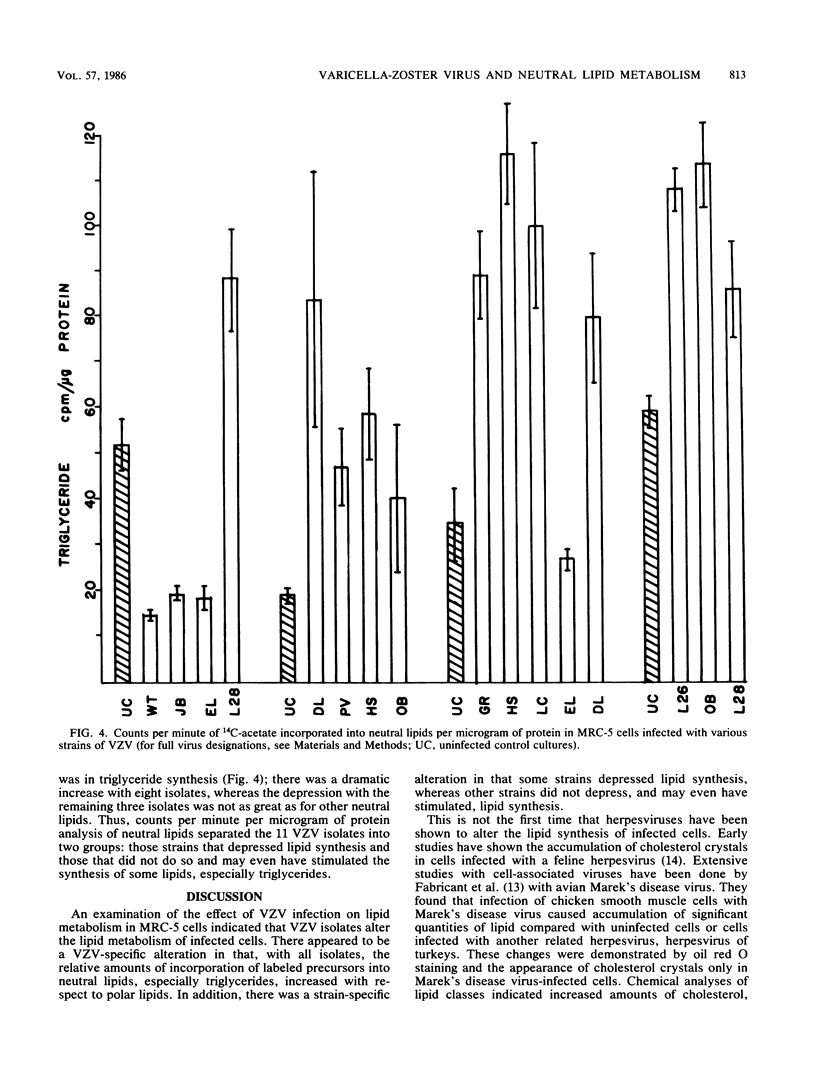
Eleven isolates of varicella-zoster virus were tested for their effects on the incorporation of [14C]acetate into lipids in infected human embryonic lung cells. By relative percent, all virus isolates demonstrated a shift from polar lipid synthesis to neutral lipid, especially triglyceride, synthesis. By data expressed as counts per minute per microgram of protein, the VZV strains could be separated into two groups: those strains which depressed lipid synthesis and those strains which did not depress, and may even have stimulated, lipid, especially triglyceride, synthesis. These results may be useful in understanding the development of lipid changes seen in children affected with Reye's syndrome following chickenpox.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen L. H., Jerkofsky M. Characterization of varicella-zoster virus enhancement by the pesticide carbaryl. Appl Environ Microbiol. 1983 May;45(5):1560–1565. doi: 10.1128/aem.45.5.1560-1565.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen L. H., Jerkofsky M. Enhancement of varicella-zoster virus replication in cultured human embryonic lung cells treated with the pesticide carbaryl. Appl Environ Microbiol. 1981 Mar;41(3):652–656. doi: 10.1128/aem.41.3.652-656.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Dales S. Biogenesis of poxviruses: glycolipid metabolism in vaccinia-infected cells. Virology. 1978 Jan;84(1):108–117. doi: 10.1016/0042-6822(78)90222-2. [DOI] [PubMed] [Google Scholar]
- Anderton P., Wild T. F., Zwingelstein G. Modification of the fatty acid composition of phospholipid in measles virus-persistently infected cells. Biochem Biophys Res Commun. 1981 Nov 16;103(1):285–291. doi: 10.1016/0006-291x(81)91691-0. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Brennan P. J. Effect of Sendai virus infection on lipid metabolism in chick embryo fibroblasts. J Virol. 1972 May;9(5):813–822. doi: 10.1128/jvi.9.5.813-822.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford W. D., Parker J. C., Jr Reye's syndrome. Possible causes and pathogenetic pathways. Clin Pediatr (Phila) 1971 Mar;10(3):148–153. doi: 10.1177/000992287101000309. [DOI] [PubMed] [Google Scholar]
- Carić-Lazar M., Schwarz R. T., Scholtissek C. Influence of the infection with lipid-containing viruses on the metabolism and pools of phospholipid precursors in animal cells. Eur J Biochem. 1978 Nov 15;91(2):351–361. doi: 10.1111/j.1432-1033.1978.tb12687.x. [DOI] [PubMed] [Google Scholar]
- Corey L., Rubin R. J., Hattwick M. A., Noble G. R., Cassidy E. A nationwide outbreak of Reye's Syndrome. Its epidemiologic relationship of influenza B. Am J Med. 1976 Nov;61(5):615–625. doi: 10.1016/0002-9343(76)90139-x. [DOI] [PubMed] [Google Scholar]
- Crocker J. F., Ozere R. L., Safe S. H., Digout S. C., Rozee K. R., Hutzinger O. Lethal interaction of ubiquitous insecticide carriers with virus. Science. 1976 Jun 25;192(4246):1351–1353. doi: 10.1126/science.179146. [DOI] [PubMed] [Google Scholar]
- Crocker J. F., Rozee K. R., Ozere R. L., Digout S. C., Hutzinger O. Insecticide and viral interaction as a cause of fatty visceral changes and encephalopathy in the mouse. Lancet. 1974 Jul 6;2(7871):22–24. doi: 10.1016/s0140-6736(74)91351-8. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Waite M., Kucera L. S., King L., Edwards I. Phospholipid synthesis in human embryo fibroblasts infected with herpes simplex virus type 2. Lipids. 1981 Sep;16(9):655–662. doi: 10.1007/BF02535060. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Hajjar D. P., Minick C. R., Fabricant J. Herpesvirus infection enhances cholesterol and cholesteryl ester accumulation in cultured arterial smooth muscle cells. Am J Pathol. 1981 Nov;105(2):176–184. [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Krook L., Gillespie J. H. Virus-induced cholesterol crystals. Science. 1973 Aug 10;181(4099):566–567. doi: 10.1126/science.181.4099.566. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., Steinberg S. P., Gelb L., Galasso G., Borkowsky W., LaRussa P., Farrara A. Live attenuated varicella vaccine. Efficacy for children with leukemia in remission. JAMA. 1984 Jul 20;252(3):355–362. doi: 10.1001/jama.252.3.355. [DOI] [PubMed] [Google Scholar]
- Halpin T. J., Holtzhauer F. J., Campbell R. J., Hall L. J., Correa-Villaseñor A., Lanese R., Rice J., Hurwitz E. S. Reye's syndrome and medication use. JAMA. 1982 Aug 13;248(6):687–691. [PubMed] [Google Scholar]
- Israel A., Audubert F., Semmel M. Phospholipids in Newcastle Disease Virus infected cells. Biochim Biophys Acta. 1975 Jan 28;375(2):224–235. doi: 10.1016/0005-2736(75)90191-1. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M., Abrahamsen L. H. Variation in human herpesvirus susceptibility to enhancement by the pesticide carbaryl. Appl Environ Microbiol. 1983 May;45(5):1555–1559. doi: 10.1128/aem.45.5.1555-1559.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linnemann C. C., Jr, Shea L., Partin J. C., Schubert W. K., Schiff G. M. Reye's syndrome: epidemiologic and viral studies, 1963-1974. Am J Epidemiol. 1975 Jun;101(6):517–526. doi: 10.1093/oxfordjournals.aje.a112123. [DOI] [PubMed] [Google Scholar]
- Portoukalian J., Bugand M., Zwingelstein G., Precausta P. Comparison of the lipid composition of rabies virus propagated in Nil 2 cells maintained in monolayer versus spinner culture. Biochim Biophys Acta. 1977 Oct 24;489(1):106–118. doi: 10.1016/0005-2760(77)90237-5. [DOI] [PubMed] [Google Scholar]
- REYE R. D., MORGAN G., BARAL J. ENCEPHALOPATHY AND FATTY DEGENERATION OF THE VISCERA. A DISEASE ENTITY IN CHILDHOOD. Lancet. 1963 Oct 12;2(7311):749–752. doi: 10.1016/s0140-6736(63)90554-3. [DOI] [PubMed] [Google Scholar]
- Rozee K. R., Lee S. H., Crocker J. F., Safe S. H. Enhanced virus replication in mammalian cells exposed to commercial emulsifiers. Appl Environ Microbiol. 1978 Feb;35(2):297–300. doi: 10.1128/aem.35.2.297-300.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder E. W., Merrick J. M. Alterations in glycosphingolipid patterns in a line of African green monkey kidney cells infected with herpesvirus. J Virol. 1979 Dec;32(3):734–740. doi: 10.1128/jvi.32.3.734-740.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starko K. M., Ray C. G., Dominguez L. B., Stromberg W. L., Woodall D. F. Reye's syndrome and salicylate use. Pediatrics. 1980 Dec;66(6):859–864. [PubMed] [Google Scholar]
- Steinhart W. L., Busch J. S., Oettgen J. P., Howland J. L. Sphingolipid metabolism during infection of human fibroblasts by herpes simplex virus type 1. Intervirology. 1984;21(2):70–76. doi: 10.1159/000149504. [DOI] [PubMed] [Google Scholar]
- Steinhart W. L., Nicolet C. M., Howland J. L. Incorporation of 32P-phosphate into membrane phospholipids during infection of cultured human fibroblasts by herpes simplex virus type 1. Intervirology. 1981;16(2):80–85. doi: 10.1159/000149251. [DOI] [PubMed] [Google Scholar]
- Waldman R. J., Hall W. N., McGee H., Van Amburg G. Aspirin as a risk factor in Reye's syndrome. JAMA. 1982 Jun 11;247(22):3089–3094. [PubMed] [Google Scholar]


