Abstract
Escherichia coli DNA polymerase III holoenzyme is composed of 10 different subunits linked by noncovalent interactions. The polymerase activity resides in the α-subunit. The ε-subunit, which contains the proofreading exonuclease site within its N-terminal 185 residues, binds to α via a segment of 57 additional C-terminal residues, and also to θ, whose function is less well defined. The present study shows that θ greatly enhances the solubility of ε during cell-free synthesis. In addition, synthesis of ε in the presence of θ and α resulted in a soluble ternary complex that could readily be purified and analyzed by NMR spectroscopy. Cell-free synthesis of ε from PCR-amplified DNA coupled with site-directed mutagenesis and selective 15N-labeling provided site-specific assignments of NMR resonances of ε that were confirmed by lanthanide-induced pseudocontact shifts. The data show that the proofreading domain of ε is connected to α via a flexible linker peptide comprising over 20 residues. This distinguishes the α : ε complex from other proofreading polymerases, which have a more rigid multidomain structure.
INTRODUCTION
The DNA polymerase III (Pol III) holoenzyme is the major chromosomal replicase in Escherichia coli (1). This enzyme complex is composed of 10 different polypeptide subunits. The catalytic core contains one each of the α (130 kDa), ɛ (27 kDa) and θ (8.8 kDa) subunits encoded by the dnaE, dnaQ and holE genes, respectively. The α-subunit contains the 5′–3′ DNA polymerase active site (2,3), the ɛ-subunit is responsible for 3′–5′ proofreading exonuclease activity (4) and the θ-subunit has no identified enzymatic activity (5). The α:ɛ:θ core complex is active alone as a proofreading DNA polymerase, and copurification of these three subunits demonstrates their tight physical association (6,7). Direct interactions between ɛ and α (8) and ɛ and θ (5) have been demonstrated using purified subunits, but no interaction has been detected between α and θ.
The θ-subunit is not essential, as a ΔholE mutant is normally viable (9) and θ has only a modest stimulatory effect on the exonuclease activity of ɛ on a mispaired primer terminus (5). Genetic studies with the temperature-sensitive dnaQ49 mutant allele indicated that θ stabilizes the structure of ɛ (10), and this effect may also be achieved by the θ homolog HOT (homolog of theta) encoded by bacteriophage P1 (11). Remarkably, the phage genome (94 kb) relies on E. coli Pol III for its replication but, with the exception of the Ban (DnaB analog) DNA helicase and SSB (single-stranded DNA-binding protein), does not encode any other replication proteins (12).
Two crystal structures of α have been determined: of a C-terminally truncated version of E. coli α (13), and of full-length Thermus aquaticus α (14). Structures are also known of the N-terminal globular domain of ɛ (ɛ186), both alone (15) and in complex with HOT (16,17). The structure of the ɛ186:θ complex determined by nuclear magnetic resonance (NMR) spectroscopy (18,19) is in agreement with the ɛ186:HOT structure. No structure has been determined of full-length ɛ but residues following ɛ186 within its C-terminal region (in the following referred to as C-terminal segment (CTS) of ɛ or ɛCTS; Figure 1) are known to be responsible for binding of α (20,21). This 57-residue segment contains a Q-linker sequence proposed to provide a flexible tether between domains (22), followed by the C-terminal 40-residue segment that has been shown to interact tightly with α when fused to maltose-binding protein (21).
Figure 1.
Amino acid sequence of full-length ɛ. Residues 7–180 have a defined conformation in the crystal structure of ɛ186 (15). The 57 residues of the ɛCTS are highlighted in bold. Secondary structure prediction suggests an α-helical segment in the ɛCTS with high propensity; the corresponding residues are underlined. Alanine and threonine residues in the CTS of ɛ are highlighted in red and yellow, respectively.
The present study was carried out to obtain structural information about the α:ɛ:θ complex. By studying the ɛ:θ, α:ɛ and α:ɛ:θ complexes and probing potential weak interactions between α and ɛ186 by a novel two-pronged NMR assignment strategy, we show that residues in the ɛCTS are flexible and that those in the Q-linker region remain so even when assembled in the Pol III core complex. This provides the first experimental evidence that ɛ is indeed tethered to α by a flexible peptide linker, suggesting a mechanism for transition between polymerization and proofreading modes in Pol III that is fundamentally different from those in other polymerases whose structures in both modes are known. In addition, experiments to produce ɛ by cell-free protein synthesis shed further light on the function of θ.
MATERIALS AND METHODS
Materials
L-[15N]Alanine and L-[15N]threonine were obtained from Spectra Stable Isotopes. Synthetic oligonucleotides were purchased from GeneWorks (Hindmarsh, SA, Australia); sequences of oligonucleotides used are listed in Table S1.
Protein purification
For large-scale isolation of α, E. coli cells (BL21::λDE3 recA) harboring the plasmid pND517 encoding the dnaE gene under control of a tac promoter (23) were grown aerobically at 30°C and in 20 l of Z medium of pH 7.3 (24) supplemented with 100 mg/l ampicillin in a fermenter with pH control (BIOSTAT C, B. Braun Biotech International, Melsungen, Germany). Overexpression of α was induced by addition of 0.5 mM isopropyl-β,d-thiogalactoside at A595 = 1.5 and the induced culture was grown to an A595 of ∼10 (4 h), yielding about 250 g of cells. The α-subunit was purified from 54 g of cells using a modified version of the procedure of Wijffels et al. (23), with two steps of chromatography on columns of DEAE-Toyopearl 650 M (2.5 × 45 cm) and phosphocellulose (5.5 × 22.5 cm). This was followed by chromatography using a heparin–Sepharose column (2.5 × 17.5 cm) to concentrate the protein. About 96 mg of purified α-subunit were obtained. The preparation of a C-terminal truncation mutant of α, α917 (13) is described in the Supplementary Data.
The Pol III θ-subunit was purified as described (25), except that cells were grown for 2 days at room temperature in an autoinduction medium (26), and the French press lysate was passed through a column of DEAE-Toyopearl in 40 mM Tris–HCl buffer, pH 7.6, prior to chromatography on a phosphocellulose column. 15N-labeled θ was produced using minimal medium supplemented with 15NH4Cl (Cambridge Isotope Laboratories, Andover, MA, USA) as described (27). T7 RNA polymerase (28) and ɛ186 (25) were as described. Concentrations of samples of soluble pure proteins (α, ɛ186 and θ) were determined spectrophotometrically at 280 nm, using calculated values of ɛ280 of 95440, 7680 and 8250/M/cm, respectively (29).
Cell-free synthesis of the ε-subunit
S30 cell extracts from either E. coli strain Rosetta::λDE3/pRARE (from Novagen, Gibbtown, NJ, USA) or BL21Star::λDE3 (from Invitrogen, Carlsbad, CA, USA) were prepared by the procedure of Pratt (24,30,31), followed by concentration with polyethylene glycol 8000 as described by Kigawa et al. (31,32), and were used interchangeably. Cell-free protein synthesis was carried out for 6–7 h either using an autoinduction system that uses plasmid pKO1166 to direct production of T7 RNA polymerase in S30 extracts (33) at 37°C, or a standard coupled transcription/translation system with purified T7 RNA polymerase at 30 or 37°C as described previously (28,31). The plasmid template pSH1017 (25) was used at a concentration of 16 μg/ml for production of ɛ. All reaction mixtures containing expressed proteins were clarified by centrifugation (30 000g, 1 h) at 4°C.
Cell-free synthesis of selectively 15N-labeled ε in complex with θ
Samples of the ɛ:θ complex containing 15N-Ala or 15N-Thr labeled ɛ were prepared by synthesis of ɛ in the cell-free system in the presence of separately purified unlabeled θ (0.5 mg/ml), with 0.6 ml reaction mixtures at 30°C for 7 h. Following centrifugation, the supernatant was dialyzed against 2 l of NMR buffer (20 mM Tris–HCl, pH 6.9, 1 mM EDTA, 1 mM dithiothreitol) and concentrated to a final volume of about 0.5 ml using Millipore Ultra-4 centrifugal filters (molecular weight cutoff 10 000). D2O was added to a final concentration of 10% (v/v) prior to NMR measurements.
Cell-free synthesis and purification of the α:ε:θ complexes with selectively 15N-labeled ε
Samples of the α:ɛ:θ complex containing 15N-Ala or 15N-Thr labeled ɛ were prepared by cell-free synthesis of ɛ in the presence of separately purified unlabeled θ (0.5 mg/ml) and α (5.0 mg/ml). Reaction mixtures (0.9 ml) were incubated at 30°C for 7 h. Following centrifugation, the supernatant was dialyzed against 2 l of buffer A (50 mM Tris–HCl, pH 7.6, 1 mM EDTA, 1 mM dithiothreitol), then loaded onto a DEAE-Toyopearl 650 M column (2.5 × 2.5 cm) pre-equilibrated with buffer A and eluted with a linear gradient of 0–1 M NaCl in 150 ml of buffer A. The combined fractions containing the α:ɛ:θ complex (eluting at about 0.22 M NaCl) were dialyzed against 2 l of buffer A, loaded onto a heparin–Sepharose column (2.5 × 17.5 cm) in buffer A and eluted with a linear gradient of 0–1 M NaCl in 300 ml of buffer A. The α:ɛ:θ complex eluted at about 0.25 M NaCl and was subsequently dialyzed against NMR buffer, concentrated and prepared for NMR spectroscopy as described above for the ɛ:θ complex. The purity of the α:ɛ:θ complex was assessed by 15% SDS–PAGE followed by staining with Coomassie brilliant blue; its concentration was estimated by the method of Bradford (34).
Cell-free synthesis and purification of the ε:θ complex containing 15N-labeled θ
Cell-free synthesis of unlabeled ɛ at 30°C for 7 h in the presence of separately purified uniformly 15N-labeled θ (0.5 mg/ml) was done in a reaction volume of 0.9 ml. Following centrifugation, the supernatant was dialyzed against buffer A, loaded onto a DEAE-Toyopearl column and eluted as above for the α:ɛ:θ complex. Excess θ remained unbound, and the first protein peak eluting at about 60 mM NaCl contained the ɛ:θ complex. Following dialysis against 2 l of NMR buffer and concentration to a final volume of about 0.5 ml as above, D2O was added to a final concentration of 10% (v/v) for NMR measurements. The purity was assessed by 15% SDS–PAGE followed by staining with Coomassie brilliant blue.
Cell-free synthesis of mutant ε:θ complexes from PCR-amplified DNA
Samples of the ɛ:θ complex containing site-specific mutants of ɛ were produced by cell-free protein synthesis from linear PCR-amplified DNA with 5′-phosphorylated primers to promote ligation to circular DNA in the reaction mixture (35). The DNA was prepared in two steps, using Vent DNA polymerase (New England Biolabs, Ipswich, MA, USA) for all PCR reactions. First, the site-specific mutation was introduced using two separate PCR reactions (50 μl each) with 20–30 ng of pSH1017 template, where the first reaction used primer 1133 and one of the reverse primers of Table S1 (containing the desired mutation) and the second reaction used one of the forward primers of Table S1 and primer 1134. The PCR products were mixed in an equimolar ratio and the residual primers removed using the Qiaquick PCR purification kit (Qiagen, Hilden, Germany). In the second step, the T7 promoter and terminator sequences were added in two separate PCR reactions (50 μl each) using the primer pairs 1131 and 1134, and 1132 and 1133, respectively (Table S1), with 20–30 ng of purified PCR product from the first step. The two PCR products were mixed in an approximately equimolar ratio and the residual primers removed as above, denatured at 95°C (5 min) and reannealed at room temperature (∼5 min). This generated DNA with complementary single-stranded 8-nt overhangs suitable for cyclization by the intrinsic ligase activity of the cell extract (35). The reannealed PCR solution was used as the template for subsequent cell-free protein synthesis at a concentration of about 10 μg/ml of reaction mixture. Four mutants of ɛ (A186G, A188G, A200G and A243G) labeled with 15N-Ala and two (T193A, T201A) labeled with 15N-Thr were thus prepared in six parallel reactions in the presence of unlabeled θ.
Genes encoding two additional ɛ mutants (the double mutant S2A/T3S and the C-terminal deletion mutant ɛ217) were prepared using PCR reactions with appropriate primer pairs (Table SI) and inserted between the NdeI and EcoRI sites of the T7 expression vector pRSET-5b (36). The resulting plasmids were used as templates to prepare PCR-amplified DNA products by following the procedure of the second step described above. The resulting purified and reannealed mixtures of PCR products were used directly as templates for cell-free protein synthesis of the mutant ɛ-subunits in the presence of θ, as above.
NMR spectroscopy
All NMR spectra were recorded at 25°C using a Bruker 800 MHz NMR spectrometer equipped with a cryoprobe. 15N-HSQC spectra were recorded using 5 mm sample tubes (except for samples of the α:ɛ:θ complex which were concentrated to 200 μl and measured in 3 mm sample tubes), t1max = 32 ms, t2max = 102 ms, and total recording times between 1 h and 13 h.
RESULTS
Cell-free synthesis of full-length ε in the presence of θ and/or α
Cell-free synthesis of full-length ɛ at 37°C produced similar yields in both the autoinduction system with coexpression of T7 RNA polymerase from plasmid pKO1166 (33) and in our standard system with purified T7 RNA polymerase (28,31). Yields were about 3–4 mg/ml at 37°C and 2 mg/ml at 30°C. At both temperatures, the protein was produced in insoluble form. Similarly, the protein was in inclusion bodies when overexpression was carried out in vivo at 30 or 37°C, using standard expression protocols in LB medium (25,37), or at room temperature using the autoinduction medium described by Studier (26).
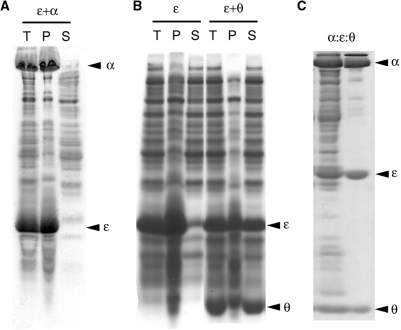
In an attempt to improve the solubility of full-length ɛ, its cell-free synthesis was carried out in the presence of either purified α or θ. At 37°C, the presence of α alone did not improve the solubility of ɛ. Instead, a coprecipitate of the α:ɛ complex was obtained (Figure 2A), although α alone remained soluble when added to the cell-free reaction mixture. Expression at 30°C produced mainly soluble α:ɛ complex (data not shown), suggesting that the effect is caused by thermal instability of ɛ. In contrast, cell-free synthesis of ɛ in the presence of 0.5–1.0 mg/ml of θ led to a soluble ɛ:θ complex (Figure 2B) even at 37°C, and the simultaneous presence of α and θ also gave a soluble α:ɛ:θ core complex (Figure 2C). The yield of expressed ɛ was unaffected by the presence of θ and/or α.
Figure 2.
Subunit θ solubilizes nascent ɛ and α:ɛ:θ forms a soluble isolable complex when ɛ is made in the presence of α and θ. The ɛ-subunit was produced by cell-free synthesis. The gels were stained with Coomassie brilliant blue. T, P and S denote the total reaction mixtures, the insoluble fractions (pellet) and the supernatants, respectively. (A) The 10% SDS–PAGE of ɛ synthesized at 37°C in the presence of 5 mg/ml of α-subunit. Nascent ɛ coprecipitates α. (B) The 15% SDS–PAGE of ɛ synthesized at 37°C in the presence of 0.1 mg/ml (lanes 1–3, labeled ɛ) or 1 mg/ml (lanes 4–6, labeled ɛ + θ) of θ-subunit. (C) The 15% SDS–PAGE of ɛ synthesized at 30°C in the presence of 5 mg/ml of the α-subunit and 0.5 mg/ml of the θ-subunit. The left and right lanes show the soluble fraction of the cell-free reaction mixture and the purified α:ɛ:θ complex, respectively.
A similar set of experiments performed with C-terminally truncated ɛ, ɛ217, and C-terminally truncated α, α917 (13), also resulted in coprecipitation of ɛ217 and α917, indicating that the interaction with α does not depend only on the C-terminal 26 residues of ɛ (Figure S1).
15N-HSQC spectra of 15N-labeled ε in complex with θ
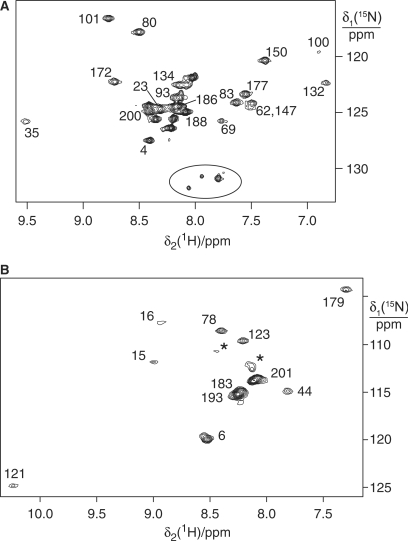
Cell-free synthesized samples of the 36 kDa ɛ:θ complex, where ɛ was selectively labeled with 15N-Ala or 15N-Thr, were found to be adequate for the acquisition of 15N-HSQC NMR spectra without chromatographic purification (Figure 3). The cross-peaks of the N-terminal domain of ɛ were readily assigned by comparison with the reported assignments of ɛ186 (38,39). Conservation of their chemical shifts indicated that neither the structure of the N-terminal domain nor its interface with θ is significantly affected by the presence of the ɛCTS, and provide no indication that the ɛCTS interacts with portions of the folded core of ɛ186. The additional cross-peaks observed must arise from Ala and Thr residues in the ɛCTS (Figure 1). Their chemical shifts are characteristic of a random-coil polypeptide chain. Many of them are also more intense than the signals from the globular N-terminal domain but their line widths are not sufficiently narrow to indicate that the segment is completely free in solution. In addition, the 15N-Ala labeled sample displays at least one more cross-peak than expected from the amino acid sequence and peak doubling is observed also in the 15N-Thr labeled sample (see below). This may be explained by a heterogeneous chemical environment due to nonspecific interaction with other components of the cell extract, considering that the cell-free reaction mixture inhibited the binding of the ɛ:θ complex to α (see below).
Figure 3.
15N-HSQC NMR spectra of ɛ in complex with θ. The cross-peaks are assigned with the residue numbers. (A) 15N-Ala labeled ɛ in complex with unlabeled θ. The circle identifies a set of unassigned peaks indicative of sample heterogeneity. They were not observed for the α:ɛ:θcomplex (Figure 5A, spectral region not shown). (B) 15N-Thr labeled ɛ in complex with unlabeled θ. The asterisks label peaks which were not observed in the spectra of ɛ186 or the α:ɛ:θ complex, indicating sample heterogeneity.
15N-HSQC spectra of 15N-labeled θ in the ε:θ complex
The 15N-HSQC spectrum of uniformly labeled θ in complex with full-length ɛ was indistinguishable from that of θ in complex with ɛ186 (data not shown). This indicates that θ does not interact with the ɛCTS in the ɛ:θ complex.
Analysis of the α:ε:θ complex containing 15N-Thr or 15N-Ala labeled ε
Initially, we attempted to form the α:ɛ:θ complex by the addition of purified α to the NMR sample containing the unpurified ɛ:θ complex. 15N-HSQC spectra of samples containing 15N-Ala or 15N-Thr labeled ɛ were, however, indistinguishable from spectra in the absence of α even after addition of α in 2-fold excess. This seemed to be due to nonspecific association of ɛ with other proteins (or nucleic acids) in the cell-free reaction mixture since after partial purification via a DEAE column, the ɛ:θ complex readily bound to α (data not shown). Therefore, we prepared the α:ɛ:θ complex by cell-free synthesis of ɛ in the presence of purified α and θ. Subsequent purification of the complex was straightforward, requiring only two steps of chromatography (Figure 2C).
The 15N-HSQC spectra of the ternary complex contained only a few cross-peaks (Figure 4A). Since nuclear magnetization relaxes faster with decreasing molecular tumbling rates, the high molecular weight of the complex (about 165 kDa) leads to excessively fast nuclear relaxation for most residues and cross-peaks can be observed only for residues located in polypeptide segments of increased mobility.
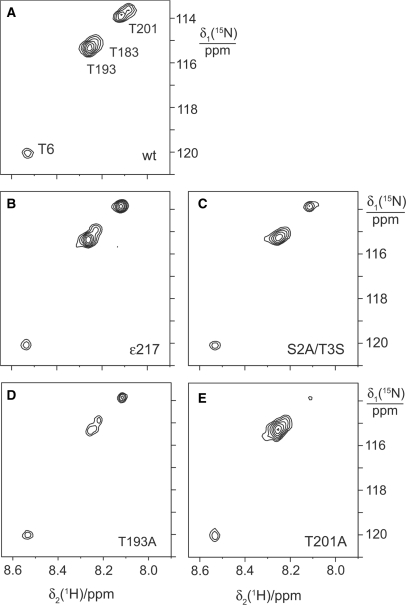
Figure 4.
15N-HSQC NMR spectra of α:ɛ:θ and mutant ɛ:θ complexes containing 15N-threonine labeled ɛ. For best comparison, the spectra were Fourier transformed with the same parameters and scaled for similar intensity of the cross-peak of Thr6 in all spectra. (A) Spectrum of the α:ɛ:θ complex. (B–E) Spectra of samples of the ɛ:θ complex prepared with mutant 15N-threonine labeled ɛ. The mutations are indicated.
We next set out to assign the cross-peaks observed for the α:ɛ:θ complex containing 15N-Thr labeled ɛ. The peaks observed for α:ɛ:θ coincide with the most intense peaks observed for the ɛ:θ complex (Figure 3B). Therefore, the assignment could be obtained in the absence of α by analyzing appropriate ɛ mutants in complex with θ.
The crystal structure of ɛ186 is missing electron density for the first six and last six residues of the protein (15) and NMR cross-peaks of these residues are narrower than average, indicating increased mobility. (The 15N-HSQC cross-peaks of the three N-terminal residues cannot be observed in ɛ186 (38,39), presumably due to line broadening arising from hydroxide-catalyzed amide-proton exchange rates near the amino terminus.) Assuming that these segments are also highly mobile in the α:ɛ:θ complex, the cross-peaks of Ala4, Thr6 and Thr183 would be expected to appear at conserved positions in the 15N-HSQC spectrum of the α:ɛ:θ complex. This is indeed the case (Figures 4A and 5). The conserved appearance of the spectrum obtained for the ɛ:θ complex containing the 15N-Thr labeled S2A/T3S double-mutant of ɛ (Figure 4C) confirms that Thr3 remains unobservable in full-length ɛ and that the unassigned cross-peaks must arise from Thr residues located in the ɛCTS.
Figure 5.
15N-HSQC NMR spectra of the α:ɛ:θ complex and PCS induced by Dy3+, Er3+ or Tb3+ ions. Resonance assignments are indicated for the cross-peaks observed in the absence of lanthanide ions. Arrows identify PCS induced by paramagnetic lanthanide ions bound to the active site of ɛ. (A) 15N-Alanine labeled ɛ in complex with α and θ. The spectra in the absence of lanthanide ion (black), with Er3+ (blue) and with Dy3+ (brown) are superimposed. (B) 15N-Threonine labeled ɛ in complex with α and θ. The spectra in the absence of lanthanide ion (black), with Er3+ (blue) and with Tb3+ (red) are superimposed.
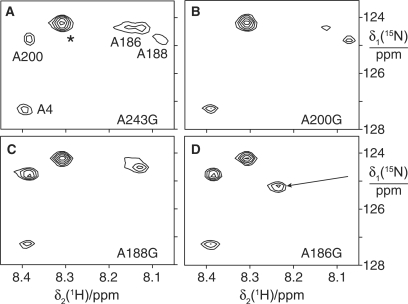
In order to assign the cross-peaks of these additional Thr residues, we synthesized ɛ in the presence of θ from DNA that had been mutated and amplified by PCR. Mutagenesis of Thr201 to Ala led to a much weaker cross-peak at the 15N-chemical shift of 114 p.p.m. (Figure 4E). Similarly, mutation of Thr193 led to a much weaker cross-peak at the 15N-chemical shift of 115.3 p.p.m. (Figure 4D). The residual cross-peak intensities arise from the presence of small amounts of wild-type protein due to residual amounts of wild-type plasmid template in the cell-free reactions (35).
The cross-peak of Thr201 appears as two peaks in the spectrum of the wild-type ɛ:θ complex (Figure 3B) and of the α:ɛ:θ complex (Figure 4A), but not in the spectra of the mutants. The origin of this peak doubling could be conformational heterogeneity or the inconsistent appearance of a cross-peak of the only unassigned threonine residue in the ɛCTS, Thr218. This was examined by truncation of ɛ following Ala217 in the deletion mutant ɛ217. The result was inconclusive: the ɛ217 sample did not show doubling of the cross-peak assigned to Thr201 (Figure 4B), but neither did the T193A mutant (Figure 4D). As expected for a more mobile peptide segment, however, the truncation led to a more intense signal for Thr201.
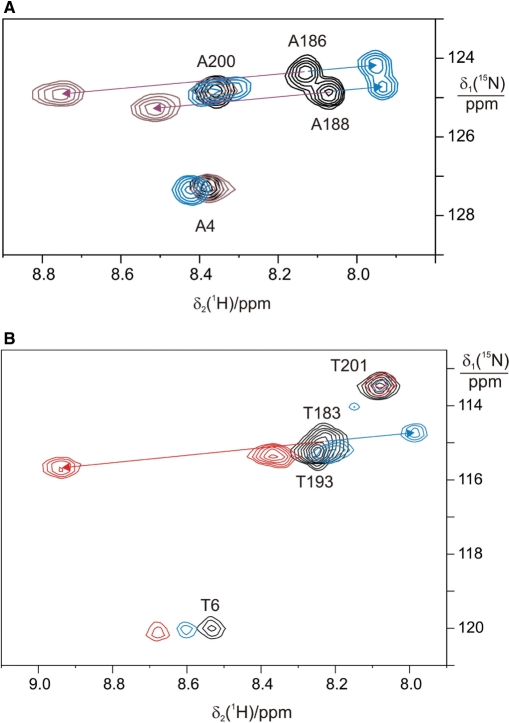
Four 15N-HSQC cross-peaks could be observed in the α:ɛ:θ complex prepared with 15N-Ala labeled ɛ (Figure 5). They were also present at conserved positions in the spectrum of the ɛ:θ complex (Figure 3A). One of the cross-peaks was assigned to Ala4 by virtue of its similar position in the NMR spectrum of ɛ186. For additional assignments of the most intense peaks, mutant samples of the ɛ:θ complex were prepared. Mutation of the C-terminal residue of ɛ (Ala243) to glycine did not remove any of the intense Ala cross-peaks observed for the wild-type protein (data not shown). In contrast, mutations of Ala200 (Figure 6B) and Ala188 (Figure 6C) enabled straightforward assignment of two of the cross-peaks to these residues. Mutation of Ala186 resulted in disappearance of one cross-peak (assigned to Ala186) and significant change in chemical shift of the cross-peak assigned to Ala188, as might be expected for a next-neighbor residue (Figure 6D). Notably, most of the chemical shifts were insensitive toward mutations in other residues, in agreement with the random-coil nature of the ɛCTS until at least Thr201.
Figure 6.
Assignment of 15N-HSQC spectra of 15N-alanine labeled ɛ:θ complexes by site-directed mutagenesis. ε was labeled with 15N-alanine and θ was unlabeled. The four spectra are of samples prepared with ɛ where different alanine residues were mutated to glycine. (A) A243G mutant. The asterisk identifies a cross-peak that was not observed in the wild-type protein (Figure 3A). It may arise from a low-molecular weight metabolite. (B) A200G mutant. (C) A188G mutant. (D) A186G mutant. The arrow identifies the putative shift of the cross-peak of Ala188 from its position in the wild-type protein.
To verify that these 15N-alanine and 15N-threonine resonance assignments also apply to the α:ɛ:θ complex, we added paramagnetic lanthanides to Pol III core complexes containing appropriately labeled ɛ (Figure 5). The ɛ-subunit has a natural metal-binding site that can bind a single lanthanide ion, resulting in substantial pseudocontact shifts (PCS) (40), which displace each 15N-HSQC cross-peak by a chemical shift increment that is similar in the 1H and 15N dimensions. PCS could indeed be measured in the α:ɛ:θ complex (Figure 5). The sign and magnitudes of the PCS observed for Ala4, Thr6, Thr183 and Ala186 with Dy3+, Tb3+ and Er3+ were in agreement with those measured previously for ɛ186 (39). Along the sequence Ala186, Ala188, Thr193, Ala200 and Thr201, the PCS steadily decreased toward zero. This would be expected for a flexible polypeptide chain for which positive and negative PCS are averaged by extensive sampling of the space around the metal ion.
In summary, we have demonstrated that the Pol III α:ɛ:θ core complex is characterized by substantially increased mobility of all Ala and Thr residues in the polypeptide segment between Thr183 and Thr201 of ɛ, whereas residues farther toward the C-terminus are immobilized by binding to α.
Lack of interaction between ε186 and α
The N-terminal domain of ɛ, ɛ186, has been shown previously to be incapable of forming an isolable complex with α (21,41). If the ɛ:θ complex is tethered to α via a long flexible linker involving the C-terminal residues of ɛ, weak binding of the globular N-terminal domain of ɛ to α may still be sufficient to put the exonuclease domain in a defined location with respect to the polymerase subunit. 15N-HSQC spectra of the ɛ186:θ complex with uniformly 15N-labeled ɛ186, however, were not affected by the presence of α (data not shown), indicating that any association between ɛ186 and α would involve a dissociation constant Kd > 0.1 mM. This lower limit for Kd is consistent with a reported estimate from gel filtration data (21).
DISCUSSION
In contrast to the soluble N-terminal domain of the ɛ-subunit, ɛ186, overexpression of full-length dnaQ leads to insoluble protein which in the past could only be solubilized by a denaturation and refolding protocol (37). Cell-free synthesis circumvents this problem elegantly by allowing the formation of protein–protein complexes from nascently produced ɛ in the presence of its natural binding partners θ and α. In addition, nascently synthesized ɛ readily formed the ternary α:ɛ:θ complex, whereas the ɛ:θ complex was unable to bind to α in the reaction mixture of cell-free synthesis. This effect appears to be due to an engagement of the ɛCTS in nonspecific interactions with other components of the cell-free reaction mixture, as partial purification of the ɛ:θ complex by anion exchange chromatography restored the binding capacity to α. The nonspecific interactions also explain the apparent heterogeneity observed for the NMR signals from the CTS (Figure 3).
Our experiments confirm the role of θ as an important factor for stabilizing the structure of ɛ. The ɛ186 domain is prone to irreversible denaturation at 25°C (42), whereas the complex with θ can be studied at 30°C without degradation of NMR signals over time (38). The temperature sensitivity of the N-terminal domain of ɛ explains why the presence of θ is required during cell-free synthesis of ɛ to obtain the full-length ɛ:θ complex in soluble form. In the absence of θ, cell-free synthesis of ɛ leads to precipitation even in the presence of α. Precipitation is more pronounced at higher temperatures, in agreement with the sensitivity of the N-terminal domain to denaturation. The fact that α coprecipitates with ɛ highlights the independent roles of the N- and C-terminal domains of ɛ, where binding to α via the ɛCTS is still possible if the globular N-terminal domain unfolds and aggregates.
Whether θ acts as a chaperone for ɛ in vivo is not clear, in view of the low cellular concentration of Pol III subunits (43). In any case, neither θ nor the N-terminal domain of ɛ seem to act as a specific receptor of the ɛCTS in the absence of α, as NMR spectra of ɛ:θ samples containing selectively labeled ɛ and uniformly labeled θ did not reveal significant differences from corresponding spectra of the ɛ186:θ complex.
Among the C-terminal 57 residues of ɛ, the segment between Ala186 and Thr201 is much more mobile than the residues closer to the C-terminus. Judging by the signal intensities of the 15N-Ala residues (Figures 3A and 5A), this holds both for the ɛ:θ and α:ɛ:θ complexes. Therefore, the ‘sticky’ part of the ɛCTS is limited to residues following Thr201. In the complex with α, the contacts must involve residues close to or even including the C-terminal alanine residue of ɛ, as no NMR signal could be observed for this residue. This is consistent with properties of the dnaQ932 mutant allele, which encodes a version of ɛ lacking only the last three residues. Its recessive phenotype suggests it is incapable of competing with wild-type ɛ for binding to α (44). Considering that ɛ217 can coprecipitate a C-terminally truncated form of α, α917 (Supplementary Data), at least some of the residues between Thr201 and Thr218 seem to be involved in binding to α. This indicates a large binding epitope, which would be difficult to explore by site-directed mutagenesis.
Residues 183–201 of ɛ thus form the core of a highly flexible tether in the α:ɛ:θ complex. Combined with the absence of detectable interactions between the ɛ186:θ complex and α, this means that the proofreading exonuclease domain enjoys an extraordinary degree of positional and orientational freedom with respect to the polymerase subunit to which it is tethered.
Similar to ɛ, the polymerase active site of the α-subunit binds a divalent metal ion (Mg2+) in a similar coordination environment (13,14). It is likely that this ion can also be replaced by a lanthanide ion, but there was no evidence for PCS induced in ɛ by a paramagnetic lanthanide bound to α in any of our experiments, even though PCS induced by Dy3+ can extend over distances >40 Å (45). As all PCS were readily explained as intramolecular effects from a lanthanide bound to the active site of ɛ (Figure 5), it is unlikely that the proofreading domain is positioned close to a lanthanide ion in the polymerase active site of α in the α:ɛ:θ complex. We do not know yet whether the presence of primer-template DNA or of any of the other subunits of the polymerase III holoenzyme complex would change this; there has certainly been no indication in published work of an interaction between ɛ and any Pol III subunit other than α and θ.
Wieczorek and McHenry (46) reported that a 320-residue N-terminal segment of α binds ɛ with the same affinity (Kd about 5 nM) as the 1160-residue full-length α-subunit. In the crystal structures of α (13,14), the N-terminal 270 residues fold into a globular (php) domain of about 40 Å diameter. Considering that a 20-residue peptide in extended conformation can span well over 60 Å, the flexible tether by which ɛ is attached to α could readily bridge the distance between almost any attachment site on the php domain and the polymerase active site.
The α:ɛ:θ Pol III core complex is distinctly different from proofreading polymerases whose structures have been determined in that the polymerase and exonuclease active sites reside in different subunits. In examples of structures of single-chain proofreading polymerases like DNA polymerase I (47,48) and RB69 DNA polymerase (49), the proofreading domain shares a large and well-defined interface with the polymerase domain, suggesting that both domains are rigidly associated. Repair of a mismatched template-primer DNA end thus involves translocation of the DNA from the polymerase to the exonuclease active sites which are located about 30 Å apart.
Our observations that neither ɛ186 nor θ interact to any appreciable degree with the ɛCTS or with α, and that the long linker between the catalytic domain of ɛ and the C-terminal region that is firmly bound to α is still flexible in the Pol III core complex, suggests a fundamentally different mechanism for transition of Pol III from the polymerization to the editing mode. The transition might involve conformational changes in α that expose a cryptic binding site for the ɛ186 domain, such that the two active sites are brought closer together, rather than protein-mediated transfer of the DNA substrate as occurs in other enzymes. Since we have shown the exonuclease domain to be relatively freely mobile in the α:ɛ:θ complex, it might thus be able to swing in closer to the polymerase active site to repair a mismatched terminus when this is required.
CONCLUDING REMARKS
In this work, we have exploited several powerful new techniques for structural biology in solution to study the structure of the Pol III core complex. The assignment of NMR resonances by site-directed mutagenesis has long been used in situations where resonance assignments by other means are difficult (50). As mutations can change the chemical shifts by perturbing the 3D protein structure, this strategy is not always successful. While this problem hardly occurs in random-coil polypeptide segments as in the C-terminal region of ɛ, chemical shift changes were indeed induced by the mutation of Ala186 (Figure 6D). In this situation, PCS induced by paramagnetic lanthanide ions provided a conclusive second line of evidence. We anticipate that the combination of mutation by PCR, cell-free protein synthesis from PCR-amplified DNA (35) and PCS induced by lanthanide tags (51) will become an attractive tool set for many hitherto difficult cases like those examined here.
The present study also illustrates the power of cell-free protein synthesis in the presence of separately purified cofactors for the production of soluble protein–protein complexes. In a similar manner, we were able to produce the complex between the χ- and ψ-subunits of E. coli Pol III in soluble form by cell-free synthesis of ψ in the presence of separately purified χ (33). In the χ:ψ complex, the N-terminal segment of 26 residues of ψ is unstructured in the complex with χ, but required for binding to the γ-subunit (33). Similarly, the Pol III τ-subunit binds to α via a C-terminal polypeptide segment which is unstructured in pure τ (52,53). The emerging picture of the polymerase III holoenzyme is that of a machine composed of globular building blocks that are linked by flexible tethers.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Dr Ruhu Qi for assistance in purifying the α-subunit, Dr Xun-Cheng Su for help with the NMR measurements and Dr Michael John for helpful discussions. This work was supported by project grants (to N.E.D. and G.O.) from the Australian Research Council (ARC), an ARC Linkage (CSIRO) Postdoctoral Fellowship to K.O., an Australian Professorial Fellowship to N.E.D. and a Federation Fellowship to G.O. Financial support by the ARC for the 800 MHz NMR Facility at the ANU is also gratefully acknowledged. Funding to pay the Open Access publication charges for this article was provided by the Australian National University.
Conflict of interest statement. None declared.
REFERENCES
- 1.Kornberg A, Baker TA. DNA Replication. New York, N.Y: W.H. Freeman & Co.; 1991. [Google Scholar]
- 2.Maki H, Horiuchi T, Kornberg A. The polymerase subunit of DNA polymerase III of Escherichia coli. I. Amplification of the dnaE gene product and polymerase activity of the α subunit. J. Biol. Chem. 1985;260:12982–12986. [PubMed] [Google Scholar]
- 3.Maki H, Kornberg A. The polymerase subunit of DNA polymerase III of Escherichia coli. II. Purification of the α subunit, devoid of nuclease activities. J. Biol. Chem. 1985;260:12987–12992. [PubMed] [Google Scholar]
- 4.Scheuermann RH, Tam S, Burgers PMJ, Echols H. Identification of the ɛ-subunit of Escherichia coli DNA polymerase III holoenzyme as the dnaQ gene product: a fidelity subunit for DNA replication. Proc. Natl Acad. Sci. USA. 1983;80:7085–7089. doi: 10.1073/pnas.80.23.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studwell-Vaughan PS, O’Donnell M. DNA polymerase III accessory proteins V. θ encoded by holE. J. Biol. Chem. 1993;268:11785–11791. [PubMed] [Google Scholar]
- 6.McHenry CS, Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J. Biol. Chem. 1979;254:1748–1753. [PubMed] [Google Scholar]
- 7.Kim DR, McHenry CS. In vivo assembly of overproduced DNA polymerase III. J. Biol. Chem. 1996;271:20681–20689. doi: 10.1074/jbc.271.34.20681. [DOI] [PubMed] [Google Scholar]
- 8.Maki H, Kornberg A. Proofreading by DNA polymerase III of Escherichia coli depends on cooperative interaction of the polymerase and exonuclease subunits. Proc. Natl Acad. Sci. USA. 1987;84:4389–4392. doi: 10.1073/pnas.84.13.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slater SC, Lifsics MR, O’Donnell M, Maurer R. holE, the gene coding for the θ subunit of DNA polymerase III of Escherichia coli: characterization of a holE mutant and comparison with a dnaQ (ɛ-subunit) mutant. J. Bacteriol. 1994;176:815–821. doi: 10.1128/jb.176.3.815-821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taft-Benz SA, Schaaper RM. The θ subunit of Escherichia coli DNA polymerase III: a role in stabilizing the ɛ proofreading subunit. J. Bacteriol. 2004;186:2774–2780. doi: 10.1128/JB.186.9.2774-2780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chikova AK, Schaaper RM. The bacteriophage P1 hot gene product can substitute for the Escherichia coli DNA polymerase III θ subunit. J. Bacteriol. 2005;187:5528–5536. doi: 10.1128/JB.187.16.5528-5536.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobocka MB, Rose D, Rusin M, Samojedny A, Lehnherr H, Yarmolinsky MB, Blattner FR. The genome of bacteriophage P1. J. Bacteriol. 2004;186:7032–7068. doi: 10.1128/JB.186.21.7032-7068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers MH, Georgescu RE, Lee S-G, O’Donnell M, Kuriyan J. Crystal structure of catalytic α subunit of E. coli replicative DNA polymerase III. Cell. 2006;126:881–892. doi: 10.1016/j.cell.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Bailey S, Wing RA, Steitz TA. The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerases. Cell. 2006;126:893–904. doi: 10.1016/j.cell.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Hamdan S, Carr PD, Brown SE, Ollis DL, Dixon NE. Structural basis for proofreading during replication of the Escherichia coli chromosome. Structure. 2002;10:535–546. doi: 10.1016/s0969-2126(02)00738-4. [DOI] [PubMed] [Google Scholar]
- 16.DeRose EF, Kirby TW, Mueller GA, Chikova AK, Schaaper RM, London RE. Phage like it HOT: solution structure of the bacteriophage P1-encoded HOT protein, a homolog of the θ subunit of E. coli DNA polymerase III. Structure. 2004;12:2221–2231. doi: 10.1016/j.str.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Kirby TW, Harvey S, DeRose EF, Chalov S, Chikova AK, Perrino FW, Schaaper RM, London RE, Pedersen LC. Structure of the Escherichia coli DNA polymerase III ɛ-HOT proofreading complex. J. Biol. Chem. 2006;281:38466–38471. doi: 10.1074/jbc.M606917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keniry MA, Park AY, Owen EA, Hamdan SM, Pintacuda G, Otting G, Dixon NE. Structure of the θ subunit of Escherichia coli DNA polymerase III in complex with the ɛ subunit. J. Bacteriol. 2006;188:4464–4473. doi: 10.1128/JB.01992-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pintacuda G, Park AY, Keniry MA, Dixon NE, Otting G. Lanthanide labeling offers fast NMR approach to 3D structure determinations of protein-protein complexes. J. Am. Chem. Soc. 2006;128:3696–3702. doi: 10.1021/ja057008z. [DOI] [PubMed] [Google Scholar]
- 20.Taft-Benz SA, Schaaper RM. The C-terminal domain of DnaQ contains the polymerase binding site. J. Bacteriol. 1999;181:2963–2965. doi: 10.1128/jb.181.9.2963-2965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrino FW, Harvey S, McNeill SM. Two functional domains of the ɛ subunit of DNA polymerase III. Biochemistry. 1999;38:16001–16009. doi: 10.1021/bi991429+. [DOI] [PubMed] [Google Scholar]
- 22.Wootton JC, Drummond MH. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989;2:535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- 23.Wijffels G, Dalrymple BP, Prosselkov P, Kongsuwan K, Epa C, Lilley PE, Jergic S, Buchardt J, Brown SE, Alewood PF. Inhibition of protein interactions with the β2 sliding clamp of Escherichia coli DNA polymerase III by peptides from β2-binding proteins. Biochemistry. 2004;43:5661–5671. doi: 10.1021/bi036229j. [DOI] [PubMed] [Google Scholar]
- 24.Pratt JM. Coupled transcription-translation in prokaryotic cell-free systems. In: Hames BD, Higgins SJ, editors. Transcription and Translation. Oxford, UK: IRL Press; 1984. pp. 179–209. [Google Scholar]
- 25.Hamdan S, Bulloch EM, Thompson PR, Beck JL, Yang J-Y, Crowther JA, Lilley PE, Carr PD, Ollis DL, Brown SE. Hydrolysis of the 5′-p-nitrophenyl ester of TMP by the proofreading exonuclease (ɛ) subunit of Escherichia coli DNA polymerase III. Biochemistry. 2002;41:5266–5275. doi: 10.1021/bi0159480. [DOI] [PubMed] [Google Scholar]
- 26.Studier FW. Protein production by auto-induction in high-density shaking cultures. Protein Express. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Keniry MA, Berthon HA, Yang J-Y, Miles CS, Dixon NE. NMR solution structure of the θ subunit of DNA polymerase III from Escherichia coli. Protein Sci. 2000;9:721–733. doi: 10.1110/ps.9.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa K, Headlam MJ, Schaeffer PM, Henderson BR, Dixon NE, Otting G. Optimization of an Escherichia coli system for cell-free synthesis of selectively 15N-labelled proteins for rapid analysis by NMR spectroscopy. Eur. J. Biochem. 2004;271:4084–4093. doi: 10.1111/j.1432-1033.2004.04346.x. [DOI] [PubMed] [Google Scholar]
- 29.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa K, Dixon NE, Otting G. Cell-free synthesis of 15N-labeled proteins for NMR studies. IUBMB Life. 2005;57:615–622. doi: 10.1080/15216540500217859. [DOI] [PubMed] [Google Scholar]
- 31.Apponyi M, Ozawa K, Dixon NE, Otting G. Cell-free protein synthesis for analysis by NMR spectroscopy. In: Kobe B, Guss M, Huber T, editors. Methods in Molecular Biology, Vol. 426, Structural Proteomics: High-Throughput Methods. Totowa, USA: Humana Press; 2008. pp. 257–268. [DOI] [PubMed] [Google Scholar]
- 32.Kigawa T, Yabuki T, Yoshida Y, Tsutsui M, Ito Y, Shibata T, Yokoyama S. Cell-free production and stable-isotope labeling of milligram quantities of proteins. FEBS Lett. 1999;442:15–19. doi: 10.1016/s0014-5793(98)01620-2. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa K, Jergic S, Crowther JA, Thompson PR, Wijffels G, Otting G, Dixon NE. Cell-free in vitro protein synthesis in an autoinduction system for NMR studies of protein-protein interactions. J. Biomol. NMR. 2005;32:235–241. doi: 10.1007/s10858-005-7946-4. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Wu PSC, Ozawa K, Lim SP, Vasudevan SG, Dixon NE, Otting G. Cell-free transcription/translation from PCR amplified DNA for high-throughput NMR studies. Angew. Chemie Int. Ed. 2007;46:3356–3358. doi: 10.1002/anie.200605237. [DOI] [PubMed] [Google Scholar]
- 36.Schoepfer R. The pRSET family of T7 promoter expression vectors for Escherichia coli. Gene. 1993;124:83–85. doi: 10.1016/0378-1119(93)90764-t. [DOI] [PubMed] [Google Scholar]
- 37.Scheuermann RH, Echols H. A separate editing exonuclease for DNA replication: the subunit ɛ of Escherichia coli DNA polymerase III holoenzyme. Proc. Natl Acad. Sci. USA. 1984;81:7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeRose EF, Darden T, Harvey S, Gabel S, Perrino FW, Schaaper RM, London RE. Elucidation of ɛ−θ subunit interface of Escherichia coli DNA polymerase III by NMR spectroscopy. Biochemistry. 2003;42:3635–3644. doi: 10.1021/bi0205451. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz C, John M, Park AY, Dixon NE, Otting G, Pintacuda G, Huber T. Efficient χ-tensor determination and NH assignment of paramagnetic proteins. J. Biomol. NMR. 2006;35:79–87. doi: 10.1007/s10858-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 40.Pintacuda G, Keniry MA, Huber T, Park AY, Dixon NE, Otting G. Fast structure-based assignment of 15N HSQC spectra of selectively 15N-labeled paramagnetic proteins. J. Am. Chem. Soc. 2004;126:2963–2970. doi: 10.1021/ja039339m. [DOI] [PubMed] [Google Scholar]
- 41.Lehtinen DA, Perrino FW. Dysfunctional proofreading in the Escherichia coli DNA polymerase III core. Biochem. J. 2004;384:337–348. doi: 10.1042/BJ20040660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamdan S, Brown SE, Thompson PR, Yang J-Y, Carr PD, Ollis DL, Otting G, Dixon NE. Preliminary X-ray crystallographic and NMR studies on the exonuclease domain of the ɛ subunit of Escherichia coli DNA polymerase III. J. Struct. Biol. 2000;131:164–169. doi: 10.1006/jsbi.2000.4291. [DOI] [PubMed] [Google Scholar]
- 43.Ishihama Y, Schmidt T, Rappsilber J, Mann M, Hartl FU, Kerner MJ, Frishman D. Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics. 2008;9:102. doi: 10.1186/1471-2164-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taft-Benz SA, Schaaper RM. Mutational analysis of the 3′–5′ proofreading exonuclease of Escherichia coli DNA polymerase III. Nucleic Acids Res. 1998;26:4005–4011. doi: 10.1093/nar/26.17.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allegrozzi M, Bertini I, Janik MBL, Lee Y-M, Liu G, Luchinat C. Lanthanide-induced pseudocontact shifts for solution structure refinements of macromolecules in shells up to 40 Å from the metal ion. J. Am. Chem. Soc. 2000;122:4154–4161. [Google Scholar]
- 46.Wieczorek A, McHenry CS. The NH2-terminal php domain of α subunit of the Escherichia coli replicase binds the ɛ proofreading subunit. J. Biol. Chem. 2006;281:12561–12567. doi: 10.1074/jbc.M513844200. [DOI] [PubMed] [Google Scholar]
- 47.Brautigam CA, Steitz TA. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr. Opin. Struct. Biol. 1998;8:54–63. doi: 10.1016/s0959-440x(98)80010-9. [DOI] [PubMed] [Google Scholar]
- 48.Steitz TA. DNA polymerases: structural diversity and common mechanism. J. Biol. Chem. 1999;274:17395–17398. doi: 10.1074/jbc.274.25.17395. [DOI] [PubMed] [Google Scholar]
- 49.Franklin MC, Wang J, Steitz TA. Structure of the replicating complex of a Pol α family DNA polymerase. Cell. 2001;105:657–667. doi: 10.1016/s0092-8674(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 50.Falzone CJ, Benkovic SJ, Wright PE. Partial proton NMR assignments of the Escherichia coli dihydrofolate reductase complex with folate: evidence for a unique conformation of bound folate. Biochemistry. 1990;29:9667–9677. doi: 10.1021/bi00493a023. [DOI] [PubMed] [Google Scholar]
- 51.Pintacuda G, John M, Su X-C, Otting G. NMR structure determination of protein-ligand complexes by lanthanide labeling. Acc. Chem. Res. 2007;40:206–212. doi: 10.1021/ar050087z. [DOI] [PubMed] [Google Scholar]
- 52.Jergic S, Ozawa K, Williams NK, Su X-C, Scott DD, Hamdan SM, Crowther JA, Otting G, Dixon NE. The unstructured C-terminus of the τ subunit of Escherichia coli DNA polymerase III holoenzyme is the site of interaction with the α subunit. Nucleic Acids Res. 2007;35:2813–2824. doi: 10.1093/nar/gkm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su X-C, Jergic S, Keniry MA, Dixon NE, Otting G. Solution structure of domains IVa and V of the τ subunit of Escherichia coli DNA polymerase III and interaction with the α subunit. Nucleic Acids Res. 2007;35:2825–2832. doi: 10.1093/nar/gkm080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.