Abstract
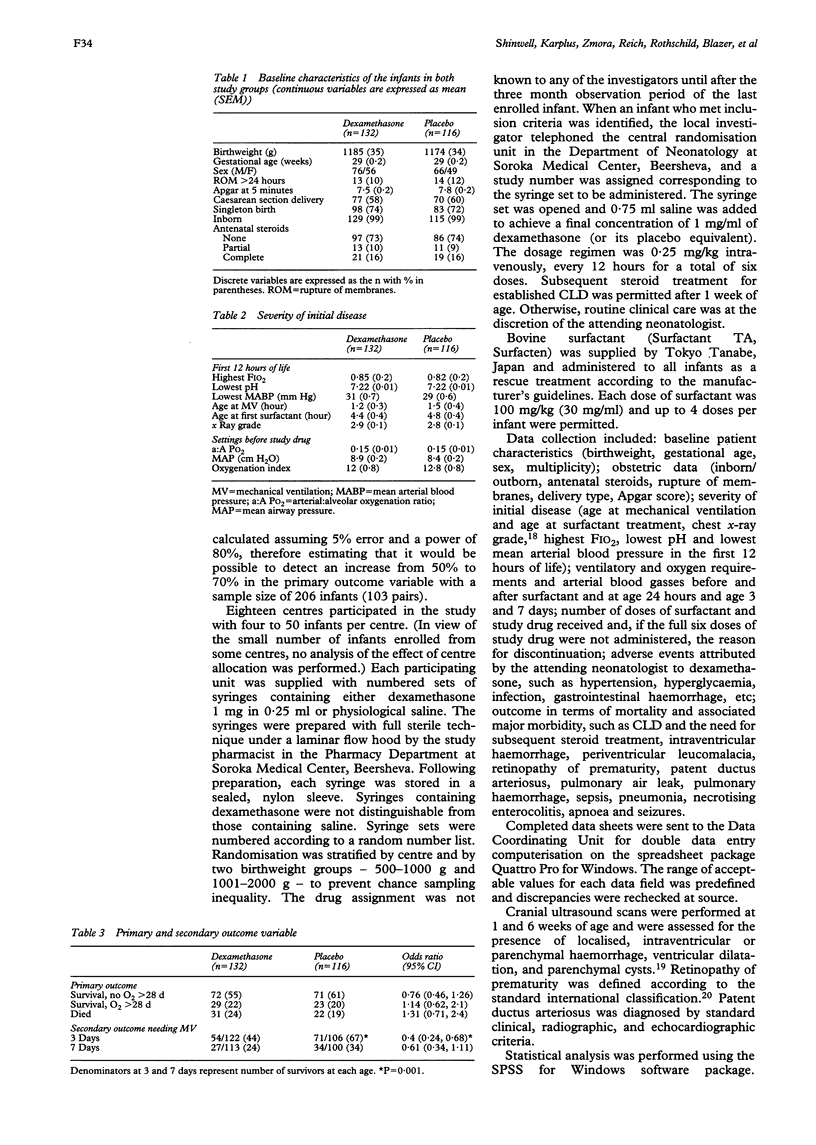
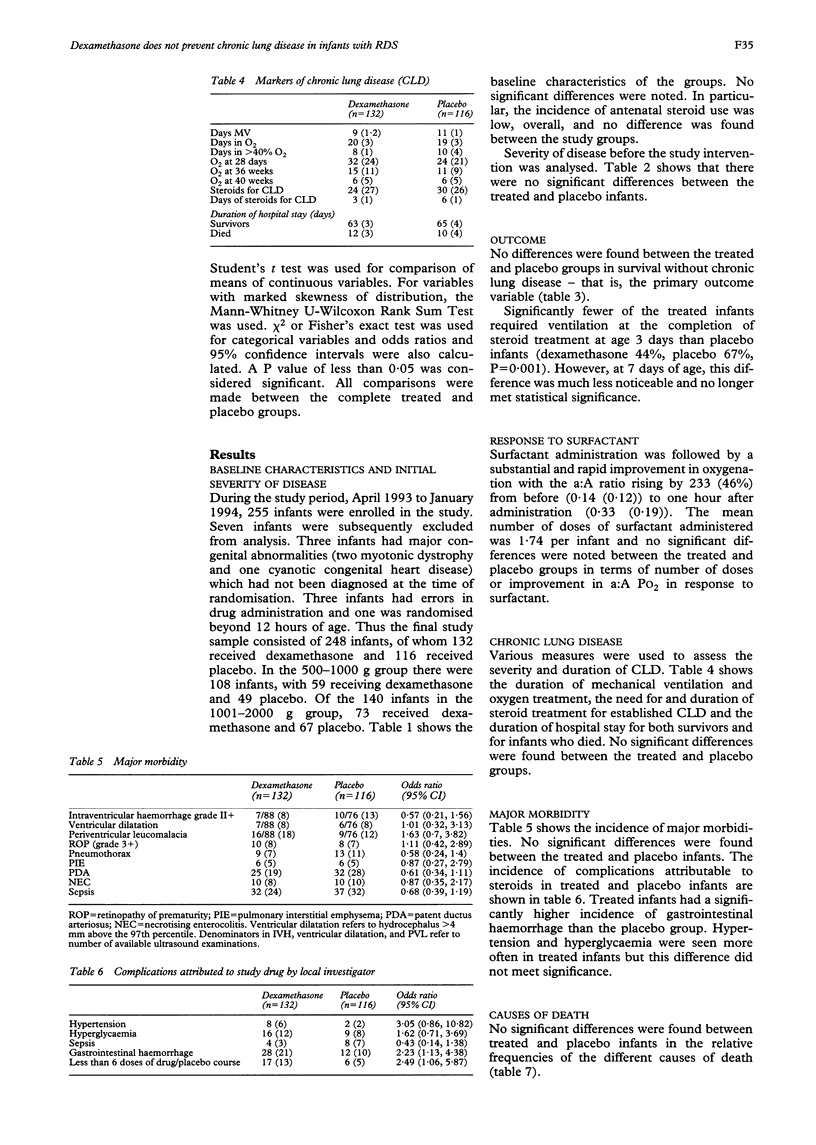
OBJECTIVE: To study the effect of early postnatal dexamethasone (days 1-3) on the incidence and severity of chronic lung disease in preterm infants with respiratory distress syndrome. METHODS: A multicentre, randomised, placebo controlled, blinded study was carried out in 18 neonatal intensive care units in Israel. The primary outcome measure was survival to discharge without requirement for supplemental oxygen therapy beyond 28 days of life. The secondary outcome measures were requirement for mechanical ventilation at 3 and 7 days, duration of ventilation or oxygen therapy, need for subsequent steroids for established chronic lung disease and incidence of major morbidities. RESULTS: The study consisted of 248 infants (dexamethasone n = 132; placebo n = 116). No differences were found in the outcome variables except for a reduction in requirement for mechanical ventilation at age 3 days in treated infants (dexamethasone 44%, placebo 67%; P = 0.001). Gastrointestinal haemorrhage, hypertension, and hyperglycaemia were more common in treated infants, but no life threatening complications, such as gastrointestinal perforation, were encountered. CONCLUSIONS: These data do no support the routine use of early postnatal steroids, but may justify further study in a selected, high risk group of infants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery G. B., Fletcher A. B., Kaplan M., Brudno D. S. Controlled trial of dexamethasone in respirator-dependent infants with bronchopulmonary dysplasia. Pediatrics. 1985 Jan;75(1):106–111. [PubMed] [Google Scholar]
- Baden M., Bauer C. R., Colle E., Klein G., Taeusch H. W., Jr, Stern L. A controlled trial of hydrocortisone therapy in infants with respiratory distress syndrome. Pediatrics. 1972 Oct;50(4):526–534. [PubMed] [Google Scholar]
- Cummings J. J., D'Eugenio D. B., Gross S. J. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med. 1989 Jun 8;320(23):1505–1510. doi: 10.1056/NEJM198906083202301. [DOI] [PubMed] [Google Scholar]
- Giedion A., Haefliger H., Dangel P. Acute pulmonary X-ray changes in hyaline membrane disease treated with artificial ventilation and positive end-expiratory pressure (PEP). Pediatr Radiol. 1973 Oct;1(3):145–152. doi: 10.1007/BF00974058. [DOI] [PubMed] [Google Scholar]
- Groneck P., Oppermann M., Speer C. P. Levels of complement anaphylatoxin C5a in pulmonary effluent fluid of infants at risk for chronic lung disease and effects of dexamethasone treatment. Pediatr Res. 1993 Nov;34(5):586–590. doi: 10.1203/00006450-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Groneck P., Reuss D., Götze-Speer B., Speer C. P. Effects of dexamethasone on chemotactic activity and inflammatory mediators in tracheobronchial aspirates of preterm infants at risk for chronic lung disease. J Pediatr. 1993 Jun;122(6):938–944. doi: 10.1016/s0022-3476(09)90024-5. [DOI] [PubMed] [Google Scholar]
- Harkavy K. L., Scanlon J. W., Chowdhry P. K., Grylack L. J. Dexamethasone therapy for chronic lung disease in ventilator- and oxygen-dependent infants: a controlled trial. J Pediatr. 1989 Dec;115(6):979–983. doi: 10.1016/s0022-3476(89)80754-1. [DOI] [PubMed] [Google Scholar]
- Levene M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child. 1981 Dec;56(12):900–904. doi: 10.1136/adc.56.12.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden B. E., Murphy S. A., Saunders G. C., Pathak D., Johnson J. D. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984 Nov;130(5):817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- Sanders R. J., Cox C., Phelps D. L., Sinkin R. A. Two doses of early intravenous dexamethasone for the prevention of bronchopulmonary dysplasia in babies with respiratory distress syndrome. Pediatr Res. 1994 Jul;36(1 Pt 1):122–128. doi: 10.1203/00006450-199407001-00022. [DOI] [PubMed] [Google Scholar]
- Yeh T. F., Torre J. A., Rastogi A., Anyebuno M. A., Pildes R. S. Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind, controlled study. J Pediatr. 1990 Aug;117(2 Pt 1):273–282. doi: 10.1016/s0022-3476(05)80547-5. [DOI] [PubMed] [Google Scholar]
- van Houten J., Long W., Mullett M., Finer N., Derleth D., McMurray B., Peliowski A., Walker D., Wold D., Sankaran K. Pulmonary hemorrhage in premature infants after treatment with synthetic surfactant: an autopsy evaluation. The American Exosurf Neonatal Study Group I, and the Canadian Exosurf Neonatal Study Group. J Pediatr. 1992 Feb;120(2 Pt 2):S40–S44. doi: 10.1016/s0022-3476(05)81232-6. [DOI] [PubMed] [Google Scholar]


