Abstract
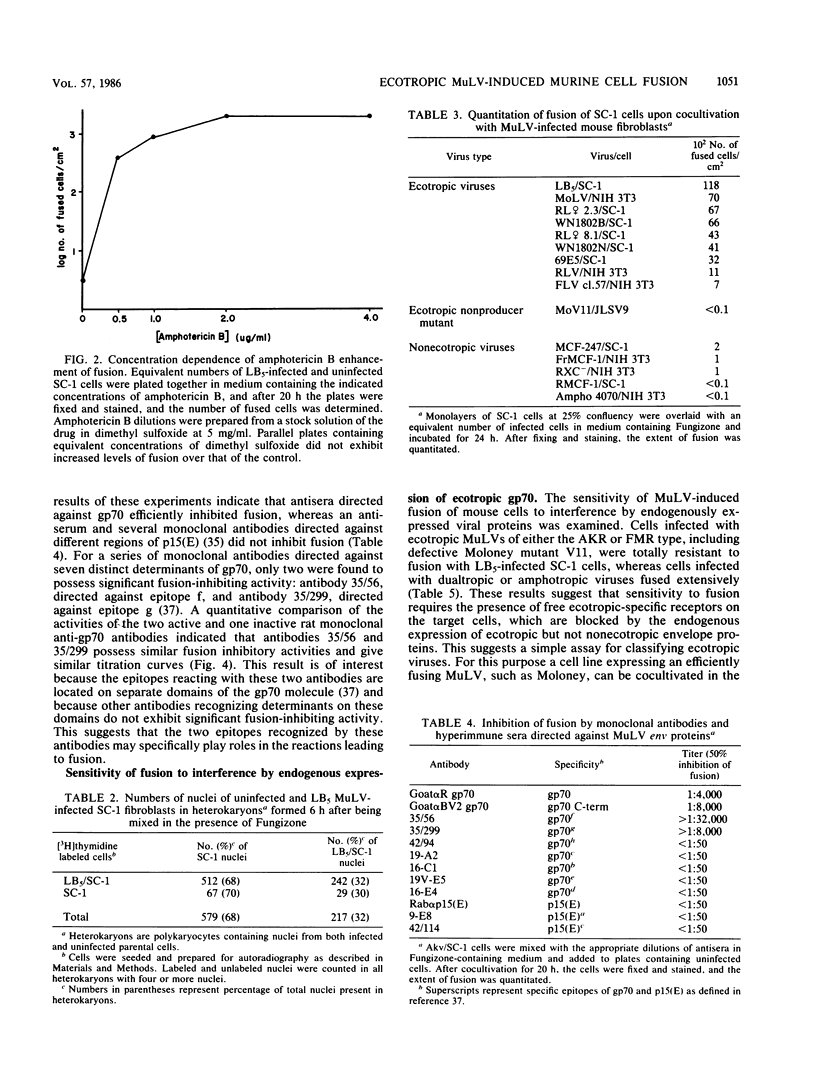
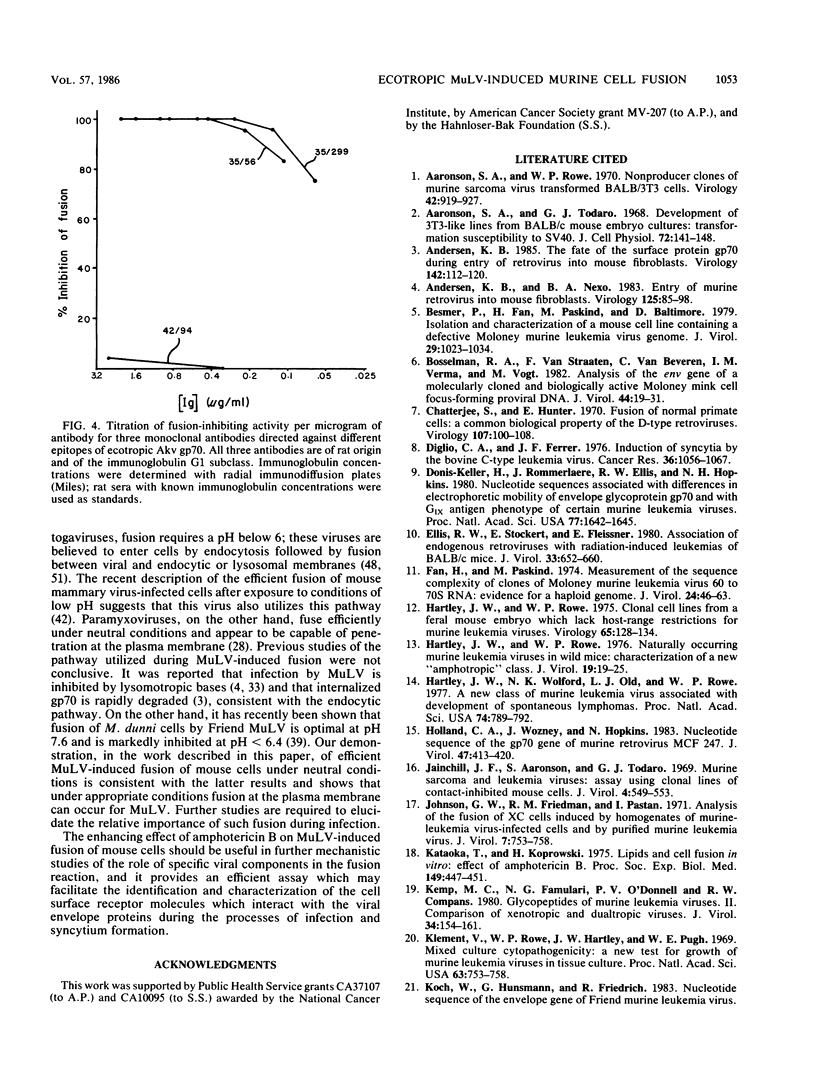
Extensive fusion occurs upon cocultivation of murine fibroblasts producing ecotropic murine leukemia viruses (MuLVs) with a large variety of murine cell lines in the presence of the polyene antibiotic amphotericin B, the active component of the antifungal agent Fungizone. The resulting polykaryocytes contain nuclei from both infected and uninfected cells, as evidenced by autoradiographic labeling experiments in which one or the other parent cell type was separately labeled with [3H]thymidine and fused with an unlabeled parent. This cell fusion specifically requires the presence of an ecotropic MuLV-producing parent and is not observed for cells producing xenotropic, amphotropic, or dualtropic viruses. Mouse cells infected with nonecotropic viruses retain their sensitivity toward fusion, whereas infection with ecotropic viruses abrogates the fusion of these cells upon cocultivation with other ecotropic MuLV-producing cells. Nonmurine cells lacking the ecotropic gp70 receptor are not fused under similar conditions. Fusion is effectively inhibited by monospecific antisera to gp70, but not by antisera to p15(E), and studies with monoclonal antibodies identify distinct amino- and carboxy-terminal gp70 regions which play a role in the fusion reaction. The enhanced fusion which occurs in the presence of amphotericin B provides a rapid and sensitive assay for the expression of ecotropic MuLVs and should facilitate further mechanistic studies of MuLV-induced fusion of murine cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Andersen K. B. The fate of the surface protein gp70 during entry of retrovirus into mouse fibroblasts. Virology. 1985 Apr 15;142(1):112–120. doi: 10.1016/0042-6822(85)90426-x. [DOI] [PubMed] [Google Scholar]
- Besmer P., Fan H., Paskind M., Baltimore D. Isolation and characterization of a mouse cell line containing a defective Moloney murine leukemia virus genome. J Virol. 1979 Mar;29(3):1023–1034. doi: 10.1128/jvi.29.3.1023-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E. Fusion of normal primate cells: a common biological property of the D-type retroviruses. Virology. 1980 Nov;107(1):100–108. doi: 10.1016/0042-6822(80)90276-7. [DOI] [PubMed] [Google Scholar]
- Diglio C. A., Ferrer J. F. Induction of syncytia by the bovine C-type leukemia virus. Cancer Res. 1976 Mar;36(3):1056–1067. [PubMed] [Google Scholar]
- Donis-Keller H., Rommelaere J., Ellis R. W., Hopkins N. Nucleotide sequences associated with differences in electrophoretic mobility of envelope glycoprotein gp70 and with GIX antigen phenotype of certain murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1642–1645. doi: 10.1073/pnas.77.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Stockert E., Fleissner E. Association of endogenous retroviruses with radiation-induced leukemias of BALB/c mice. J Virol. 1980 Feb;33(2):652–660. doi: 10.1128/jvi.33.2.652-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Hopkins N. Nucleotide sequence of the gp70 gene of murine retrovirus MCF 247. J Virol. 1983 Sep;47(3):413–420. doi: 10.1128/jvi.47.3.413-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Analysis of the fusion of XC cells induced by homogenates of murine leukemia virus-infected cells and by purified murine leukemia virus. J Virol. 1971 Jun;7(6):753–758. doi: 10.1128/jvi.7.6.753-758.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Koprowski H. Lipids and cell fusion in vitro: effect of amphotericin B. Proc Soc Exp Biol Med. 1975 Jun;149(2):447–451. doi: 10.3181/00379727-149-38825. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Famulari N. G., O'Donnell P. V., Compans R. W. Glycopeptides of murine leukemia viruses. II. Comparison of xenotropic and dual-tropic viruses. J Virol. 1980 Apr;34(1):154–161. doi: 10.1128/jvi.34.1.154-161.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M. Titration of murine leukemia viruses with rat cell line RFL. J Virol. 1977 Aug;23(2):436–438. doi: 10.1128/jvi.23.2.436-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander M. R., Chattopadhyay S. K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984 Nov;52(2):695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin M. Intracellular potassium and macromolecular synthesis in mammalian cells. Nature. 1967 Feb 4;213(5075):451–453. doi: 10.1038/213451a0. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973 Mar;3(3):441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Hamaguchi M., Toyoda T., Yoshida T. The uncoating of paramyxoviruses may not require a low pH mediated step. Virology. 1983 Oct 15;130(1):263–268. doi: 10.1016/0042-6822(83)90138-1. [DOI] [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Pickering R., O'Donnell P. V., Pinter A., Hammerling U. Selective neutralization of ecotropic murine leukemia virus by monoclonal antibodies: localization of a site on the gp70 protein associated with ecotropism. Virology. 1981 May;111(1):84–92. doi: 10.1016/0042-6822(81)90655-3. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Deitch C. J., Pincus T. Multiplicity-dependent kinetics and murine leukemia virus infection in Fv-1-sensitive and Fv-1-resistant cells. Virology. 1976 Aug;73(1):23–35. doi: 10.1016/0042-6822(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazmiño N. H., Yuhas J. M., Tennant R. W. Inhibition of murine RNA tumor virus replication and oncogenesis by chloroquine. Int J Cancer. 1974 Sep 15;14(3):379–385. doi: 10.1002/ijc.2910140312. [DOI] [PubMed] [Google Scholar]
- Pierotti M., DeLeo A. B., Pinter A., O'Donnell P. V., Hämmerling U., Fleissner E. The GIX antigen of murine leukemia virus: an analysis with monoclonal antibodies. Virology. 1981 Jul 30;112(2):450–460. doi: 10.1016/0042-6822(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. Comparison of structural domains of gp70s of ecotropic Akv and dualtropic MCF-247 MuLVs. Virology. 1983 Aug;129(1):40–50. doi: 10.1016/0042-6822(83)90394-x. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. Topography of murine leukemia virus envelope proteins: characterization of transmembrane components. J Virol. 1983 Jun;46(3):1056–1060. doi: 10.1128/jvi.46.3.1056-1060.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tung J. S., O'Donnell P. V., Hämmerling U. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology. 1982 Jan 30;116(2):499–516. doi: 10.1016/0042-6822(82)90143-x. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Evans L. H. Infectious entry of murine retroviruses into mouse cells: evidence of a postadsorption step inhibited by acidic pH. J Virol. 1985 Sep;55(3):806–812. doi: 10.1128/jvi.55.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Redmond S., Peters G., Dickson C. Mouse mammary tumor virus can mediate cell fusion at reduced pH. Virology. 1984 Mar;133(2):393–402. doi: 10.1016/0042-6822(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Choppin P. W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983 Dec;131(2):518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Scheid A., Choppin P. W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980 Aug;105(1):205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Tung J. S., Hopkins N., Robbins P. W. Relationship of GIX antigen expression to the glycosylation of murine leukemia virus glycoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6420–6424. doi: 10.1073/pnas.77.11.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagi S. Effects of 5-bromodeoxyuridine on tumorigenicity, immunogenicity, virus production, plasminogen activator, and melanogenesis of mouse melanoma cells. Int Rev Cytol. 1976;45:65–111. doi: 10.1016/s0074-7696(08)60078-9. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Yuan E., Linemeyer D., Ruscetti S., Scolnick E. M. Helper-independent mink cell focus-inducing strains of Friend murine type-C virus: potential relationship to the origin of replication-defective spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):639–653. doi: 10.1084/jem.148.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Virus cloned from the Rauscher virus complex induces erythroblastosis and thymic lymphoma. Virology. 1982 Apr 15;118(1):225–228. doi: 10.1016/0042-6822(82)90336-1. [DOI] [PubMed] [Google Scholar]
- Vänänen P., Käriäinen L. Fusion and haemolysis of erythrocytes caused by three togaviruses: Semliki Forest, Sindbis and rubella. J Gen Virol. 1980 Feb;46(2):467–475. doi: 10.1099/0022-1317-46-2-467. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., Kaufman S. J. Mechanism of murine leukemia virus-induced fusion in rat myoblasts defective in differentiation. J Virol. 1977 Sep;23(3):768–775. doi: 10.1128/jvi.23.3.768-775.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling D. A., Keshet I. Fusion activity of virions of murine leukemia virus. Virology. 1979 May;95(1):185–196. doi: 10.1016/0042-6822(79)90413-6. [DOI] [PubMed] [Google Scholar]
- van Griensven L. J., Vogt M. Rauscher "mink cell focus-inducing" (MCF) virus causes erythroleukemia in mice: its isolation and properties. Virology. 1980 Mar;101(2):376–388. doi: 10.1016/0042-6822(80)90451-1. [DOI] [PubMed] [Google Scholar]