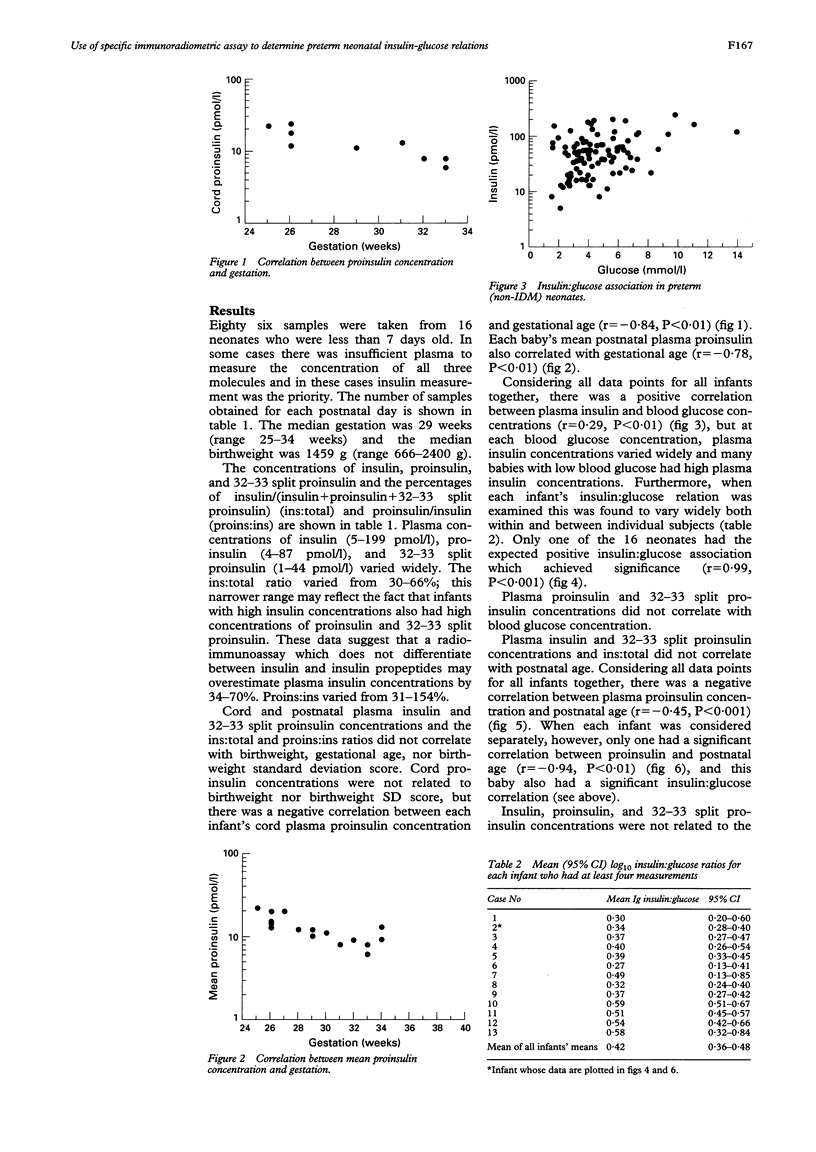
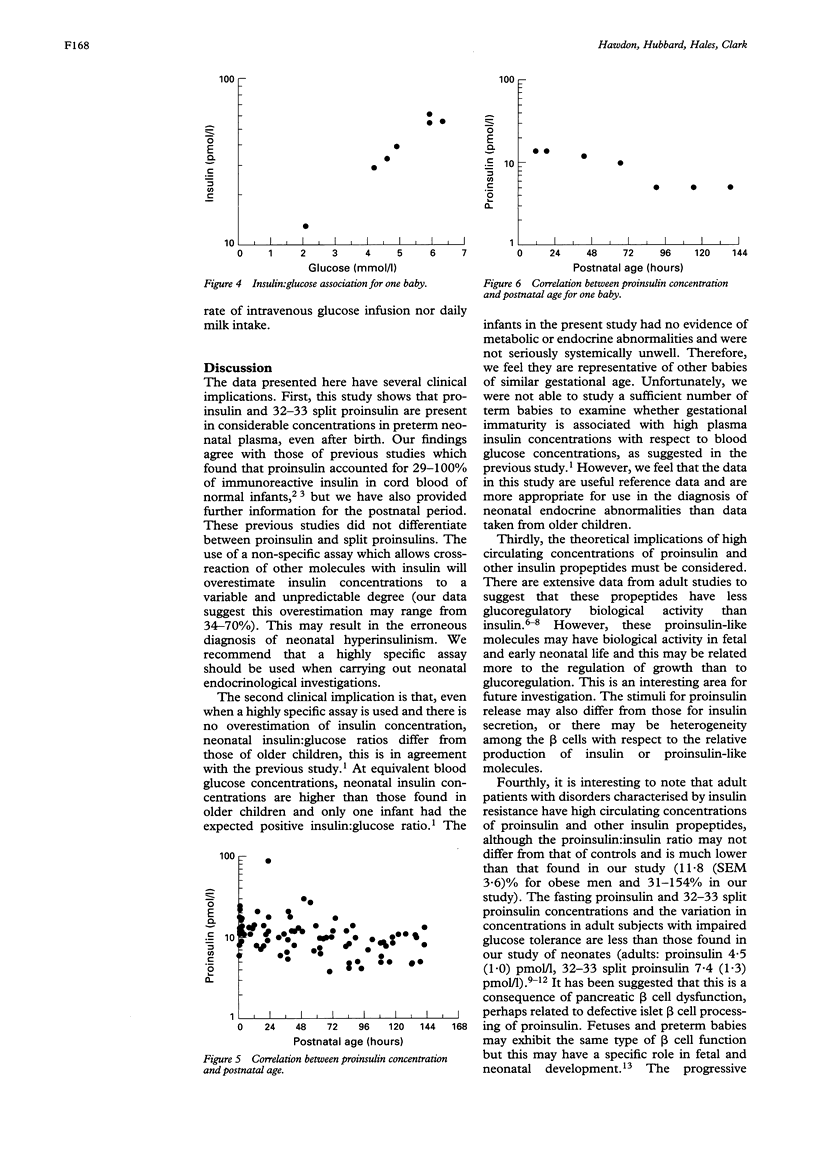
Abstract
Highly specific immunoradiometric assays were used to measure plasma concentrations of insulin, proinsulin, and 32-33 split proinsulin in neonates (n = 16). Neonatal plasma insulin concentrations were high relative to blood glucose concentrations and compared with adult insulin-glucose relations. Concentrations of proinsulin and 32-33 split proinsulin together accounted for 34-70% of the total concentration of insulin and pro-peptides. This study confirms the need to use a specific assay and neonatal reference data in the diagnosis of neonatal hyperinsulinism, and shows that neonatal pancreatic beta cell function may differ from that of older subjects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aynsley-Green A., Jenkins P., Tronier B., Heding L. G. Plasma proinsulin and C-peptide concentrations in children with hyperinsulinaemic hypoglycaemia. Acta Paediatr Scand. 1984 May;73(3):359–363. doi: 10.1111/j.1651-2227.1994.tb17748.x. [DOI] [PubMed] [Google Scholar]
- Cook J. T., Levy J. C., Page R. C., Shaw J. A., Hattersley A. T., Turner R. C. Association of low birth weight with beta cell function in the adult first degree relatives of non-insulin dependent diabetic subjects. BMJ. 1993 Jan 30;306(6873):302–306. doi: 10.1136/bmj.306.6873.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Metcalfe J., Gray I. P., Day J. L., Hales C. N. Insulin deficiency rather than hyperinsulinaemia in newly diagnosed type 2 diabetes mellitus. Diabet Med. 1993 May;10(4):305–312. doi: 10.1111/j.1464-5491.1993.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Rayman G., Gray I. P., Day J. L., Hales C. N. Insulin deficiency and increased plasma concentration of intact and 32/33 split proinsulin in subjects with impaired glucose tolerance. Diabet Med. 1993 May;10(4):313–320. doi: 10.1111/j.1464-5491.1993.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Glauber H. S., Revers R. R., Henry R., Schmeiser L., Wallace P., Kolterman O. G., Cohen R. M., Rubenstein A. H., Galloway J. A., Frank B. H. In vivo deactivation of proinsulin action on glucose disposal and hepatic glucose production in normal man. Diabetes. 1986 Mar;35(3):311–317. doi: 10.2337/diab.35.3.311. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Barker D. J., Clark P. M., Cox L. J., Fall C., Osmond C., Winter P. D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991 Oct 26;303(6809):1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N. Use of terminology related to fetal insulin secretion. Diabetologia. 1995 Jan;38(1):124–124. doi: 10.1007/BF02369366. [DOI] [PubMed] [Google Scholar]
- Hawdon J. M., Aynsley-Green A., Alberti K. G., Ward Platt M. P. The role of pancreatic insulin secretion in neonatal glucoregulation. I. Healthy term and preterm infants. Arch Dis Child. 1993 Mar;68(3 Spec No):274–279. doi: 10.1136/adc.68.3_spec_no.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G., Persson B., Stangenberg M. B-cell function in newborn infants of diabetic mothers. Diabetologia. 1980 Nov;19(5):427–432. doi: 10.1007/BF00281821. [DOI] [PubMed] [Google Scholar]
- Krentz A. J., Clark P. M., Cox L., Nattrass M. Hyperproinsulinaemia in impaired glucose tolerance. Clin Sci (Lond) 1993 Jul;85(1):97–100. doi: 10.1042/cs0850097. [DOI] [PubMed] [Google Scholar]
- McCance D. R., Pettitt D. J., Hanson R. L., Jacobsson L. T., Knowler W. C., Bennett P. H. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994 Apr 9;308(6934):942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. E., Brunner M. R., Duckworth W. C., Hooker C. S., Frank B. H. Receptor binding and biological potency of several split forms (conversion intermediates) of human proinsulin. Studies in cultured IM-9 lymphocytes and in vivo and in vitro in rats. J Biol Chem. 1985 Nov 15;260(26):13989–13994. [PubMed] [Google Scholar]
- Proudler A. J., Godsland I. F., Stevenson J. C. Insulin propeptides in conditions associated with insulin resistance in humans and their relevance to insulin measurements. Metabolism. 1994 Apr;43(4):446–449. doi: 10.1016/0026-0495(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Henry R., Schmeiser L., Kolterman O., Cohen R., Bergenstal R., Polonsky K., Jaspan J., Rubenstein A., Frank B. The effects of biosynthetic human proinsulin on carbohydrate metabolism. Diabetes. 1984 Aug;33(8):762–770. doi: 10.2337/diab.33.8.762. [DOI] [PubMed] [Google Scholar]
- Sobey W. J., Beer S. F., Carrington C. A., Clark P. M., Frank B. H., Gray I. P., Luzio S. D., Owens D. R., Schneider A. E., Siddle K. Sensitive and specific two-site immunoradiometric assays for human insulin, proinsulin, 65-66 split and 32-33 split proinsulins. Biochem J. 1989 Jun 1;260(2):535–541. doi: 10.1042/bj2600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Wilkin T. J. Early nutrition and diabetes mellitus. BMJ. 1993 Jan 30;306(6873):283–284. doi: 10.1136/bmj.306.6873.283. [DOI] [PMC free article] [PubMed] [Google Scholar]


