Abstract
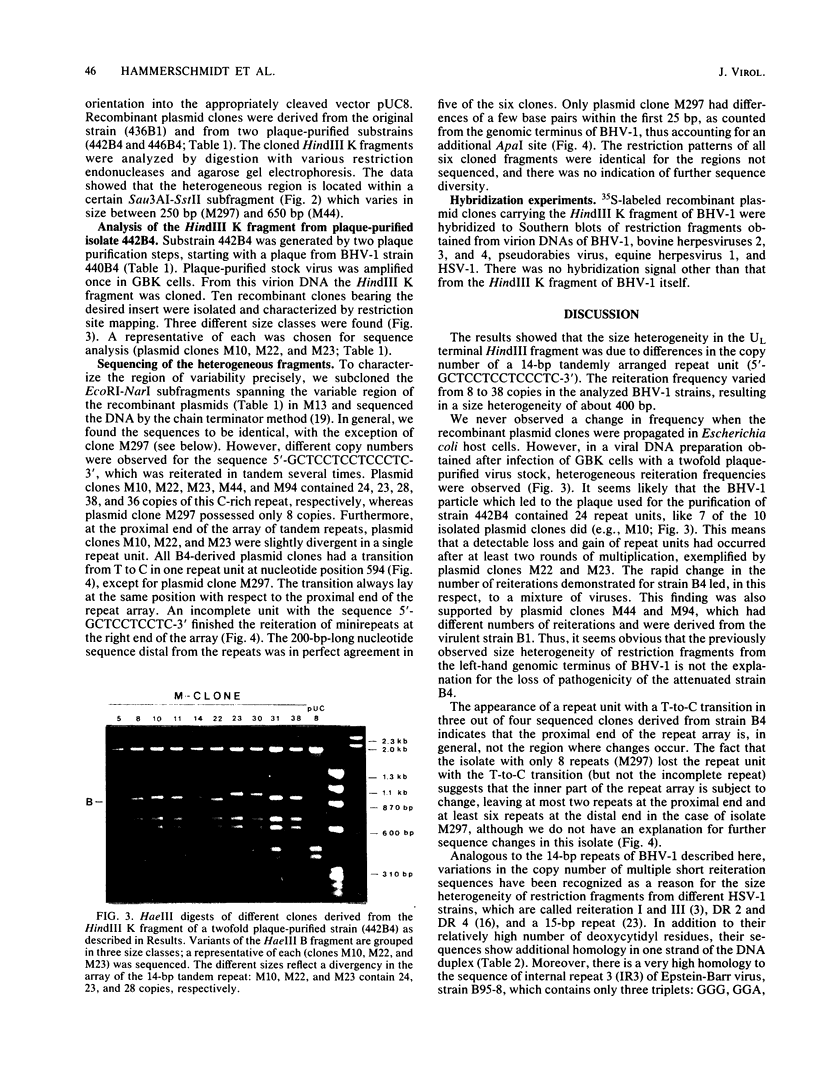
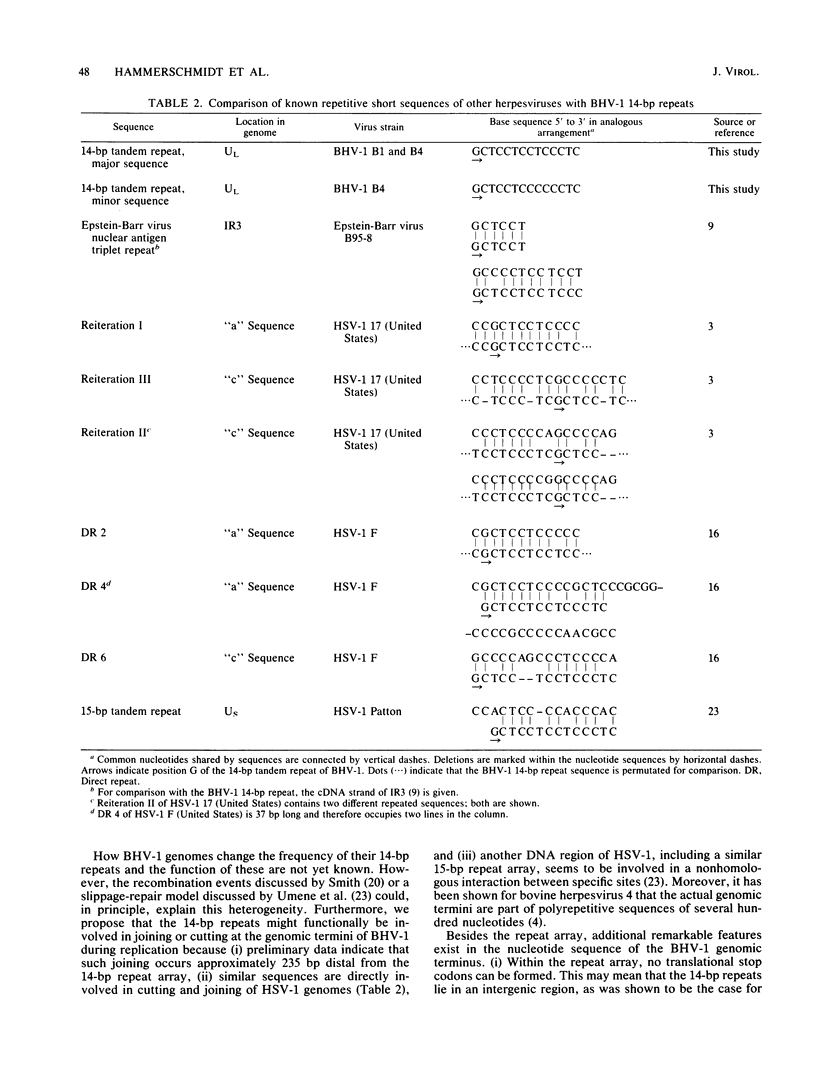
Analysis of the genomes of different bovine herpesvirus 1 strains revealed a UL terminal HindIII fragment differing in size (from 2.4 to 2.8 kilobases). This fragment polymorphism occurred in the DNA of a wild-type isolate, in highly passaged, apathogenic tissue culture derivatives, and in plaque-purified substrains. This heterogeneity was due to variations in the copy number of a 14-base-pair tandem repeat comprising the base sequence 5'-GCTCCTCCTCCCTC-3', which also exists, with some differences, in other short reiteration sequences of herpes simplex virus type 1, Epstein-Barr virus, and related human cellular DNA. Furthermore, the tandem repeat array was located in close proximity to the left end of the viral genome and may functionally be involved in viral replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Rixon F. J., Blankenship M. L. Analysis of the structure of the genome of pseudorabies virus. Virology. 1979 Jun;95(2):285–294. doi: 10.1016/0042-6822(79)90484-7. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Structure of the genome termini of varicella-zoster virus. J Gen Virol. 1984 Nov;65(Pt 11):1969–1977. doi: 10.1099/0022-1317-65-11-1969. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Ehlers B., Buhk H. J., Ludwig H. Analysis of bovine cytomegalovirus genome structure: cloning and mapping of the monomeric polyrepetitive DNA unit, and comparison of European and American strains. J Gen Virol. 1985 Jan;66(Pt 1):55–68. doi: 10.1099/0022-1317-66-1-55. [DOI] [PubMed] [Google Scholar]
- Engels M., Steck F., Wyler R. Comparison of the genomes of infectious bovine rhinotracheitis and infectious pustular vulvovaginitis virus strains by restriction endonuclease analysis. Arch Virol. 1981;67(2):169–174. doi: 10.1007/BF01318601. [DOI] [PubMed] [Google Scholar]
- Gregersen J. P., Pauli G., Ludwig H. Bovine herpesvirus 1: differentiation of IBR- and IPV-viruses and identification and functional role of their major immunogenic components. Arch Virol. 1985;84(1-2):91–103. doi: 10.1007/BF01310556. [DOI] [PubMed] [Google Scholar]
- Heller M., Flemington E., Kieff E., Deininger P. Repeat arrays in cellular DNA related to the Epstein-Barr virus IR3 repeat. Mol Cell Biol. 1985 Mar;5(3):457–465. doi: 10.1128/mcb.5.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., van Santen V., Kieff E. Simple repeat sequence in Epstein-Barr virus DNA is transcribed in latent and productive infections. J Virol. 1982 Oct;44(1):311–320. doi: 10.1128/jvi.44.1.311-320.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. E., Robinson R. A., Dauenhauer S. A., Atherton S. S., Hayward G. S., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 1. Virology. 1981 Nov;115(1):97–114. doi: 10.1016/0042-6822(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Good P. J., VanOort H. J., Campbell A. R., Reed D. E. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (Cooper strain). J Virol. 1983 Jul;47(1):259–264. doi: 10.1128/jvi.47.1.259-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Misra V., Babiuk L. A., Darcel C. L. Analysis of bovine herpes virus-type 1 isolates by restriction endonuclease fingerprinting. Arch Virol. 1983;76(4):341–354. doi: 10.1007/BF01311201. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982 Nov;31(1):89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan D. C., Atherton S. S., Staczek J., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 3. Virology. 1984 Jan 30;132(2):352–367. doi: 10.1016/0042-6822(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Umene K., Watson R. J., Enquist L. W. Tandem repeated DNA in an intergenic region of herpes simplex virus type 1 (Patton). Gene. 1984 Oct;30(1-3):33–39. doi: 10.1016/0378-1119(84)90102-1. [DOI] [PubMed] [Google Scholar]




