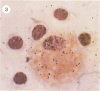
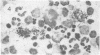
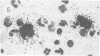
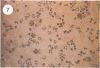
Abstract
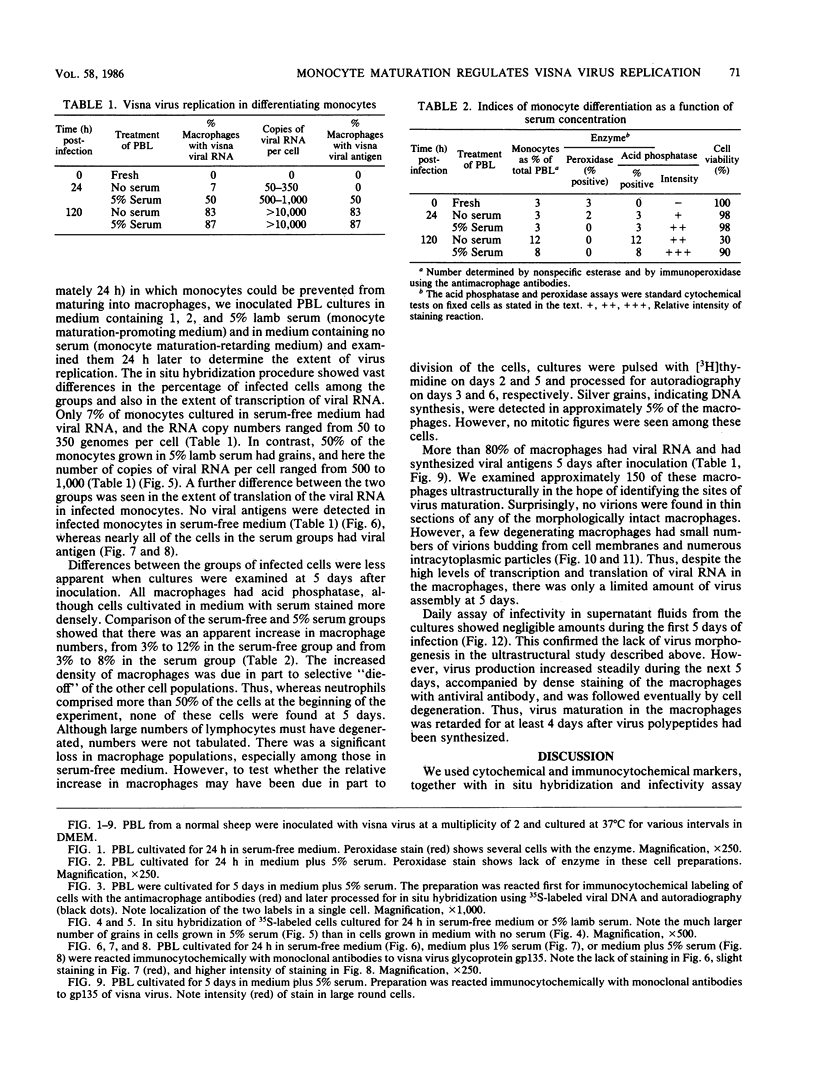
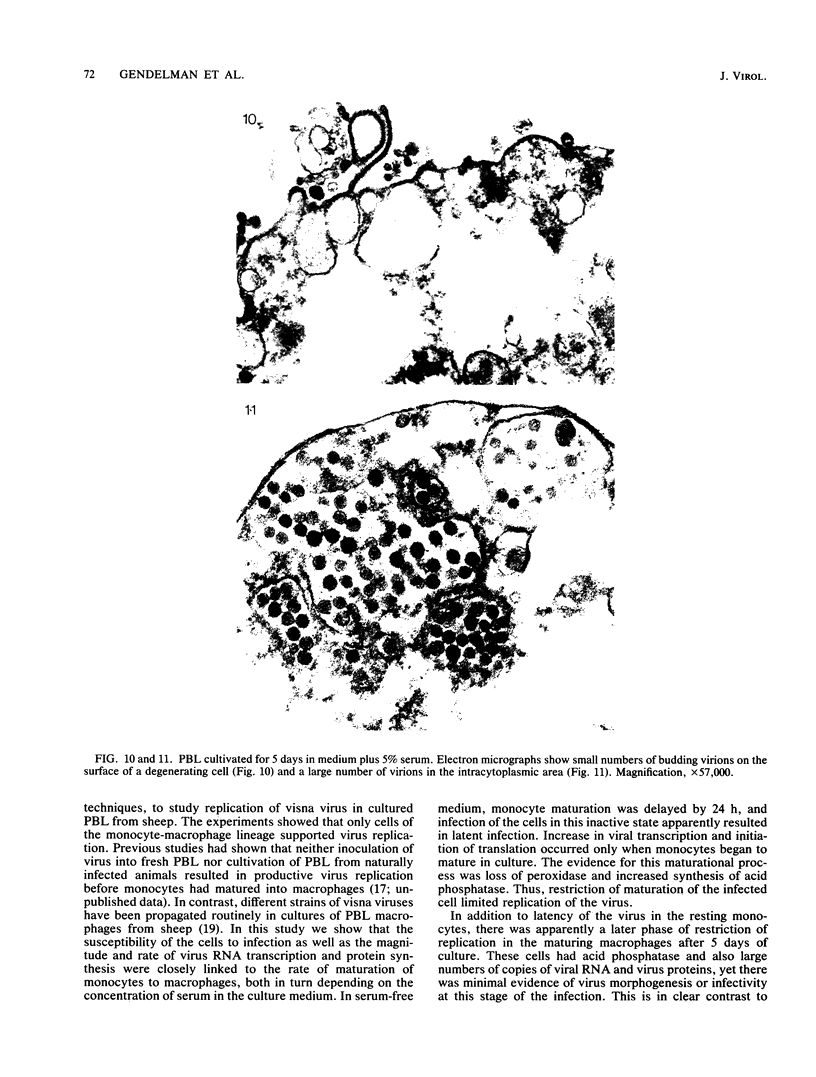
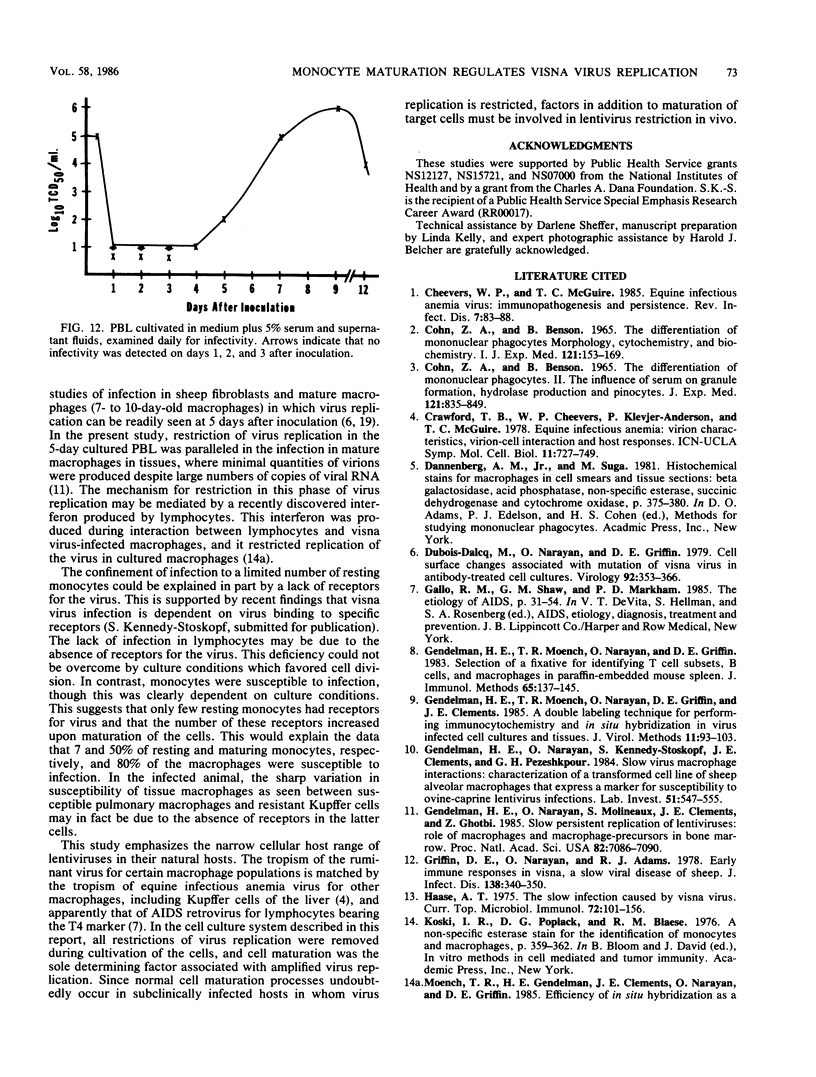
Visna lentiviruses have a natural tropism for cells of the macrophage lineage of sheep and goats, but virus replication in these cells in vivo is restricted so that only small quantities of virus are produced. One restricting factor suggested in previous studies is that virus replication is dependent on the maturity of the cells: the more mature the cell, the less restrictive the replication of the virus. Since monocytes in peripheral blood are precursors of macrophages, we investigated the effect of cell maturation on virus replication under limited control conditions in vitro by inoculating blood leukocytes with virus and retarding the maturation of monocytes to macrophages during cultivation in serum-free medium. Using enzyme markers that identified the cells in their resting monocytic stage (peroxidase) and mature macrophage stage (acid phosphatase) along with quantitative in situ hybridization and immunocytochemistry with viral reagents to trace the efficiency of virus replication, we correlated virus replication with cell maturation. Only a few monocytes were susceptible to infection, and virus replication did not extend beyond a low level of transcription of viral RNA. In the acid phosphatase-positive, maturing macrophage, susceptibility of the cells to infection was increased and virus replication was greatly amplified to the level of translation of viral polypeptides. However, virus maturation was delayed by 3 days until further cell maturation had occurred. Thus, the entire life cycle of the virus, from its attachment to the target cell to its maturation in the cell, was dependent on the level of maturation/differentiation of the monocytic cell.
Full text
PDF


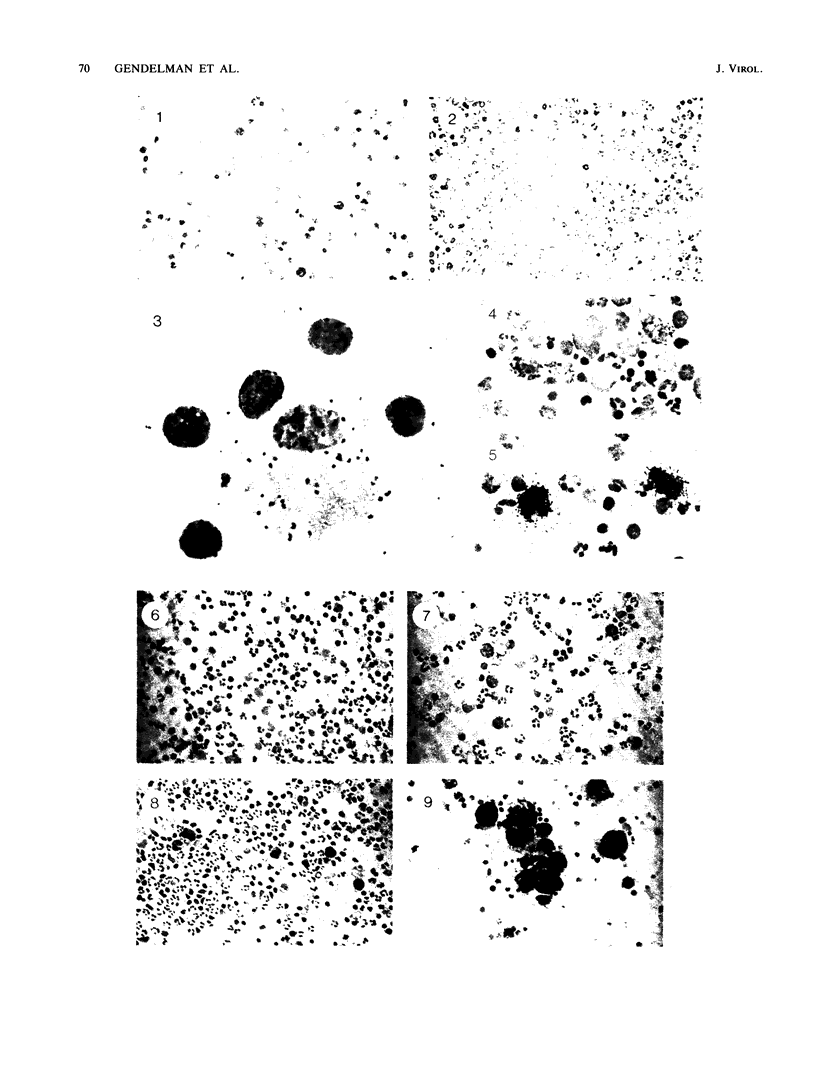

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. II. THE INFLUENCE OF SERUM ON GRANULE FORMATION, HYDROLASE PRODUCTION, AND PINOCYTOSIS. J Exp Med. 1965 May 1;121:835–848. doi: 10.1084/jem.121.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., McGuire T. C. Equine infectious anemia virus: immunopathogenesis and persistence. Rev Infect Dis. 1985 Jan-Feb;7(1):83–88. doi: 10.1093/clinids/7.1.83. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Narayan O., Griffin D. E. Cell surface changes associated with mutation of visna virus in antibody-treated cell cultures. Virology. 1979 Jan 30;92(2):353–366. doi: 10.1016/0042-6822(79)90140-5. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E., Clements J. E. A double labeling technique for performing immunocytochemistry and in situ hybridization in virus infected cell cultures and tissues. J Virol Methods. 1985 Jun;11(2):93–103. doi: 10.1016/0166-0934(85)90033-3. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E. Selection of a fixative for identifying T cell subsets, B cells, and macrophages in paraffin-embedded mouse spleen. J Immunol Methods. 1983 Dec 16;65(1-2):137–145. doi: 10.1016/0022-1759(83)90310-1. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Clements J. E., Pezeshkpour G. H. Slow virus-macrophage interactions. Characterization of a transformed cell line of sheep alveolar macrophages that express a marker for susceptibility to ovine-caprine lentivirus infections. Lab Invest. 1984 Nov;51(5):547–555. [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., Narayan O., Adams R. J. Early immune responses in visna, a slow viral disease of sheep. J Infect Dis. 1978 Sep;138(3):340–350. doi: 10.1093/infdis/138.3.340. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Sheffer D., Clements J. E., Tennekoon G. Restricted replication of lentiviruses. Visna viruses induce a unique interferon during interaction between lymphocytes and infected macrophages. J Exp Med. 1985 Dec 1;162(6):1954–1969. doi: 10.1084/jem.162.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Oliver R. E., Gorham J. R., Parish S. F., Hadlow W. J., Narayan O. Ovine progressive pneumonia: pathologic and virologic studies on the naturally occurring disease. Am J Vet Res. 1981 Sep;42(9):1554–1559. [PubMed] [Google Scholar]
- Stevenson H. C., Katz P., Wright D. G., Contreras T. J., Jemionek J. F., Hartwig V. M., Flor W. J., Fauci A. S. Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand J Immunol. 1981 Sep;14(3):243–256. doi: 10.1111/j.1365-3083.1981.tb00561.x. [DOI] [PubMed] [Google Scholar]