Abstract
Basal fat oxidation decreases with age. In obesity it is not known whether this age-related process occurs independently of changes in body composition and insulin sensitivity. Therefore, body composition, resting energy expenditure (REE), basal substrate oxidation, and maximal oxygen consumption (VO2max) were measured in ten older (age 60 ± 4 years; mean ± S.E.M.) and ten younger (age 35 ± 4 years) body mass index-matched, obese, normal glucose tolerant individuals. Fasting blood samples were also collected. Older subjects had slightly elevated fat mass (32.2 ± 7.1 vs. 36.5 ± 6.7 kg; P = 0.16), however waist circumference (WC) was not different between groups (104.3 ± 10.3 vs. 102.1 ± 12.6 cm; P = 0.65). Basal fat oxidation was 22% lower (1.42 ± 0.14 vs. 1.17 ± 0.22 mg/kg fat-free mass (FFM)/min; P = 0.03) in older subjects. VO2max was also decreased in older individuals (44.6 ± 7.1 vs. 38.3 ± 6.0 ml/kgFFM/min; P = 0.03), but neither insulin sensitivity, lipemia, nor leptinemia were different between groups (P > 0.05). Fat oxidation was most related to age (r = −0.61, P = 0.003) and VO2max (r = 0.52, P = 0.01). These data suggest that aging per se is responsible for reduced basal fat oxidation and maximal oxidative capacity in older obese individuals, independent of changes in insulin resistance, body mass, and abdominal fat. This indicates that age, in addition to obesity, is an independent risk factor for weight gain and for the metabolic complications of elevated body fat.
Keywords: aging, substrate oxidation, energy expenditure
1. Introduction
Aging and obesity are both associated with increased risk of developing type 2 diabetes (T2DM) and cardiovascular disease (CVD) [1, 2]. Obesity also tends to increase with advancing age [3], which may explain the association between age and such diseases. Impaired glucose tolerance and insulin resistance, which arises in obesity, is due in part to increased appearance of free fatty acids (FFA) and reduced rates of skeletal muscle fat utilization, leading to accumulation of intracellular fatty acid metabolites that interfere with insulin signaling pathways [4]. These metabolic defects are also more prevalent in older populations [3]. In addition, basal fat oxidation (Fox) is decreased in obese and insulin resistant individuals [5], and has been shown to deteriorate with age in cross-sectional studies of lean healthy individuals as a result of changes in body composition (increased fat mass and decreased fat-free mass (FFM)) [6–8]. These observations indicate that the impairments in substrate utilization that occur in obesity may be greater in older obese individuals, and therefore they may be more susceptible to the metabolic abnormalities associated with increased body fat. Skeletal muscle mitochondrial function and protein synthesis is impaired in older individuals [9, 10], and in obesity and insulin resistance [11, 12]; suggesting that in older groups, age per se may be responsible for alterations in substrate utilization at the mitochondrial level. Understanding age-related changes in oxidative capacity may be important to formulating optimal therapeutic strategies for older populations affected by abnormalities in substrate utilization. However, examination of the direct effects of aging alone requires control of several variables (e.g. physical activity, body mass, FFM, and gender). This has not been done effectively in previous studies. To our knowledge, the effects of aging per se on substrate utilization has not been fully examined in obese individuals. Therefore this study fills a gap in the literature by investigating the hypothesis that basal Fox is decreased in older obese individuals as a function of age alone. To examine the effects of age per se, we chose sedentary, normal glucose tolerant (NGT), and body mass index- (BMI) and gender-matched individuals to minimize the effects of physical activity, insulin resistance, body composition, and sex on substrate utilization measures.
2. Subjects and Methods
2.1 Subjects
Twenty obese (BMI > 30 kg/m2), normal glucose tolerant (fasting plasma glucose < 100 mg/dl), male and female volunteers were recruited from the general population. Volunteers were divided into two gender- and BMI-matched groups of ten younger (five male, five female, age 35 ± 4 years, BMI 32.1 ± 1.4 kg/m2; mean ± S.D.) and ten older (five male, five female, age 60 ± 4 years, BMI 31.0 ± 5.0 kg/m2) individuals. A medical screening was performed to exclude subjects with heart, kidney, liver, intestinal, and pulmonary diseases, or those taking medications for hypertension, diabetes, or other obesity-related conditions. During this screening, volunteers also underwent basal metabolic rate (BMR) measures via indirect calorimetry (as described in Section 2.3) to calculate their individual caloric requirements (using the Weir equation multiplied by a factor of 1.25 for sedentary lifestyle correction [13, 14]). All volunteers were sedentary (as assessed by PARQ and Minnesota Leisure Time Physical Activity questionnaires [15]), and had been weight stable for six months prior to the study. All young, female, pre-menopausal volunteers were studied during the early-follicular phase (days 1–5) of their menses in order to minimize the effects of reproductive hormones on metabolism. Dietary records were collected for three days prior to the study to monitor adequate nutrient intake, during which time subjects were requested to refrain from non-habitual exercise, and caffeinated and alcoholic beverages. The study was approved by the Institutional Review Board, and signed informed consent was obtained from all volunteers prior to commencing the study.
2.2 Body composition
Anthropometric data were measured using standard techniques. Height was measured using a stadiometer to the nearest 1.0 cm without shoes. Body weight was measured to the nearest 0.1 kg with the subject wearing their underclothing and a hospital gown. Waist circumference (WC) was measured midway between the lower rib margin and iliac crest in a horizontal position to the nearest 1.0 cm. Body density was determined by hydrostatic weighing after an overnight fast as previously described [16].Underwater weight was determined using electronic load cells, and residual lung volume was determined by open-circuit nitrogen washout. Percent body fat was calculated using Siri equations as previously described [16].
2.3 Metabolic Measurements
Study subjects were admitted to the inpatient ward of the General Clinical Research Centre the day prior to testing and fed a standardized diet (55% carbohydrate, 35% fat, 10% protein), the caloric load of which was calculated from each volunteer’s BMR measured during medical screening. Following a twelve hour overnight fast, subjects were awakened at 0600 hours and taken by wheelchair to void and to be weighed, and then reclined in a semi-darkened, thermoneutral (22 ± 1°C) environment under a clear plastic hood (Brooks Instruments, Hatfield, PA) for 30 mins for indirect calorimetry measurements [17]. Air was pulled through the hood at a rate of 50 l/min to maintain a slight negative pressure, allowing fresh air movement through the hood at all times. Continuous open-circuit exhaled gas was analyzed using Hartmann-Braun (Frankfurt, Germany) differential paramagnetic O2 (Magnos 4 G) and nondispersive infrared CO2 (Uras 4) analyzers. The analyzers were calibrated prior to the collection with known gas concentrations. The molar ratio of O2 consumed to CO2 produced was used to derive a measure (respiratory quotient, RQ) of the relative amounts of substrate that were being oxidized. Energy expenditure was calculated using equations of Weir [13], and substrate oxidation rates were calculated according to Frayn [18]. Timed urinary nitrogen excretion measurements (Roche Modular Diagnostics, Indianapolis, IN) were also made for estimates of protein oxidation rates [18].
2.4 Exercise capacity
Following the metabolic measurements, each subject performed an incremental treadmill exercise test to determine their maximal oxygen consumption (VO2max), a marker of maximal aerobic capacity. The test was performed at speeds between 2–4 mph and the incline of the treadmill was increased 2.5% every two minutes until fatigue. Exhaled air was collected through a two-way Hans-Rudolph mouthpiece into a FITGO mixing chamber. Concentrations of O2 and CO2 were measured using an electrochemical O2 analyzer (Applied Electrochemistry, S-3A) and infrared CO2 analyzer (Beckman LB-2), respectively. Subjects were considered to have reached their maximum aerobic capacity when at least two of the following three criteria were achieved: (i) a plateau in VO2, (ii) a heart rate within 10 beats/min of age-predicted maximum, and/or (iii) a respiratory exchange ratio > 1.0.
2.5 Blood Measurements
During the inpatient stay, a fasting blood sample was drawn from an antecubital vein for the measurement of triglycerides (TG), total cholesterol (T-Chol), glucose (FPG), insulin (FSI), and leptin. Two further samples were collected for glucose and insulin at ten minute intervals to calculate an insulin resistance index according to Matthews et al. (1985); see Equation 1 [19].
| [1] |
Where FPG represents fasting plasma glucose in mg/dl, and FSI is fasting serum insulin in µU/ml.
2.6 Statistical analysis
Statistical analyses were carried out using Statview (SAS Institute, NC, USA), and all data are expressed as mean ± S.D. All data comparisons in parentheses represent values for young versus old. Between-group (young vs. old, and male vs. female) comparisons for all variables were analyzed using one-way ANOVA and Fisher's least significant difference (LSD) post hoc test. No gender differences were found in any of the dependent variables. Bivariate correlations were used to examine the relationships between age and other measured variables, and between fat oxidation and other variables. Additionally, bivariate analyses were also applied to fat oxidation and fat mass in both young and old groups to assess fat balance. To examine the direct relationships of age on energy expenditure, fat oxidation, and maximal oxygen uptake, partial correlations were performed whilst controlling for FFM. Statistical significance was accepted at P < 0.05.
3. Results
3.1 Dietary Control
Analysis of the three day diet records showed no differences in caloric intake or macronutrient composition between groups prior to metabolic testing. In the younger vs. older groups, total caloric intake was 1862 ± 610 vs. 1933 ± 169 kcal (P = 0.83), percentage of calories from carbohydrate was 47 ± 10 vs. 43 ± 11 % (P = 0.45), percentage calories from fat was 35 ± 6 vs. 36 ± 9 % (P = 0.90), percentage calories from protein was 17 ± 6 vs. 19 ± 4 % (P = 0.56).
3.2 Body Composition
Demographic and body composition information are presented in Table 1. The age groups displayed no differences in BMI, body mass, fat mass, FFM, or WC (all P > 0.05). Percentage fat mass however, was elevated in older individuals (young vs. old; 34.3 ± 6.4 vs. 40.3 ± 7.4 %; P = 0.04), whilst percentage fat-free mass was reduced in the older group (65.7 ± 6.4 vs. 58.0 ± 9.0 %; P = 0.05).
Table 1.
Subject Characteristics
| Variable | YOUNGER | OLDER | P-value | |
|---|---|---|---|---|
| Age | years | 35 ± 4 | 60 ± 4 | <0.001 *** |
| Height | cm | 171 ± 9 | 172 ± 10 | 0.69 |
| Weight | Kg | 93.9 ± 115 | 91.4 ± 9.3 | 0.57 |
| BMI | Kg/m2 | 32.2 ± 7.1 | 31.0 ± 5.0 | 0.47 |
| FM | Kg | 32.2 ± 7.1 | 36.5 ± 6.7 | 0.16 |
| FFM | Kg | 61.8 ± 10.0 | 53.1 ± 11.0 | 0.07 |
| WC | cm | 104.3 ± 10.3 | 102.1 ± 12.6 | 0.65 |
Data are mean ± S.D. BMI is body mass index. FM is fat mass. FFM is fat-free mass. WC is waist circumference.
represents P < 0.001.
3.3 Indirect Calorimetry
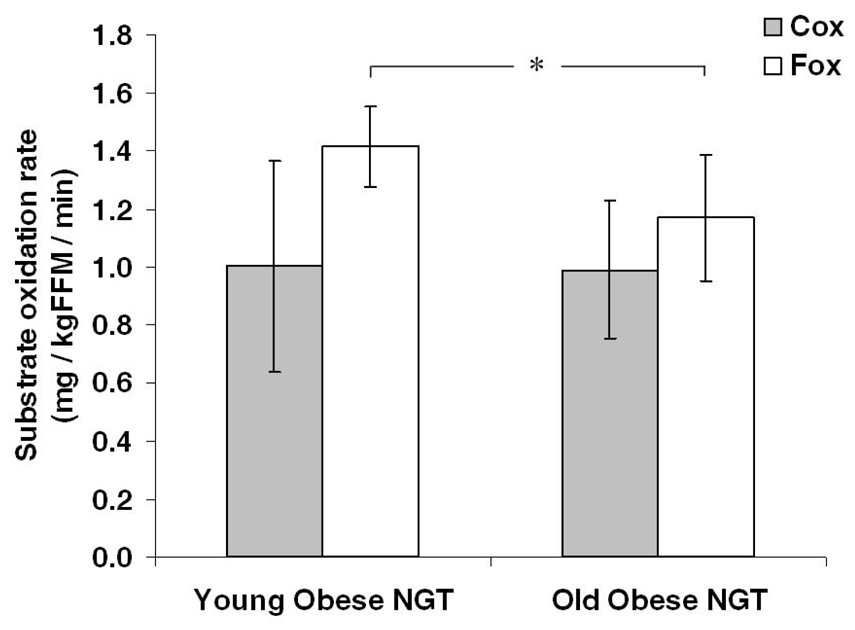
Figure 1 and Table 2 illustrate data from the indirect calorimetry measurements taken at rest following an overnight fast, plus data from the subjects' maximal exercise test. No differences in resting RQ (P = 0.12), carbohydrate oxidation (Cox; P = 0.96), protein oxidation (Pox; P = 0.39), or energy expenditure (REE) (P = 0.15) were noted between age groups. Basal Fox, expressed per unit of FFM, was significantly decreased in the older age group (−22.0 ± 7.8 %; P = 0.03), as was VO2max (−16.0 ± 4.7 %; P = 0.03) and maximal heart rate (HRmax; −12 ± 1.4 %; P = 0.0007) recorded during exhaustive exercise.
Fig. 1. Energy Metabolism.
Substrate oxidation rates were estimated by indirect calorimetry in two BMI- and gender-matched obese NGT age groups. Carbohydrate oxidation (Cox) was not different between younger and older individuals, however fat oxidation (Fox) was significantly reduced in the older cohort (*; P < 0.05). Error bars represent mean ± S.D.
Table 2.
Metabolic data
| Variable | YOUNGER | OLDER | P-value | |
|---|---|---|---|---|
| RQ | 0.77 ± 0.02 | 0.79 ± 0.02 | 0.12 | |
| REE | Kcal/KgFFM/min | 0.021 ± 0.002 | 0.019 ± 0.002 | 0.15 |
| Cox | mg/KgFFM/min | 1.00 ± 0.36 | 0.99 ± 0.24 | 0.96 |
| Fox | mg/KgFFM/min | 1.42 ± 0.14 | 1.17 ± 0.22 | 0.03 * |
| Pox | mg/KgFFM/min | 0.76 ± 0.22 | 0.66 ± 0.29 | 0.39 |
| VO2max | mg/KgFFM/min | 44.6 ± 7.1 | 38.3 ± 6.0 | 0.03 * |
| HRmax | beast/min | 182 ± 10 | 163 ± 14 | <0.001 *** |
Data are mean ± S.D. Younger, n = 10; Older, n = 10. RQ is respiratory quotient. REE is resting energy expenditure. Cox is basal carbohydrate oxidation. Fox is basal fat oxidation. Pox is basal protein oxidation. VO2max is maximal oxygen uptake during exhaustive exercise. HRmax is maximum heart rate recorded during exhaustive exercise.
represents P < 0.05
represents P < 0.001.
3.4 Blood Measurements
Changes in blood chemistry with age are shown in Table 3. Measures of FPG confirmed the subjects’ normal fasting glucose tolerance (< 100 mg/dl). No differences in insulin resistance, TG, T-Chol, or leptin were seen between younger and older individuals (all comparisons P > 0.05).
Table 3.
Blood chemistry
| Variable | YOUNGER | OLDER | P-value | |
|---|---|---|---|---|
| FPG | mg/dl | 94.0 ± 17.8 | 95.5 ± 5.8 | 0.70 |
| FSI | µU/ml | 12.8 ± 5.9 | 15.2 ± 4.3 | 0.21 |
| HOMA-IR | 3.03 ± 1.88 | 3.61 ± 1.10 | 0.33 | |
| TG | mg/dl | 139.2 ± 56.4 | 145.2 ± 69.5 | 0.49 |
| T-Chol | mg/dl | 200.8 ± 46.2 | 183.6 ± 41.6 | 0.76 |
| Leptin | mg/dl | 23.0 ± 15.2 | 24.5 ± 15.5 | 0.98 |
Data are mean ± S.D. FPG is fasting plasma glucose; divide mg/dl by 18 to convert to mmol/l. FSI is fasting serum insulin. HOMA-IR is insulin resistance according to Matthews et al. (1985). TG is triglycerides. T-Chol is total cholesterol.
3.5 Correlation analyses
To investigate the association between age and other measured variables, bivariate correlations were performed. Table 4 shows that basal Fox (P < 0.01), VO2max (P < 0.01), and HRmax (P < 0.001) each showed a significant inverse association with age. The significant relationships between age and basal Fox, and age and VO2max persisted when the effect of FFM was controlled for (partial correlation analysis): age versus basal Fox, r = −0.58, P = 0.007; age versus VO2max, r = −0.44, P = 0.04. Table 5 demonstrates that of the variables previously documented to affect fat utilization, age and VO2max were the best predictors of basal Fox in our obese cohorts (r = −0.61, P = 0.003; and r = 0.52, P = 0.01, respectively). A bivariate analysis was also performed to assess the relationship of fat mass and basal Fox. No association between fat mass and basal fat oxidation was found in either group: younger, r = 0.40, P = 0.20; older, r = −0.29, P = 0.45.
Table 4.
Bivariate correlations between age and other variables
| Age vs. | r-value | P-value |
|---|---|---|
| Weight | −0.11 | 0.61 |
| BMI | −0.06 | 0.80 |
| FFM | −0.41 | 0.06 |
| FM | 0.32 | 0.14 |
| WC | −0.07 | 0.75 |
| REE | −0.40 | 0.07 |
| RQ | 0.38 | 0.09 |
| Cox | −0.05 | 0.92 |
| Fox | −0.61 | 0.003 ** |
| Pox | −0.27 | 0.260 |
| VO2max | −0.57 | 0.004 ** |
| HRmax | −0.73 | 0.0001 *** |
| FPG | −0.01 | 0.98 |
| FSI | −0.02 | 0.47 |
| HOMA-IR | −0.05 | 0.38 |
| TG | −0.15 | 0.50 |
| T-Chol | −0.18 | 0.42 |
| Leptin | −0.01 | 0.09 |
Abbreviations and units of measurement follow the same legend as for previous tables.
represents P < 0.01
represents P < 0.001.
Table 5.
Bivariate correlations between fat oxidation and other variables
| Fox vs. | r-value | P-value |
|---|---|---|
| Age | −0.61 | 0.003 ** |
| Weight | −0.31 | 0.17 |
| BMI | −0.37 | 0.10 |
| FFM | −0.14 | 0.57 |
| FM | −0.22 | 0.34 |
| WC | −0.41 | 0.07 |
| REE | 0.65 | 0.001 ** |
| VO2max | 0.52 | 0.01 * |
| FPG | −0.25 | 0.28 |
| FSI | −0.04 | 0.89 |
| HOMA-IR | −0.06 | 0.84 |
| TG | −0.04 | 0.88 |
| T-Chol | 0.07 | 0.75 |
| Leptin | 0.19 | 0.42 |
Abbreviations and units of measurement follow the same legend as for previous tables.
represents P < 0.05
represents P < 0.01.
4. Discussion
This study provides preliminary evidence that basal fat oxidation is reduced in older obese NGT individuals as a function of age per se. In addition, it was shown that aerobic fitness, as indicated by reduced maximal oxygen uptake, also declines with age. These metabolic alterations were independent of absolute differences in body composition, insulin sensitivity, fasting lipemia, and leptinemia, physiological components that are related to substrate oxidation. It was also demonstrated that in obese NGT humans, basal fat oxidation is not related to body fat mass in either younger or older individuals.
Obesity is a determinant of decreased fat oxidative capacity in the basal state [5]; however, increasing age may also be an independent factor associated with impairment in substrate oxidation. Fat-free mass, which represents numerous metabolically-active tissues (skin, bone, muscle) and organs (heart, liver, kidney, brain), is thought to be a major determinant of energy expenditure and fat utilization in lean healthy humans [20]. Several studies have indicated that FFM, and indeed muscle mass, decline with age [21], therefore, it is reasonable to assume that this may be responsible for the reduction in REE and Fox seen in older populations. Previous studies investigating the effects of age on energy expenditure and fat utilization have focused on differences in lean healthy individuals [22–24]; to our knowledge, no studies have investigated age-related variation in obesity whilst ruling out the effects of physical activity, body composition, insulin sensitivity, lipemia, and leptinemia, factors independently associated with substrate oxidation. To assess the effects of age per se, it is imperative that factors associated with substrate utilization be controlled. In this study, we chose two BMI-matched, sedentary, NGT groups, representative of a younger and an older obese population, who were similar with respect to body composition (including WC, a marker of abdominal adiposity), insulin resistance (HOMA-IR), leptinemia, and lipemia (TG and T-Chol). Under such conditions, despite a trend toward lower FFM (Table 1; P = 0.07), we have demonstrated that basal Fox is significantly lower in older obese individuals when expressed as a function of FFM, demonstrating a direct impact of aging on substrate utilization. Interpreting this finding with the knowledge that fat utilization is impaired in obesity indicates that increasing age plus elevated fat mass may have an additive detrimental effect on metabolic substrate use. Changes in substrate utilization may be explained by substrate availability, yet previous publications have failed to show a difference in serum FFA concentrations between young and old BMI-matched groups [22, 25], reducing the likelihood that this theory is correct. In addition, reduced tissue perfusion may also explain reduced substrate utilization, as obesity and insulin resistance are related to impaired capillary perfusion [26, 27]. Yet, in this study, no differences in Cox were found, suggesting that the cause of the impairment in Fox may lie downstream of tissue substrate availability and uptake. An age-related impairment in substrate oxidative capacity, as a result of reduced mitochondrial fatty acid uptake or respiratory chain dysfunction, is perhaps more likely. Rimbert et al. (2004) demonstrated that skeletal muscle carnitine palmitoyltranferase 1 (CPT1) activity is unchanged in older lean individuals [24], illustrating that long chain fatty acid uptake into the mitochondria is not age-related; however, the same group also noted that citrate synthase (CS) activity was decreased in their older cohort, indicating reduced mitochondrial density and thus reduced total oxidative capacity. No similar data exist in obese older groups. Further to this, Conley et al. (2000), Boffoli et al. (1994), Short et al. (2005), and Trounce et al. (1989) have shown that mitochondrial complex activity and oxidative capacity are reduced in skeletal muscle of elderly individuals [28–31]. In addition, reductions in skeletal muscle mitochondrial protein synthesis have also been documented in older groups [32]. These alterations in mitochondrial function in older individuals are likely attributed to accumulative oxidative damage by reactive oxygen species [30, 33, 34], and indicate a probable cause of age-related impairment in substrate oxidation. Such explanations of the current data set are, however, speculative, and further work is required. Additionally, age- and gender-related differences in fat oxidation, whilst not significant here (one-way ANOVA: age*gender interaction, P = 0.40), should be investigated in future studies of larger cohorts. The absence of an effect of gender was also true for all other variables (all P > 0.05).
Maximal oxygen consumption (or aerobic fitness; VO2max) is an additional marker of mitochondrial function. Aerobic fitness has been shown to decline with age [24], but this is most likely attributed to the general decrease in physical activity levels and maximal cardiac output seen in the older population [20]. In our study, in order to minimize the effects of physical activity, we chose only sedentary subjects, and yet VO2max was reduced in the older cohort when expressed as a function of FFM. Maximal oxygen uptake is largely related to oxygen exchange capacity, tissue perfusion capacity, and mitochondrial capacity [35], and improvements in VO2max are associated with increased skeletal muscle mitochondrial concentration and capillarity [36]. Our finding that maximal oxygen consumption is impaired in older individuals, adds to the evidence that mitochondrial dysfunction may be a key component in the aging-related dysregulation of substrate oxidation.
Leptin, an adipocytokine that circulates in the blood in relation to fat mass, is also a marker of fat balance and energy metabolism [37]. Previous studies demonstrate that leptin directly stimulates fatty acid oxidation in skeletal muscle via an adenosine monophosphate-activated protein kinase (AMPK) pathway, activating acetyl coenzyme A carboxylase (ACC), an enzyme directly involved in mitochondrial long chain fatty acid uptake [38]. However, whilst these findings are prominent in lean individuals, it appears that in obesity, where high circulating concentrations are found, a state of leptin resistance occurs [39]. In this study we demonstrated elevated leptin concentrations in our obese cohort similar to those documented in previous reports [39], yet we found no differences between BMI-matched younger and older individuals. This suggests that, with age, either leptin production or leptin sensitivity is unchanged, and therefore leptin’s direct effects on fat oxidative capacity via cytosolic enzymes in skeletal muscle are also not altered. This adds to the hypothesis that changes in substrate utilization in older individuals are probably dictated by factors downstream of fatty acid uptake and availability, i.e. mitochondrial dysfunction.
Our correlation analyses indicate that age is related to basal Fox (P = 0.003) and maximal exercise capacity (VO2max; P = 0.004) in obese individuals. These relationships persisted when the effect of FFM was controlled, suggesting a direct involvement of aging on differences in substrate oxidative capacity. Our data also demonstrate that basal Fox is best predicted by age, maximal exercise capacity, and resting energy expenditure in obese individuals, emphasizing the effects of age per se, and highlighting the probable implication of mitochondrial function. Investigations of energy expenditure and Fox in lean healthy groups indicate that older individuals are more susceptible to weight gain [6–8, 40], but that Fox appears to be related to the level of body fat mass, indicating an adaptive oxidative response to fat intake in lean individuals [8, 41]. In this study, no relationships were demonstrated between Fox and fat mass, indicating that in obesity, there maybe a loss of lipolytic homeostasis in relation to body composition. Further to this, fat oxidation and fat mass appear to be less related in our older cohort, again demonstrating an effect of age per se on the loss of adaptive fat oxidation with increases in fat mass.
Obesity is associated with several metabolic disturbances (e.g. dyslipidemia, glucose intolerance, and hypertension) that increase the risk of developing macrovascular complications. It is known that obesity is related to decreased fat oxidative capacity, and our current data indicate that basal Fox is reduced in older obese individuals as a function of age per se whilst controlling for physical activity, glucose tolerance, and body composition. This indicates that older obese individuals may be more susceptible to the metabolic disturbances caused by increased body fat. Given the small size of the study groups in this investigation, the findings should be treated as preliminary data until further work using large cohorts and greater statistical power has been carried out. Nonetheless, this work creates an interesting direction for metabolic research. Future work should attempt to identify the mechanisms at play, and should focus on age-related differences in tissue perfusion, fatty acid transport, and mitochondrial function in particular, to improve the understanding of disease onset in the aged, and to identify therapeutic targets for “at risk” individuals.
Acknowledgments
We wish to thank the nursing/dietary staff of the General Clinical Research Center and the research participants for their cooperation and commitment. This research was supported by NIH grants AG12834, RR10732, RR00080, and RR018390; and the Diabetes Association of Greater Cleveland (467-R-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PW, Morrato EH, Ghushchyan V, et al. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28:1599–1603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Krebs M, Roden M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes Obes Metab. 2005;7:621–632. doi: 10.1111/j.1463-1326.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 6.Melanson KJ, Saltzman E, Russell RR, et al. Fat oxidation in response to four graded energy challenges in younger and older women. Am J Clin Nutr. 1997;66:860–866. doi: 10.1093/ajcn/66.4.860. [DOI] [PubMed] [Google Scholar]
- 7.Nagy TR, Goran MI, Weinsier RL, et al. Determinants of basal fat oxidation in healthy Caucasians. J Appl Physiol. 1996;80:1743–1748. doi: 10.1152/jappl.1996.80.5.1743. [DOI] [PubMed] [Google Scholar]
- 8.Horber FF, Gruber B, Thomi F, et al. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13:524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CK, Allison DB, Brand J, et al. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley DE. Skeletal muscle triglycerides: An aspect of regional adiposity and insulin resistance. Ann N Y Acad Sci. 2002;967:135–145. [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Befroy D, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks GA, Butte NF, Rand WM, et al. Chronicle of the Institute of Medicine physical activity recommendation: how a physical activity recommendation came to be among dietary recommendations. Am J Clin Nutr. 2004;79:921S–930S. doi: 10.1093/ajcn/79.5.921S. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary VB, Marchetti CM, Krishnan RK, et al. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson DL, Kirwan JP. A single bout of concentric resistance exercise increases basal metabolic rate 48 hours after exercise in healthy 59-77-year-old men. J Gerontol A Biol Sci Med Sci. 1997;52:M352–M355. doi: 10.1093/gerona/52a.6.m352. [DOI] [PubMed] [Google Scholar]
- 18.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetes. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Poehlman ET. Energy expenditure and requirements in aging humans. J Nutr. 1992;122:2057–2065. doi: 10.1093/jn/122.11.2057. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 22.Calles-Escandon J, Arciero P, Gardner AW, et al. Basal fat oxidation decreases with aging in women. J Appl Physiol. 1995;78:266–271. doi: 10.1152/jappl.1995.78.1.266. [DOI] [PubMed] [Google Scholar]
- 23.Toth MJ, Arciero PJ, Gardner AW, et al. Rates of free fatty acid appearance and fat oxidation in healthy younger and older men. J Appl Physiol. 1996;80:506–511. doi: 10.1152/jappl.1996.80.2.506. [DOI] [PubMed] [Google Scholar]
- 24.Rimbert V, Boirie Y, Bedu M, et al. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18:737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- 25.Hagenfeldt L, Wahren J, Pernow B, et al. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest. 1972;51:2324–2330. doi: 10.1172/JCI107043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jongh RT, Serne EH, Ijzerman RG, et al. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–2882. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- 27.Gudbjornsdottir S, Sjostrand M, Strindberg L, et al. Decreased muscle capillary permeability surface area in type 2 diabetic subjects. J Clin Endocrinol Metab. 2005;90:1078–1082. doi: 10.1210/jc.2004-0947. [DOI] [PubMed] [Google Scholar]
- 28.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boffoli D, Scacco SC, Vergari R, et al. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226:73–82. doi: 10.1016/0925-4439(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 30.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 32.Rooyackers OE, Adey DB, Ades PA, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 35.Terjung RL, Zarzeczny R, Yang HT. Muscle blood flow and mitochondrial function: influence of aging. Int J Sport Nutr Exerc Metab. 2002;12:368–378. doi: 10.1123/ijsnem.12.3.368. [DOI] [PubMed] [Google Scholar]
- 36.Hoppeler H, Weibel ER. Limits for oxygen and substrate transport in mammals. J Exp Biol. 1998;201:1051–1064. doi: 10.1242/jeb.201.8.1051. [DOI] [PubMed] [Google Scholar]
- 37.Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: focus on adiponectin. Curr Opin Pharmacol. 2006;6:580–585. doi: 10.1016/j.coph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg GR, Parolin ML, Heigenhauser GJ, et al. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab. 2002;283:E187–E192. doi: 10.1152/ajpendo.00542.2001. [DOI] [PubMed] [Google Scholar]
- 40.Aberg W, Thorne A, Olivecrona T, et al. Fat oxidation and plasma removal capacity of an intravenous fat emulsion in elderly and young men. Nutrition. 2006;22:738–743. doi: 10.1016/j.nut.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Schutz Y, Tremblay A, Weinsier RL, et al. Role of fat oxidation in the long-term stabilization of body weight in obese women. Am J Clin Nutr. 1992;55:670–674. doi: 10.1093/ajcn/55.3.670. [DOI] [PubMed] [Google Scholar]