Abstract
In mammals, light input from the retina entrains central circadian oscillators located in the suprachiasmatic nuclei (SCN). The phase of circadian activity rhythms with respect to the external light:dark cycle is reversed in diurnal and nocturnal species, although the phase of SCN rhythms relative to the light cycle remains unchanged. Neural mechanisms downstream from the SCN are therefore believed to determine diurnality or nocturnality. Here, we report a switch from nocturnal to diurnal entrainment of circadian activity rhythms in double-knockout mice lacking the inner-retinal photopigment melanopsin (OPN4) and RPE65, a key protein used in retinal chromophore recycling. These mice retained only a small amount of rod function. The change in entrainment phase of Rpe65−/−;Opn4−/− mice was accompanied by a reversal of the rhythm of clock gene expression in the SCN and a reversal in acute masking effects of both light and darkness on activity, suggesting that the nocturnal to diurnal switch is due to a change in the neural response to light upstream from the SCN. A switch from nocturnal to diurnal activity rhythms was also found in wild-type mice transferred from standard intensity light:dark cycles to light:dark cycles in which the intensity of the light phase was reduced to scotopic levels. These results reveal a novel mechanism by which changes in retinal input can mediate acute temporal-niche switching.
Keywords: circadian, diurnality, melanopsin, retina, Rpe65
In mammals, the external light:dark cycle is the predominant environmental cue that synchronizes the central circadian clock in the suprachiasmatic nuclei (SCN) of the hypothalamus to the 24-h solar day. The temporal niche (nocturnal, diurnal, crepuscular, etc.) occupied by an animal reflects the phase relationship between activity and the synchronized clock. Several fundamental features of the relationship of the SCN to light, such as the association between the timing of resetting light pulses and phase changes in the clock (i.e., phase-response curves), are very similar among mammalian species regardless of their temporal niche (1, 2). Daily rhythms of SCN electrical activity, metabolism, and expression of genes that comprise the core molecular clock mechanism are also similarly timed with respect to the external light:dark cycle in both nocturnal and diurnal mammals [for review, see Smale et al. (3)]. These similarities have led to the widely accepted idea that temporal niche is not controlled through pathways that communicate light information to the clock or by the response of the clock to light, but rather by neural mechanisms downstream from the SCN.
However, temporal-niche switching has recently been reported in mouse models of photoreceptor dysfunction, suggesting that input pathways may play a more important role in determining temporal niche than was previously believed (4–7). Retinal rods, cones, and intrinsically photosensitive retinal ganglion cells containing the photopigment melanopsin (OPN4) all provide light information for mammalian photo-entrainment (8, 9). We have previously reported a robust temporal-niche-switching phenotype in Rpe65−/−;Opn4−/− mice with targeted deletion of both melanopsin and RPE65, the retinoid isomerohydrolase essential for recycling the 11-cis-retinaldehyde chromophore of rods and cones (7). Rpe65−/−;Opn4−/− mice show either diurnal or free-running phenotypes, with the majority of animals (≈80%) displaying a diurnal activity pattern in 12-h-light:12-h-dark (LD12:12) cycles of 150 μW/cm2 (7).
The sensitivity of the rod system in Rpe65−/− mice is decreased by more than three log units (10–12) and cone function is believed to be completely lost (10, 13, 14). In the absence of melanopsin, the remaining light input to the circadian system of Rpe65−/−;Opn4−/− mice is most likely mediated by highly insensitive rods. Here, we characterized circadian and masking behaviors of Rpe65−/−;Opn4−/− mice and tested a possible nonphotic mechanism for temporal-niche switching in these animals. Using Rpe65−/−;Opn4−/− mice carrying the mPer2luciferase knockin reporter (15), we demonstrate a reversal in the phase relationship between SCN clock gene expression in the SCN and the light cycle. We further show that in wild-type mice, dim light:dark conditions that should mimic the rod-only retinal phenotype of Rpe65−/−;Opn4−/− mice lead to diurnal entrainment. Our data suggest that the temporal-niche switching in these two models results from channeling light through rod pathways and raises the possibility that intensity-dependent alterations in retinal light signaling may be a widespread mechanism for acutely regulating temporal niche.
Results
Reentrainment, Short Photoperiod Response, and Free-Running Period.
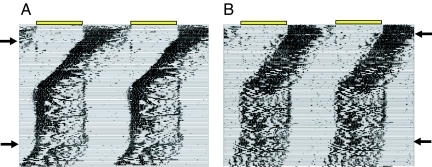
When released into constant darkness (DD), Rpe65−/−;Opn4−/− mice with diurnal activity patterns free-ran from the time of lights-on, indicating that their circadian clock was diurnally entrained (7). Clock entrainment in Rpe65−/−;Opn4−/− mice was also seen when animals with diurnal activity patterns were subjected to a 4-h delay of the light:dark cycle (Fig. 1A). A slow reentrainment (43.8 ± 7 days, n = 6) of the circadian activity rhythm to the shifted light:dark cycle was apparent as activity onset moved from the beginning of the original light phase to the shifted light phase.
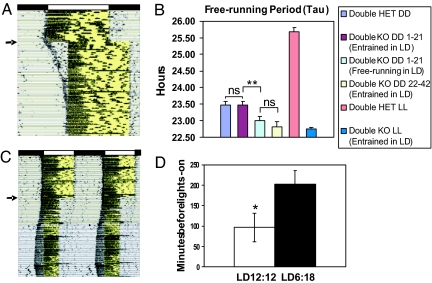
Fig. 1.
Entrainment and free-running periods of Rpe65−/−;Opn4−/− mice. (A) Wheel-running record of a representative Rpe65−/−;Opn4−/− mouse housed for 19 days in a LD12:12 cycle before receiving a 4-h phase delay of the light:dark cycle (the arrow indicates the day of delay). Each horizontal line represents 24 h, with subsequent daily records plotted beneath each other. The original LD12:12 cycle is indicated by black (dark) and white (light) bars at the top of the record, and the light phase is highlighted in yellow within the activity record. Note the slow reentrainment and the immediate positive masking response. (B) Analysis of free-running periods in DD or LL for Rpe65−/−;Opn4−/− (Double KO) and Rpe65+/−;Opn4+/− (Double HET) mice. The free-running period was calculated over days 1–21 or days 22–42 in DD from animals previously entrained (entrained in LD) or free-running (free-running in LD) for 4 weeks in a LD12:12 cycle. The free-running period in LL was calculated over days 1–21 in LL after transfer from DD ns, not significantly different. **, P < 0.01. ANOVA, Tukey's post hoc comparison. (C) Running-wheel activity record of a representative Rpe65−/−;Opn4−/− mouse maintained in a LD12:12 cycle for 40 days before being exposed to a short day photoperiod of 8-h-light:16-h-dark for 100 days. The arrow indicates the day of light cycle change. The record is double plotted so that each horizontal line represents 48 h, and successive days are shown to the right and beneath the previous day. The light:dark cycle is the same as that in A. (D) Histograms showing mean number of minutes (±SEM) by which activity onset preceded lights-on in Rpe65−/−;Opn4−/− mice (n = 7) in LD12:12 (open bar) or LD6:18 (filled bar). *, P < 0.05, Student's t test. Activity onsets were calculated over the entire 40-day period in LD12:12 and the 100-day period in LD6:18.
Because differences in free-running period length (τ) may affect the ability of Rpe65−/−;Opn4−/− mice to entrain to light:dark cycles (16), we compared τ in DD in Rpe65−/−;Opn4−/− and Rpe65+/−;Opn4+/− mice that were entrained in a light:dark cycle and in Rpe65−/−;Opn4−/− mice that free-ran throughout the light:dark cycle (Fig. 1B). τ over days 1–21 in DD did not differ between nocturnal double-heterozygous controls and diurnally entrained double knockouts (23.53 ± 0.10 h, n = 6 vs. 23.47 ± 0.11 h, n = 8), suggesting that clock function was normal in the double-knockout mice. However, τ in Rpe65−/−;Opn4−/− mice that free-ran through the light:dark cycle was significantly shorter than that of the entrained groups (23.00 ± 0.13 h, n = 10 vs. 23.47 ± 0.11 h, n = 8; P < 0.005 ANOVA, Tukey's post hoc comparison). Because after-effects of entrainment may be responsible for the longer τ observed in the entrained groups, we also measured τ from diurnally entrained Rpe65−/−;Opn4−/− mice maintained in DD for >3 weeks. τ of mice in 22–42 h DD (22.82 ± 0.16 h, n = 4) did not differ from that observed in Rpe65−/−;Opn4−/− mice that free-ran in light:dark cycles (LD). The similarity in the free-running period of these two groups suggests that τ of double knockouts that free-ran throughout the light:dark cycle is not intrinsically different from that of diurnally entrained double-knockout mice once the after-effects of entrainment wore off. Upon transfer from DD to constant light (LL), Rpe65−/−;Opn4−/− mice (n = 4) showed an absence of any free-running period change, whereas robust period lengthening was observed in nocturnal double-heterozygous controls (n = 4) (Fig. 1B). The amplitude of rhythmicity in LL, measured over a period of 30 days, was also greater for double knockouts than for double heterozygotes (fast Fourier transform relative power: 0.06 ± 0.01, n = 6 vs. 0.02 ± 0.009, n = 5; P < 0.05).
Because of the lack of RPE65, the retinoid isomerohydrolase critical for chromophore regeneration, the remaining functional photoreceptors in the Rpe65−/−;Opn4−/− retina may bleach rapidly in the presence of light, producing the neural equivalent of one pulse of light every day instead of a full 12-h photophase. In mice, a single pulse of light given once a day will entrain circadian activity rhythms such that the beginning of daily activity coincides roughly with the light pulse (16). To test the possibility that this type of abnormal photoreceptor response to light may produce the observed diurnal phenotype, we transferred Rpe65−/−;Opn4−/− mice from a LD12:12 cycle into a short-day photoperiod of 6-h-light:18-h-dark (LD6:18) (Fig. 1C). Under these conditions, photoperiodic changes in circadian activity rhythms were observed. The phase of activity onset became significantly earlier with lights-on compared to LD12:12 (96.4 ± 34 min before lights-on in LD12:12 vs. 203.2 ± 32 min in LD6:18; P < 0.05, t test, n = 7) (Fig. 1 C and D). Because the time of lights-on remained the same, these results indicate that Rpe65−/−;Opn4−/− mice detect the duration, and not simply the onset, of the light phase. They further suggest that these mice are able to regenerate chromophore through an RPE65- and melanopsin-independent mechanism.
Masking to Light and Dark Pulses.
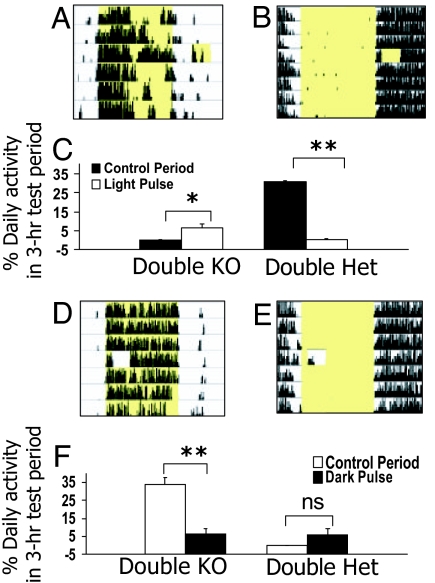
In addition to its effect in entraining the circadian oscillator, light can also have direct stimulatory or suppressive effects on activity. These have been termed “masking” effects because although they do not entrain the clock, they can obscure (or mask) its true phase. In nocturnal species such as the mouse, light normally inhibits activity, whereas darkness stimulates it. To determine whether masking responses might be reversed in Rpe65−/−;Opn4−/− mice, we tested masking to 3-h pulses of both light (150 μW/cm2 white fluorescent light) and darkness in diurnally entrained Rpe65−/−; Opn4−/− mice and nocturnal double-heterozygous controls. A light pulse given during the early night stimulated wheel running in Rpe65−/−;Opn4−/− mice while suppressing it in controls (Fig. 2 A–C). Dark pulses, on the other hand, strongly suppressed activity in double knockouts (Fig. 2 D and F) but did not significantly increase the activity of controls (Fig. 2 E and F). These data show that masking responses to both light and dark are reversed in Rpe65−/−;Opn4−/− mice. Moreover, the fact that circadian entrainment and masking, two separate nonvisual light responses, are reversed in Rpe65−/−;Opn4−/− mice suggests a general reversal of the way in which the light signal is interpreted.
Fig. 2.
Masking responses to 3-h light or dark pulses. (A, B, D, and E) Representative 24-h running-wheel records of a double-knockout Rpe65−/−;Opn4−/− (A and D) and a double-heterozygous Rpe65+/−;Opn4+/− (B and E) mouse administered either a 3-h light pulse in the early night or a 3-h dark pulse in the early part of a LD12:12 cycle. Subsequent days are plotted one beneath the other on the y axis. Yellow shading indicates time of lights-on. (C and F) Histograms showing mean percentage of daily wheel revolutions (±SEM) during the 3-h light or dark pulse and during the same 3-h control period on the day before the pulse. *, P < 0.05; **, P < 0.001, Student's t test.
Activity-Dependent Entrainment.
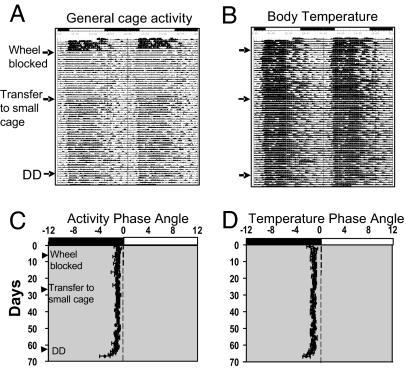
The apparent reversal of masking responses to light in Rpe65−/−;Opn4−/− mice raises the question of whether activity that is directly stimulated by light can feed back onto the circadian clock to entrain it to a diurnal phase. Such activity-dependent entrainment has previously been demonstrated in rodents in response to vigorous wheel-running activity (17–19). We tested for activity-induced entrainment in diurnally entrained Rpe65−/−;Opn4−/− mice by blocking access to running wheels while simultaneously recording circadian rhythms of body temperature and overall cage activity by using intraabdominal transponders (Fig. 3). In LD12:12 with wheels freely available, Rpe65−/−;Opn4−/− mice exhibited diurnal rhythms of both activity and body temperature. When wheels were blocked, an expected decrease in amplitude of the activity rhythm was observed; however, the phase of entrainment was unchanged and remained unchanged even after the animals were transferred to smaller cages without a wheel. Upon release into DD, the mice began to free-run from their diurnal phase. These data indicate that the diurnal entrainment of Rpe65−/−;Opn4−/− mice did not depend on the stimulation provided by wheel-running activity.
Fig. 3.
Wheel blocking does not affect the entrainment phase of Rpe65−/−;Opn4−/− mice. (A and B) Double-plotted records showing daily cage activity (A) and body temperature rhythms (B) from a representative Rpe65−/−;Opn4−/− mouse implanted with a transponder. The arrows indicate the day of wheel blocking, transfer to a small cage without a wheel, and release into DD. (C and D) Mean phase angles (±SEM) of daily cage activity (C) and body temperature elevation (D) (n = 5). Phase angle is defined as the difference in hours between the daily onset of activity or body temperature increase and the light:dark cycle (where the time of lights on is 0 h). The light:dark cycle (lights on from 04:00–16:00) is shown by black (dark) and white (light) bars at the top of each record.
Clock Phase in SCN and Peripheral Tissues.
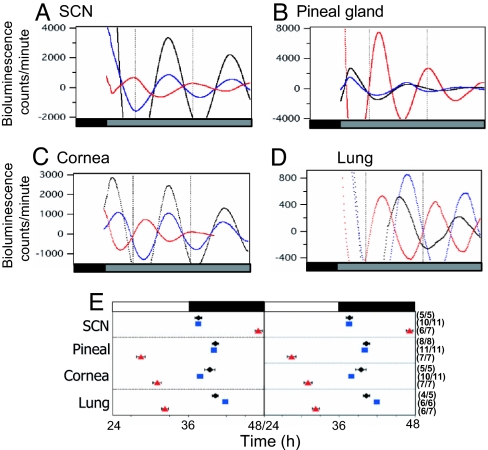
In addition to the SCN, many peripheral tissues contain self-sustained circadian oscillators whose rhythms maintain specific phase relationships with the SCN. These phase relationships are lost after SCN lesion, indicating that the SCN coordinates the phases of peripheral oscillators (15). To assess the level at which the nocturnal-to-diurnal switch had occurred in Rpe65−/−;Opn4−/− mice, we measured bioluminescence rhythms from cultured SCN and peripheral tissues of diurnally entrained Rpe65−/−;Opn4−/− and nocturnal control mice carrying the real-time clock gene reporter mPer2luc (15). Prior to culture, both experimental and control groups were stably entrained to LD12:12 for a minimum of 3 weeks. In static culture, explanted SCN, pineal gland, cornea, and lung exhibited robust circadian rhythms of bioluminescence over several days. A clear phase difference was apparent between all cultured tissues of diurnally entrained Rpe65−/−;Opn4−/−;mPer2luc mice and those of the nocturnal control animals (Fig. 4 A–D). We examined the phase of PER2::LUC-expression rhythms in the SCN and in the periphery by measuring the peak of the bioluminescence rhythm on the second day of culture (Fig. 4E). Peak PER2::LUC expression in the SCN of control mice occurred near the time of the light-to-dark transition, in agreement with data from the original characterization of this reporter by Yoo et al. (15). In contrast, SCN peak phase in the Rpe65−/−;Opn4−/−;mPer2luc mice was 9.5 h out of phase compared with wild-type and double-heterozygous controls. These phase differences are consistent with the difference in the phase angle of locomotor activity observed between Rpe65−/−;Opn4−/− mice and control animals. As previously reported by Yoo et al., we found that peak expression of PER2 in peripheral tissues was phase-delayed relative to the SCN. However, the magnitude of the phase differences was significantly greater in Rpe65−/−;Opn4−/− mice compared with control animals (P < 0.005; ANOVA, Tukey's post hoc comparison). These differences may reflect a more decentralized regulation of circadian oscillators in the periphery. Peak phases of tissues from Rpe65+/−;Opn4−/−;mPer2luc and Rpe65−/−;Opn4+/−;mPer2luc mice that also entrain nocturnally in LD cycles were not different from those of the nocturnal control animals shown in Fig. 4 (data not shown).
Fig. 4.
Real-time monitoring of mPER2::LUCIFERASE expression. (A–D) Representative records of detrended mPER2::LUC bioluminescence rhythms from different tissues of one diurnally entrained Rpe65−/−;Opn4−/−; mPer2luc mouse (red) and two nocturnal controls, a mPer2luc knockin mouse (black) and one Rpe65+/−;Opn4+/−;mPer2luc mouse (blue). (E) Peak phases of mPER2::LUC bioluminescence rhythms from SCN and pineal gland oscillators of mPer2luc knockin (black diamonds), Rpe65+/−;Opn4+/−;mPer2luc (blue squares), and Rpe65−/−;Opn4−/−;mPer2luc (red triangles) mice. Data are double plotted for ease of interpretation. The mean time of peak (±SEM) is plotted against the time of the light:dark cycle, which is indicated by black (dark) and white (light) bars above the graph, on the day of the tissue explant. The phase of the rhythm peak was determined during the 24–48 h interval after the start of each culture. The number of rhythmic cultures out of the number of total cultures tested is indicated in parentheses to the right of each data point.
Diurnal Entrainment of Wild-Type Mice in Dim LD Cycles.
Diurnal entrainment occurs in Rpe65−/−;Opn4−/− mice in which rods are believed to provide the only photoreceptor input to the circadian system. We therefore hypothesized that reducing light levels below the thresholds of cones and melanopsin would produce a diurnal phenotype in wild-type mice. To test this hypothesis, we placed 12 C57BL/6J wild-type mice into running wheels in a standard LD12:12 cycle of 150 μW/cm2 for 2 weeks and then decreased the intensity of the light during the 12-h light phase to 0.0001 μW/cm2. This intensity is approximately three orders of magnitude below the threshold estimated for cone pathways (20 and Maureen McCall, personal communication) and the threshold of the intrinsic melanopsin light response (21). We found that after entraining nocturnally to the standard-intensity LD cycle, 4 of 12 wild-type mice reentrained diurnally to the dim LD cycle. Diurnal entrainment occurred after 6–8 weeks of what appeared to be a free-run through the LD cycle (Fig. 5 A and B). When released into DD, the activity rhythms of these mice free-ran from the diurnal phase, indicating that the circadian clock was indeed diurnally entrained. Although 8 of 12 mice did not entrain diurnally, their activity was nonetheless strongly affected by the dim light:dark cycle. Two of these mice free-ran while exhibiting relative coordination to the light phase [supporting information (SI) Fig. S1 A and B]. In the remaining six mice, changes in the phase angle of activity and increases in activity at the light:dark transitions were observed (Fig. S1 C and D).
Fig. 5.
Double-plotted wheel-running records of two wild-type C57BL/6J mice that entrained diurnally to the dim-light:dark cycle. Yellow bars at the top of each record indicate the time of lights-on. For the first 2 weeks of each record, animals were housed in a standard-intensity (150 μW/cm2) LD12:12 cycle. On the days indicated by the upper arrows, the light intensity during the light phase was dimmed to 0.0001 μW/cm2 by covering the fluorescent bulbs with a neutral density filter. On the day indicated by the lower arrow, mice were released into DD.
Discussion
We have previously reported that ≈80% of Rpe65−/−;Opn4−/− mice show diurnal entrainment in a light:dark cycle of 150 μW/cm2,whereas 20% free-ran (7). When the light intensity was reduced to 40 μW/cm2, only 30% of mice entrained with a diurnal phase, whereas 70% free-ran (7), suggesting that the light intensity used in our experiments is close to the threshold which these animals are able to perceive. Here, we show that diurnally entrained Rpe65−/−;Opn4−/− mice exhibit a reversal of both masking responses to light and the timing of SCN mPER2 expression with respect to the external LD cycle and that ≈30% of wild-type mice housed in dim LD cycles also exhibit diurnal entrainment. Collectively, these data suggest that the diurnal entrainment of both Rpe65−/−;Opn4−/− mice and wild-type mice in dim LD is the result of channeling light through rod pathways and indicate that retinal pathways themselves can influence an organism's temporal niche.
In wild-type mice, two types of light-dependent masking have been described: suppression of activity by high intensity light (negative masking), mediated primarily by the melanopsin system (22), and stimulation of activity by lower intensity light (positive masking), driven by rods and/or cones (6, 23). In Rpe65−/− mice, the rod system remains functional even under photopic light conditions, although sensitivity is greatly reduced (10). The high levels of activity observed during the light phase in Rpe65−/−;Opn4−/− mice might result from the loss of melanopsin-driven negative masking while rod-mediated positive masking is promoted (7). Here, we showed that Rpe65−/−; Opn4−/− mice exhibited positive masking (i.e., enhancement of activity) by light but also a remarkable suppression of activity by darkness. Two recent studies have reported that masking responses to light do not require an intact SCN or a functional circadian clock (24, 25). One of these studies (24) was carried out in hamsters; however, both suggest that circadian entrainment and masking may be mediated by different brain areas. If so, then the reversal of both of these nonvisual responses in Rpe65−/−; Opn4−/− mice suggests that a general reversal in the response to the light signal at the level of the retina has occurred.
Entrainment of the free-running circadian clock by wheel-running activity has been demonstrated in wild-type mice and in anophthalmic mice (17–19). In these animals, intervals of wheel availability that recur every 24 h entrain the circadian system in such a way that onset of daily activity precedes the wheel-running bout by several hours. In mice entrained by periods of wheel availability, activity occurred on average 9 h after the onset of general activity. This phase relationship and the ability to entrain also varied as a function of each animal's individual free-running period (17, 18). However, in diurnally entrained Rpe65−/−; Opn4−/− mice, the onset of general activity, wheel running, and body temperature rhythms coincided. Moreover, free-running periods in long-term DD did not differ between Rpe65−/−; Opn4−/− mice that entrained to LD cycles and those that failed to entrain, and upon wheel blocking, we observed no change in the phase or period of the activity rhythms of diurnally entrained mice. These results indicate that wheel-running activity is not required to entrain the circadian clock of Rpe65−/−;Opn4−/− mice to a diurnal phase and argue against a nonphotic mechanism of entrainment.
The question of whether there are differences in the phase of SCN clock gene expression between diurnal and nocturnal mammals that might lead to diurnal or nocturnal activity patterns has been investigated in studies examining clock gene phase in several different day-active species (26–31). In all of these species, rhythms of Period gene expression in the SCN peaked at approximately the same time of day as in nocturnal rodents, suggesting that the phase of clock gene expression in the SCN relative to the light:dark cycle is conserved across mammalian groups regardless of whether they are diurnal or nocturnal. We found that the PER2::LUC rhythm in the SCN of diurnally entrained Rpe65−/−;Opn4−/− mice peaked near the time of lights-on instead of near lights-off, as has been previously reported in nocturnal mice (15). This reversal in the phase relationship between the LD cycle and the PER2 peak with respect to what has been described for both nocturnal and diurnal animals suggests that the nocturnal-to-diurnal switch in Rpe65−/−;Opn4−/− mice is the result of changes upstream of the SCN.
A reversal of the phase relationship between Period gene expression in the SCN and the LD cycle has also been found in a subset of mole rats, Spalax ehrenbergi, a subterranean rodent with atrophied eyes and massive reduction in visual sensitivity (30). Individuals in this subset display nocturnal activity patterns in contrast to the diurnal pattern typical of this species (30). The authors of that study suggested that the upstream switch is a unique characteristic of the S. ehrenbergi clock, reflecting the animal's subterranean ecotope. Our data suggest that such a switching mechanism may be more widespread. In support of this idea, Bachleitner et al. (32) recently reported a shift of morning and evening activity peaks in Drosophila melanogaster exposed to dim-light:bright-light cycles. The shift in activity was accompanied by a shift in the phase of clock gene expression in a subset of pacemaker neurons.
In addition to the diurnal entrainment that we report here in Rpe65−/−;Opn4−/− and wild-type mice, there are several documented examples of complete reversals of temporal niche in nocturnal rodents (6, 33–35) and in a nocturnal primate (Eulemur albifrons) exposed to dim LD (36). Increased activity during the light phase also occurs in mice with severe vitamin-A depletion (4, 5) and in mice lacking cryptochromes (37). Because rods, cones, and melanopsin require a vitamin-A chromophore, vitamin-A depletion may reduce both outer retinal photoreceptor and melanopsin function to produce a phenotype similar to that of Rpe65−/−;Opn4−/− mice. It is unclear, but very interesting, how the loss of cryptochromes, which are key components of the molecular clock mechanism in mammals (38–40), might produce a diurnal phenotype. Mice lacking Bmal1, another component of the molecular clock, also show an increase in the percentage of activity that occurs during the light phase in light:dark cycles (41).
The finding that manipulation of light intensity can produce temporal-niche switching in wild-type animals suggests that a common mechanism produces phase reversals in Rpe65−/−; Opn4−/− mice and in wild-type mice during dim LD cycles. It further demonstrates that normal photoreceptor input channels can be manipulated in such a way as to affect entrainment phase. It is conceivable that species-specific changes in phase resulting from changes in light intensity may be a widespread mechanism for regulating temporal niche.
Materials and Methods
Animals.
All procedures used in this study were approved by the University of Virginia Animal Care and Use Committee and are in compliance with guidelines established by the Association for Assessment of Laboratory Animal Care. Mixed strain (C57BL6 × 129s) Rpe65−/−;Opn4−/− mice from our colony and double-heterozygous littermate controls were used in these experiments. For real-time reporting of PERIOD2::LUCIFERASE, Rpe65−/−;Opn4−/− mice were crossed with mPer2luc knockin mice kindly provided by Joseph Takahashi (Northwestern University, Evanston, IL). The resulting Rpe65+/−;Opn4+/−; mPer2luc mice were crossed with Rpe65−/−;Opn4−/− mice to obtain experimental Rpe65−/−;Opn4−/−;mPer2luc mice and double-heterozygous littermate controls. PCR genotyping was performed according to published protocols (15, 42, 43). All mice used in these experiments were 2–4 months of age.
Running-Wheel Activity Recording.
Mice were housed in individual running-wheel cages in light-tight boxes with temperature and relative humidity set at 21°C and 50%, respectively. Food and water were available ad libitum. Locomotor activity was measured as the number of wheel revolutions recorded in 1-min bins and analyzed with Clocklab software (Actimetrics). Mean intensity from fluorescent bulbs (Philips F40CW/RS/EW) was 150 μW/cm2 measured at the level of the mice in their cages. To reduce light intensity, fluorescent bulbs were wrapped with black plastic sheeting. Transmittance over the visible spectrum was measured with a USB4000 Spectrometer (Ocean Optics) and remained unchanged after the addition of these filters. Procedures in dim light and in the dark were carried out with the aid of infrared viewers.
Masking to Light and Dark Pulses.
Three-hour pulses of either light (white fluorescent, 150 μW/cm2) or dark were administered to mice that had been maintained in LD12:12 for at least 2 weeks, by either turning lights on for 3 h from 1–4 h after the time of lights-off or by turning lights off for 3 h from 1–4 h after the time of lights-on. Masking responses were determined by calculating the percentage of daily activity (i.e., the number of wheel revolutions during the pulse divided by the total number of revolutions for that day times 100) during the 3-h pulse and during the same 3-h control period on the previous day.
Wheel Blocking.
Five Rpe65−/−;Opn4−/− mice that had been diurnally entrained for at least 3 weeks were implanted intraabdominally with a battery-free transponder (model G2 E-mitter, Mini Mitter) for telemetric recording of body temperature and gross motor activity. Immediately after recovery from surgery, animals were returned to their home running-wheel cages (47 × 26.5 × 20.5 cm). During this time, animals had free access to the running wheel. Body temperature and gross motor activity were collected in 10 min bins by using a Vital View telemetry system (Mini Mitter). After 5 days, running wheels were blocked and mice were monitored for ≈3 additional weeks. The mice were then placed into small cages (29 × 18 × 12.5 cm) without a running wheel and monitored for 5 more weeks. After this time period, lights were turned off and body temperature and activity were monitored for 5 days in DD to assess the phase of circadian entrainment. Data analysis was performed by using Clocklab and ActiView (Mini Mitter) software. Phase angles for general cage activity and body temperature were determined by measuring the number of minutes between activity onset or body-temperature rise and the time of lights-on.
PER2::LUC Recordings.
The running-wheel activity of Rpe65−/−;Opn4−/−; mPer2luc mice, control Rpe65+/−;Opn4+/−;mPer2luc mice, and mPer2luc mice in LD12:12 was recorded for 3–4 weeks. Animals were anesthetized with CO2 and decapitated approximately 1 h before lights-off, a time of day chosen for previous experiments of this type to minimize disruption to tissues caused by a light-to-dark transition in the middle of the day (44). For pineal dissection, the pineal on the skull cap was removed and placed in cold Hanks' balanced salt solution (HBSS). The pineal gland was identified and separated from the meninges under a dissecting microscope. For SCN dissection, brains were rapidly removed and placed in cold HBSS. Coronal sections (300 μm) were cut on a vibratome, and the SCN-containing region was dissected from each slice. Whole cornea and hand-sliced pieces of lung ≈1 mm square were dissected in cold HBSS. Tissues were cultured in sealed 35-mm Petri dishes containing 1.2 ml of culture medium and maintained in a light-tight incubator at 37°C. Culture medium consisted of DMEM (D2902, Sigma) with 10 mM Hepes, 2% B27 supplement, 352.5 μg/ml NaHCO3, 3.5 mg/ml d-glucose, 25 μg/ml penicillin, 25 units/ml streptomycin, and 0.1 mM beetle luciferin, the substrate of the luciferase enzyme. The SCN and pineal were cultured on a Millicell culture insert, and other tissues were cultured free-floating. Bioluminescence was measured with a photomultiplier tube (Hamamatsu) or a Lumicycle photomultiplier tube detector assembly (Actimetrics), beginning immediately after tissue placement into an incubator and continuing for 3–5 days. Photon counts were collected in either 1- or 10-min bins, and detrended by subtracting the 24-h running average from the normalized data and then smoothing the data to 3-h running means. Peak phase was calculated as the highest point of the smoothed data in the 24–48-h interval after the initiation of the culture. Mean peak phases of tissues were calculated for each experimental group, and statistical differences were determined with ANOVA followed by a post hoc Tukey's test.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant MH56647 (to M.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12645.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801728105/DCSupplemental.
References
- 1.Lee TM, Labyak SE. Free-running rhythms and light- and dark-pulse phase response curves for diurnal Octodon degus (Rodentia) Am J Physiol. 1997;273:R278–R286. doi: 10.1152/ajpregu.1997.273.1.R278. [DOI] [PubMed] [Google Scholar]
- 2.Kas MJ, Edgar DM. Photic phase response curve in Octodon degus: Assessment as a function of activity phase preference. Am J Physiol. 2000;278:R1385–R1389. doi: 10.1152/ajpregu.2000.278.5.R1385. [DOI] [PubMed] [Google Scholar]
- 3.Smale L, Lee T, Nunez AA. Mammalian diurnality: Some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CL, et al. Preservation of light signaling to the suprachiasmatic nucleus in vitamin A-deficient mice. Proc Natl Acad Sci USA. 2001;98:11708–11713. doi: 10.1073/pnas.201301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson CL, et al. Effect of vitamin A depletion on nonvisual phototransduction pathways in cryptochromeless mice. J Biol Rhythms. 2004;19:504–517. doi: 10.1177/0748730404270519. [DOI] [PubMed] [Google Scholar]
- 6.Mrosovsky N, Hattar S. Diurnal mice (Mus musculus) and other examples of temporal niche switching. J Comp Physiol A. 2005;191:1011–1241. doi: 10.1007/s00359-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 7.Doyle SE, Castrucci AM, McCall M, Provencio I, Menaker M. Nonvisual light responses in the Rpe65 knockout mouse: Rod loss restores sensitivity to the melanopsin system. Proc Natl Acad Sci USA. 2006;103:10432–10437. doi: 10.1073/pnas.0600934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 10.Seeliger MW, et al. New views on RPE65 deficiency: The rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29:70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- 11.Woodruff ML, et al. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain G. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Znoiko SL, et al. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel A, et al. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest Ophthalmol Vis Sci. 2007;48:534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- 15.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- 17.Laemle LK, Ottenweller JE. Nonphotic entrainment of activity and temperature rhythms in anophthalmic mice. Physiol Behav. 1999;66:461–471. doi: 10.1016/s0031-9384(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 18.Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol. 1991;261:R928–R933. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- 19.Marchant EG, Mistlberger RE. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav. 1996;60:657–663. doi: 10.1016/s0031-9384(96)80045-x. [DOI] [PubMed] [Google Scholar]
- 20.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 22.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 23.Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol A. 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- 24.Redlin U, Mrosovsky N. Masking by light in hamsters with SCN lesions. J Comp Physiol A. 1999;184:439–448. doi: 10.1007/s003590050343. [DOI] [PubMed] [Google Scholar]
- 25.Mrosovsky N. Further characterization of the phenotype of mCry1/mCry2-deficient mice. Chronobiol Int. 2001;18:613–625. doi: 10.1081/cbi-100106076. [DOI] [PubMed] [Google Scholar]
- 26.Mrosovsky N, Edelstein K, Hastings MH, Maywood ES. Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): Implications for nonphotic phase shifting. J Biol Rhythms. 2001;16:471–478. doi: 10.1177/074873001129002141. [DOI] [PubMed] [Google Scholar]
- 27.Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: Evidence for an internal coincidence timer. Proc Natl Acad Sci USA. 2002;99:13890–13895. doi: 10.1073/pnas.212517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldelas I, Poirel VJ, Sicard B, Pevet P, Challet E. Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei. Neuroscience. 2003;116:583–591. doi: 10.1016/s0306-4522(02)00654-1. [DOI] [PubMed] [Google Scholar]
- 29.Dardente H, Klosen P, Caldelas I, Pevet P, Masson-Pevet M. Phenotype of Per1- and Per2-expressing neurons in the suprachiasmatic nucleus of a diurnal rodent (Arvicanthis ansorgei): Comparison with a nocturnal species, the rat. Cell Tissue Res. 2002;310:85–92. doi: 10.1007/s00441-002-0609-9. [DOI] [PubMed] [Google Scholar]
- 30.Oster H, Avivi A, Joel A, Albrecht U, Nevo E. A switch from diurnal to nocturnal activity in S. ehrenbergi is accompanied by an uncoupling of light input and the circadian clock. Curr Biol. 2002;12:1919–1922. doi: 10.1016/s0960-9822(02)01263-0. [DOI] [PubMed] [Google Scholar]
- 31.Ramanathan C, Nunez AA, Martinez GS, Schwartz MD, Smale L. Temporal and spatial distribution of immunoreactive PER1 and PER2 proteins in the suprachiasmatic nucleus and peri-suprachiasmatic region of the diurnal grass rat (Arvicanthis niloticus) Brain Res. 2006;1073–1074:348–358. doi: 10.1016/j.brainres.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 32.Bachleitner W, Kempinger L, Wulbeck C, Rieger D, Helfrich-Forster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci USA. 2007;104:3538–3543. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavanau JL. Activity and orientational responses of white-footed mice to light. Nature. 1968;218:245–252. doi: 10.1038/218245a0. [DOI] [PubMed] [Google Scholar]
- 34.Argamaso-Hernan S. Charlottesville: Univ of Virginia; 1996. Light-evoked behavior in mice with inherited retinal degeneration: An analysis of circadian photoentrainment. PhD dissertation. [Google Scholar]
- 35.Kavanau JL. Behavior of captive white-footed mice. Science. 1967;155:1623–1639. doi: 10.1126/science.155.3770.1623. [DOI] [PubMed] [Google Scholar]
- 36.Erkert HG, Cramer B. Chronobiological background to cathemerality: Circadian rhythms in Eulemur fulvus albifrons (Prosimii) and Aotus azarai boliviensis (Anthropoidea) Folia Primatol (Basel) 2006;77:87–103. doi: 10.1159/000089697. [DOI] [PubMed] [Google Scholar]
- 37.Van Gelder RN, et al. Pleiotropic effects of cryptochromes 1 and 2 on free-running and light-entrained murine circadian rhythms. J Neurogenet. 2002;16:181–203. doi: 10.1080/01677060215306. [DOI] [PubMed] [Google Scholar]
- 38.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 39.Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 40.van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 41.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 43.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms. 2005;20:500–512. doi: 10.1177/0748730405280775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.