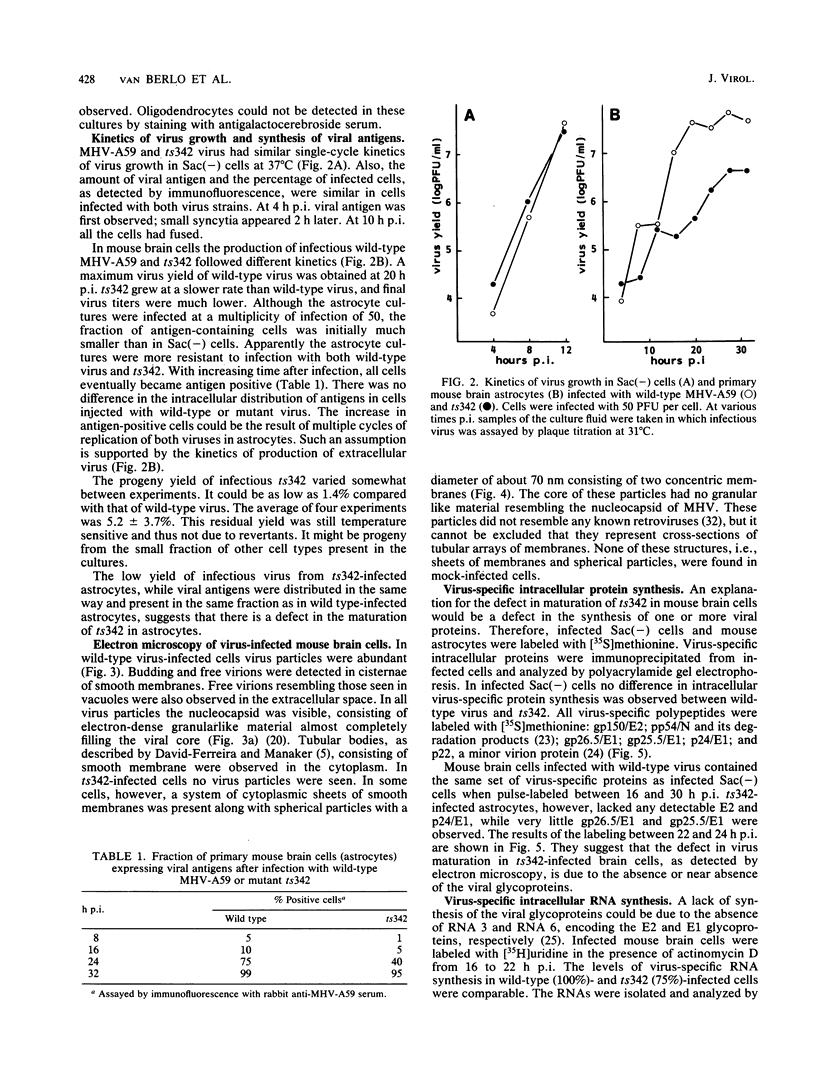
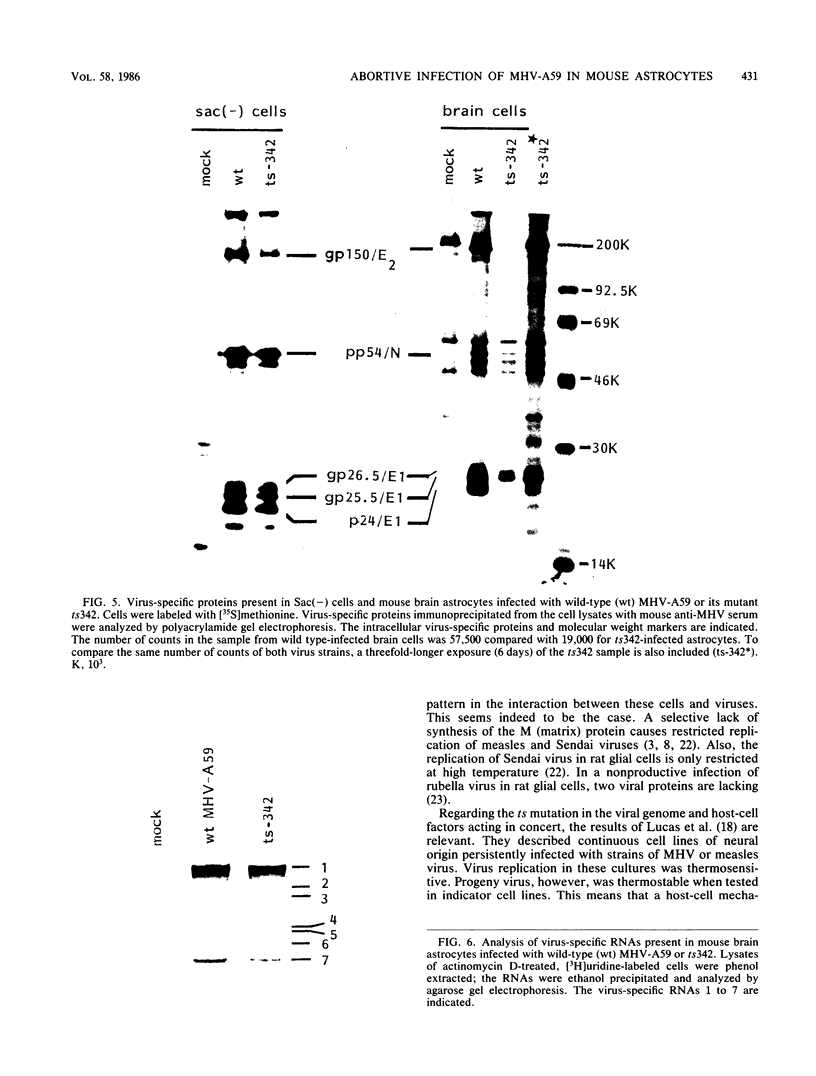
Abstract
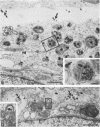
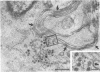
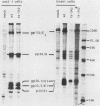
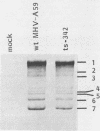
Temperature-sensitive (ts) mutants of mouse hepatitis virus A59 (MHV-A59) are drastically attenuated in their pathogenic properties. Intracerebral inoculation of mice with 10(5) PFU of mutant ts342 results in prolonged infection of the central nervous system, whereas 100 PFU of wild-type virus are lethal (M. J. M. Koolen, A. D. M. E. Osterhaus, G. van Steenis, M. C. Horzinek, and B. A. M. van der Zeijst, Virology 125:393-402, 1983). In the Sac(-) cell line ts342 grows as well at 37 degrees C (the body temperature of mice) as at 31 degrees C (the permissive temperature). There is, however, a difference in primary cultures of mouse brain astrocytes. After infection with ts342, astrocytes produced low levels of infectious virus (5.2 +/- 3.7%) compared with virus yields after infection with wild-type virus. The fraction of wild-type virus- and ts342-infected cells was similar. Electron microscopy showed in wild-type virus-infected cells abundant virions in smooth vesicles usually closely associated with a well-developed Golgi apparatus. In mutant-infected cells no mature ts342 virus particles were found. There was no difference between ts342 and wild-type virus regarding the intracellular virus-specific RNAs. In ts342-infected cells the viral glycoproteins E2 and E1 were not detectable or were barely detectable. Either the mRNAs for the glycoproteins are not translated or the proteins are rapidly broken down. Revertants of ts342 were isolated. They grew as well as wild-type virus in astrocytes, indicating that they apparently produced sufficient amounts of E2 and E1, the ts defect itself rather than a second site mutation is responsible for the defect in replication, and the ts defect acts in unison with host-cell factors. The revertants also regained the lethal properties of wild-type virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID-FERREIRA J. F., MANAKER R. A. AN ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF A MOUSE HEPATITIS VIRUS IN TISSUE CULTURE CELLS. J Cell Biol. 1965 Jan;24:57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Takenaka S., Shumiya S. Carrier state of antibody and viruses in a mouse breeding colony persistently infected with Sendai and mouse hepatitis viruses. Lab Anim Sci. 1976 Apr;26(2 Pt 50):153–159. [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hansson E. Cellular composition of a cerebral hemisphere primary culture. Neurochem Res. 1984 Feb;9(2):153–172. doi: 10.1007/BF00964164. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Lampert P. W., Oldstone M. B. Temperature-sensitive mutants of mouse hepatitis virus produce a high incidence of demyelination. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4033–4036. doi: 10.1073/pnas.75.8.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Synthesis of subgenomic mRNA's of mouse hepatitis virus is initiated independently: evidence from UV transcription mapping. J Virol. 1981 Aug;39(2):401–406. doi: 10.1128/jvi.39.2.401-406.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Dubois-Dalcq M., Haspel M. V., Claysmith A. P., Lampert P. W., Oldstone M. B. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J Neuroimmunol. 1981 Mar;1(1):81–92. doi: 10.1016/0165-5728(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Koolen M. J., Osterhaus A. D., Van Steenis G., Horzinek M. C., Van der Zeijst B. A. Temperature-sensitive mutants of mouse hepatitis virus strain A59: isolation, characterization and neuropathogenic properties. Virology. 1983 Mar;125(2):393–402. doi: 10.1016/0042-6822(83)90211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Lavi E., Gilden D. H., Wroblewska Z., Rorke L. B., Weiss S. R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984 May;34(5):597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- Lucas A., Coulter M., Anderson R., Dales S., Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978 Jul 15;88(2):325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANAKER R. A., PICZAK C. V., MILLER A. A., STANTON M. F. A hepatitis virus complicating studies with mouse leukemia. J Natl Cancer Inst. 1961 Jul;27:29–51. [PubMed] [Google Scholar]
- Massalski A., Coulter-Mackie M., Knobler R. L., Buchmeier M. J., Dales S. In vivo and in vitro models of demyelinating diseases. V. Comparison of the assembly of mouse hepatitis virus, strain JHM, in two murine cell lines. Intervirology. 1982;18(3):135–146. doi: 10.1159/000149316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H., Sato H., Tanaka J., Kamiya S., Yoshie T., Hatano M. Synthesis of M protein of HVJ (Sendai virus) in rat glial cells is selectively restricted at a non-permissive temperature. J Gen Virol. 1984 Mar;65(Pt 3):639–643. doi: 10.1099/0022-1317-65-3-639. [DOI] [PubMed] [Google Scholar]
- Pope D. D., Van Alstyne D. Evidence for restricted replication of Rubella virus in rat glial cells in culture. Virology. 1981 Sep;113(2):776–780. doi: 10.1016/0042-6822(81)90207-5. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., CAPPS W. I. Mouse hepatitis virus infection as a highly contagious, prevalent, enteric infection of mice. Proc Soc Exp Biol Med. 1963 Jan;112:161–165. doi: 10.3181/00379727-112-27980. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S., Wege H., ter Meulen V. The structure and replication of coronaviruses. Curr Top Microbiol Immunol. 1982;99:131–163. doi: 10.1007/978-3-642-68528-6_4. [DOI] [PubMed] [Google Scholar]
- Smith A. L. An immunofluorescence test for detection of serum antibody to rodent coronaviruses. Lab Anim Sci. 1983 Apr;33(2):157–160. [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Van Alstyne D., Paty D. W. The effect of dibutyryl cyclic AMP on restricted replication of rubella virus in rat glial cells in culture. Virology. 1983 Jan 15;124(1):173–180. doi: 10.1016/0042-6822(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Weiland E., Mussgay M., Weiland F. Nonproducer malignant tumor cells with rescuable sarcoma virus genome isolated from a recurrent Moloney sarcoma. J Exp Med. 1978 Aug 1;148(2):408–423. doi: 10.1084/jem.148.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]