Abstract
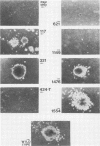
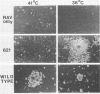
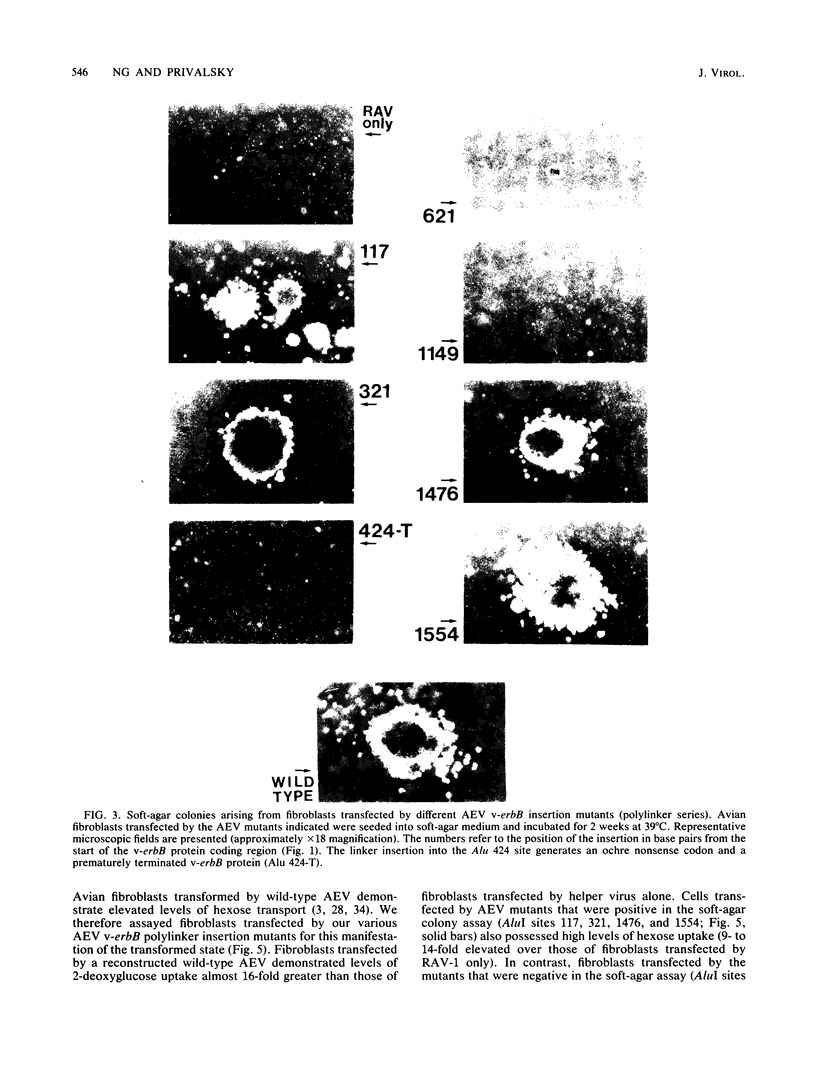
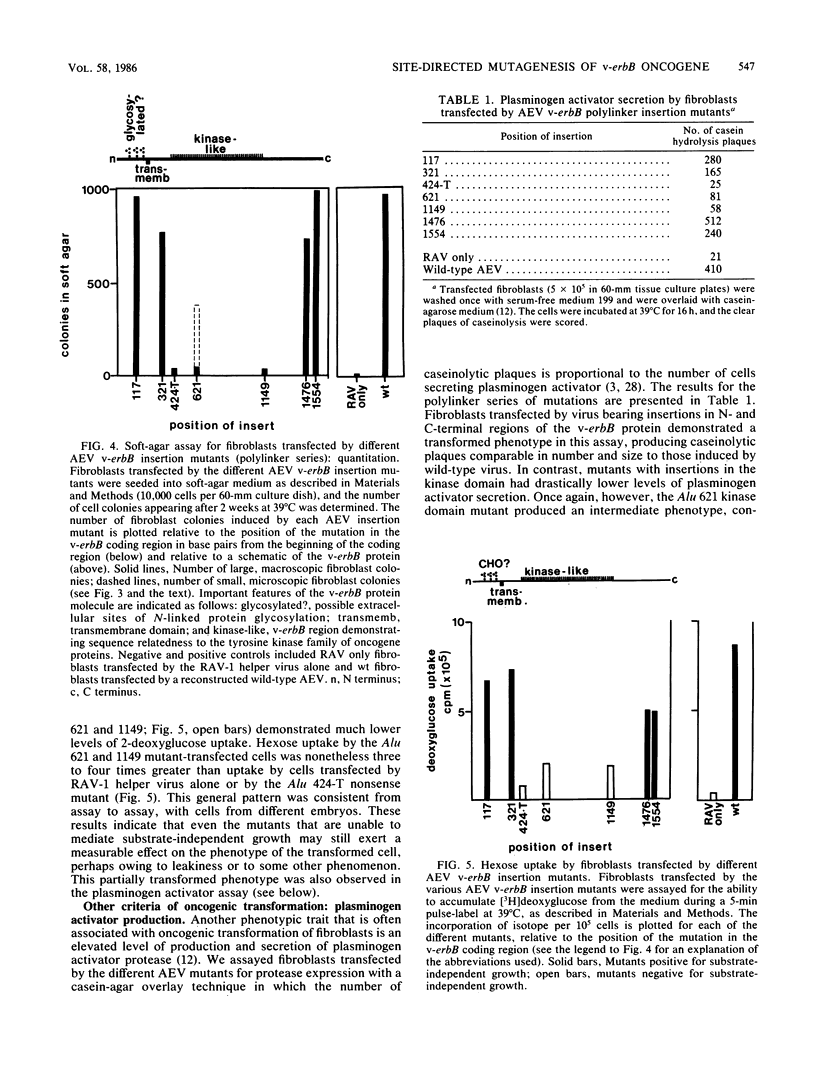
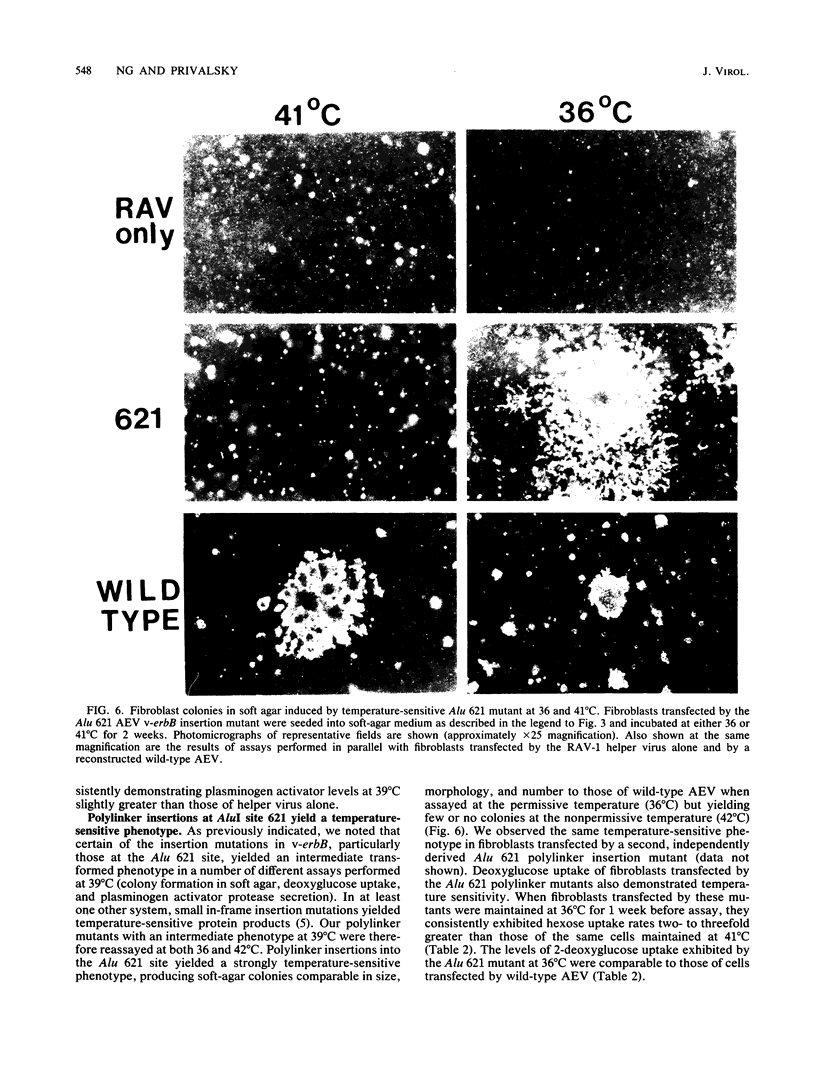
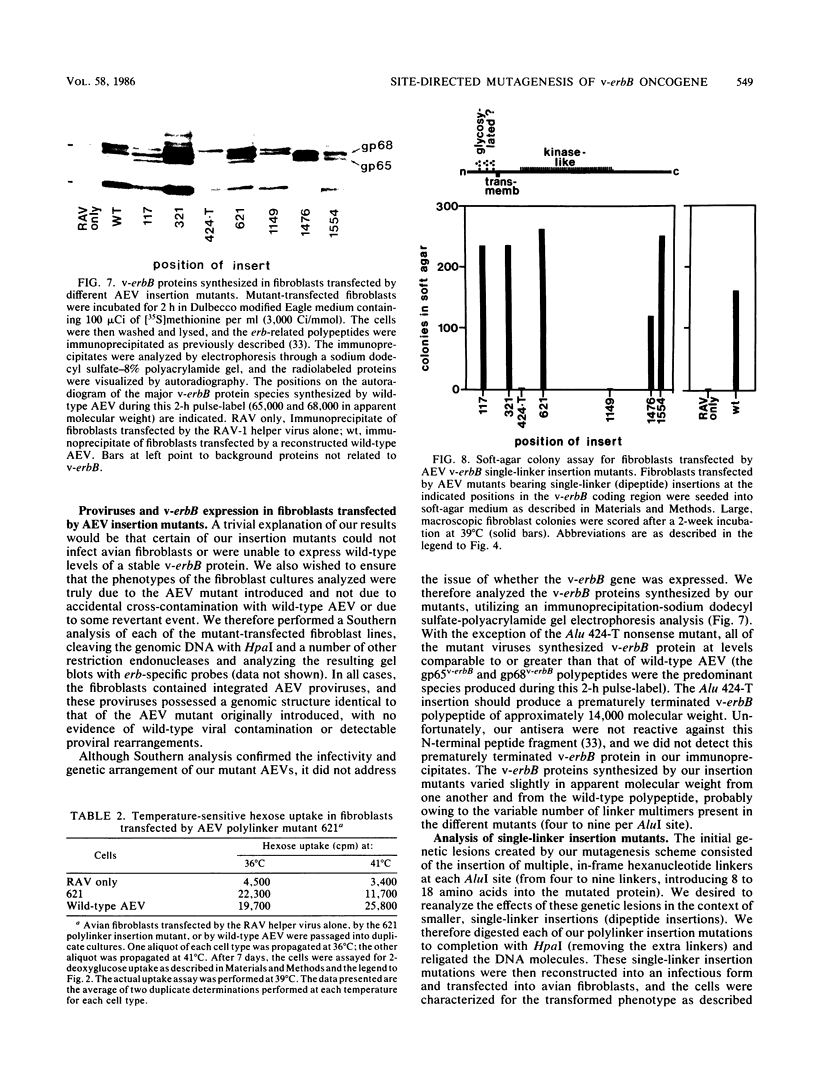
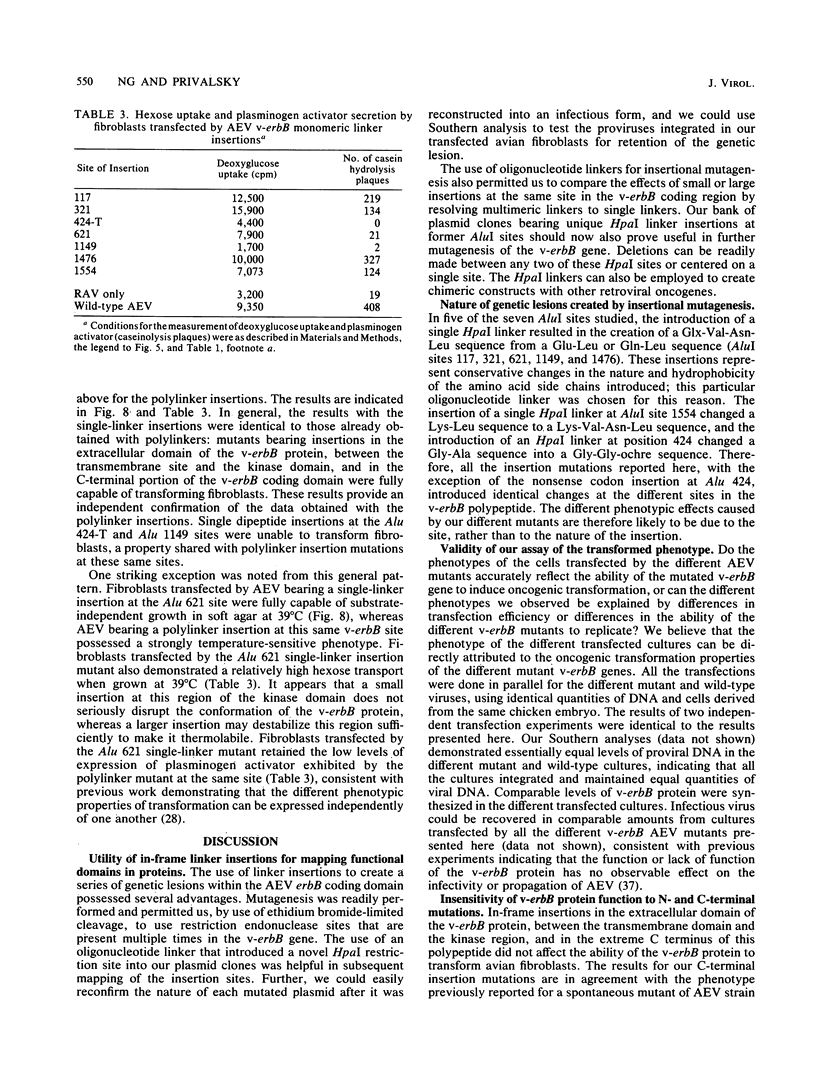
Avian erythroblastosis virus (AEV) induces erythroblastosis and fibrosarcomas. The viral erbB protein is required for AEV-mediated oncogenesis. To explore the structural aspects of the v-erbB polypeptide necessary for its oncogenic function, we created a series of small in-frame insertions in different domains of the v-erbB oncogene. AEV genomes bearing lesions within the v-erbB kinase domain demonstrated a drastically decreased ability to transform avian fibroblasts, establishing a functional role for this structurally conserved oncogene domain. In contrast, mutations in the extracellular domain, between the transmembrane region and the kinase domain, or at the extreme C terminus of the v-erbB protein had no effect on AEV-mediated fibroblast transformation. One lesion within the v-erbB kinase domain, a 10-amino acid insertion, produced a temperature-sensitive mutant capable of fibroblast transformation at 36 degrees C but not at 41 degrees C, suggesting that small in-frame insertions have general utility for the in vitro creation of conditional mutants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Graf T. Transformation parameters of chicken embryo fibroblasts infected with the ts34 mutant of avian erythroblastosis virus. Virology. 1980 Jan 30;100(2):348–356. doi: 10.1016/0042-6822(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Beug H., Hayman M. J. Temperature-sensitive mutants of avian erythroblastosis virus: surface expression of the erbB product correlates with transformation. Cell. 1984 Apr;36(4):963–972. doi: 10.1016/0092-8674(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Boeke J. D. One and two codon insertion mutants of bacteriophage f1. Mol Gen Genet. 1981;181(3):288–291. doi: 10.1007/BF00425599. [DOI] [PubMed] [Google Scholar]
- Bramson H. N., Thomas N., Matsueda R., Nelson N. C., Taylor S. S., Kaiser E. T. Modification of the catalytic subunit of bovine heart cAMP-dependent protein kinase with affinity labels related to peptide substrates. J Biol Chem. 1982 Sep 25;257(18):10575–10581. [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Gilmore T., DeClue J. E., Martin G. S. Protein phosphorylation at tyrosine is induced by the v-erbB gene product in vivo and in vitro. Cell. 1985 Mar;40(3):609–618. doi: 10.1016/0092-8674(85)90209-0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N., Reynolds F. H., Jr, Stephenson J. R. Homology between phosphotyrosine acceptor site of human c-abl and viral oncogene products. Nature. 1983 Jul 14;304(5922):167–169. doi: 10.1038/304167a0. [DOI] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Takio K., Krebs E. G. Amino acid sequence at the ATP-binding site of cGMP-dependent protein kinase. J Biol Chem. 1982 Jan 25;257(2):727–733. [PubMed] [Google Scholar]
- Hayman M. J., Beug H. Identification of a form of the avian erythroblastosis virus erb-B gene product at the cell surface. 1984 May 31-Jun 6Nature. 309(5967):460–462. doi: 10.1038/309460a0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Shimizu T. Heterogeneity of strain R avian (erythroblastosis) virus. Cancer Res. 1970 Dec;30(12):2827–2831. [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Primary structure of v-raf: relatedness to the src family of oncogenes. Science. 1984 Apr 20;224(4646):285–289. doi: 10.1126/science.6324342. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Palmieri S., Beug H., Graf T. Isolation and characterization of four new temperature-sensitive mutants of avian erythroblastosis virus (AEV). Virology. 1982 Dec;123(2):296–311. doi: 10.1016/0042-6822(82)90263-x. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L., Bishop J. M. Proteins specified by avian erythroblastosis virus: coding region localization and identification of a previously undetected erb-B polypeptide. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3958–3962. doi: 10.1073/pnas.79.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L., Bishop J. M. Subcellular localization of the v-erb-B protein, the product of a transforming gene of avian erythroblastosis virus. Virology. 1984 Jun;135(2):356–368. doi: 10.1016/0042-6822(84)90192-2. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Ralston R., Bishop J. M. The membrane glycoprotein encoded by the retroviral oncogene v-erb-B is structurally related to tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Feb;81(3):704–707. doi: 10.1073/pnas.81.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L., Sealy L., Bishop J. M., McGrath J. P., Levinson A. D. The product of the avian erythroblastosis virus erbB locus is a glycoprotein. Cell. 1983 Apr;32(4):1257–1267. doi: 10.1016/0092-8674(83)90307-0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Sealy L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: v-erb-A is not required for transformation of fibroblasts. Virology. 1983 Oct 15;130(1):179–194. doi: 10.1016/0042-6822(83)90126-5. [DOI] [PubMed] [Google Scholar]
- Sealy L., Privalsky M. L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: erb-B is required for oncogenicity. Virology. 1983 Oct 15;130(1):155–178. doi: 10.1016/0042-6822(83)90125-3. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Stone J. C., Atkinson T., Smith M., Pawson T. Identification of functional regions in the transforming protein of Fujinami sarcoma virus by in-phase insertion mutagenesis. Cell. 1984 Jun;37(2):549–558. doi: 10.1016/0092-8674(84)90385-4. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Hihara H., Nishida T., Kawai S., Toyoshima K. A new avian erythroblastosis virus, AEV-H, carries erbB gene responsible for the induction of both erythroblastosis and sarcomas. Cell. 1983 Aug;34(1):225–232. doi: 10.1016/0092-8674(83)90153-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]