Abstract
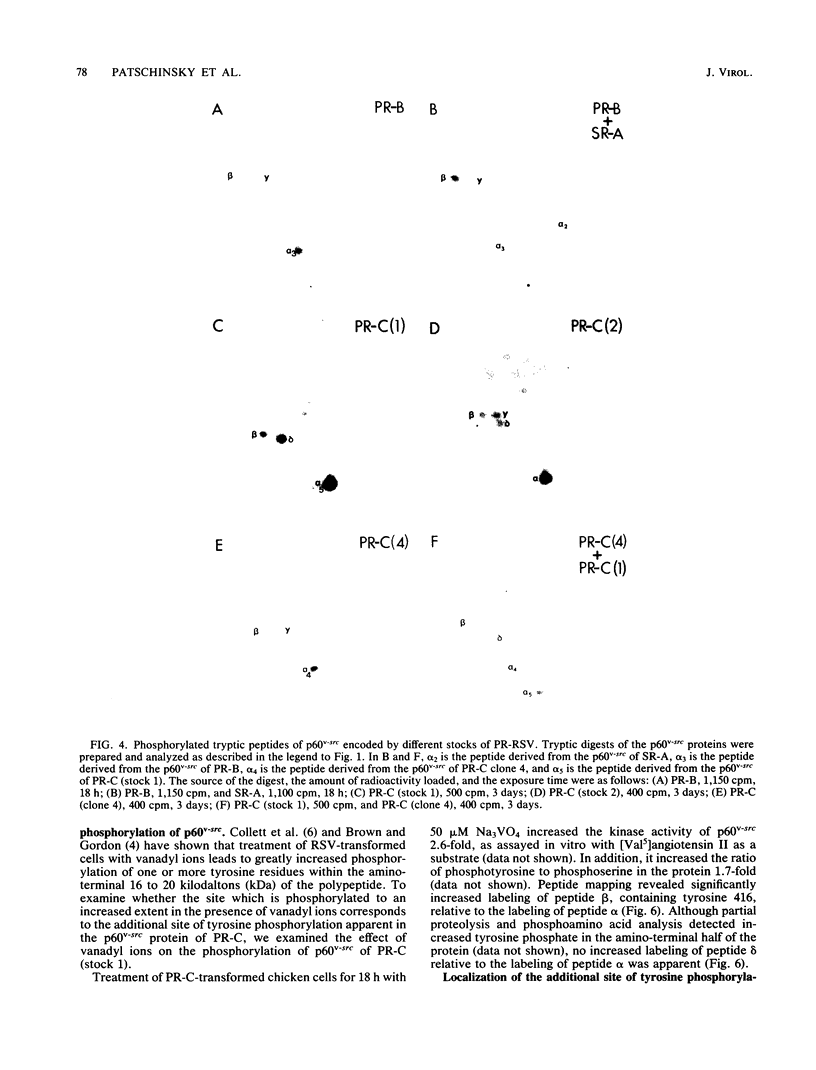
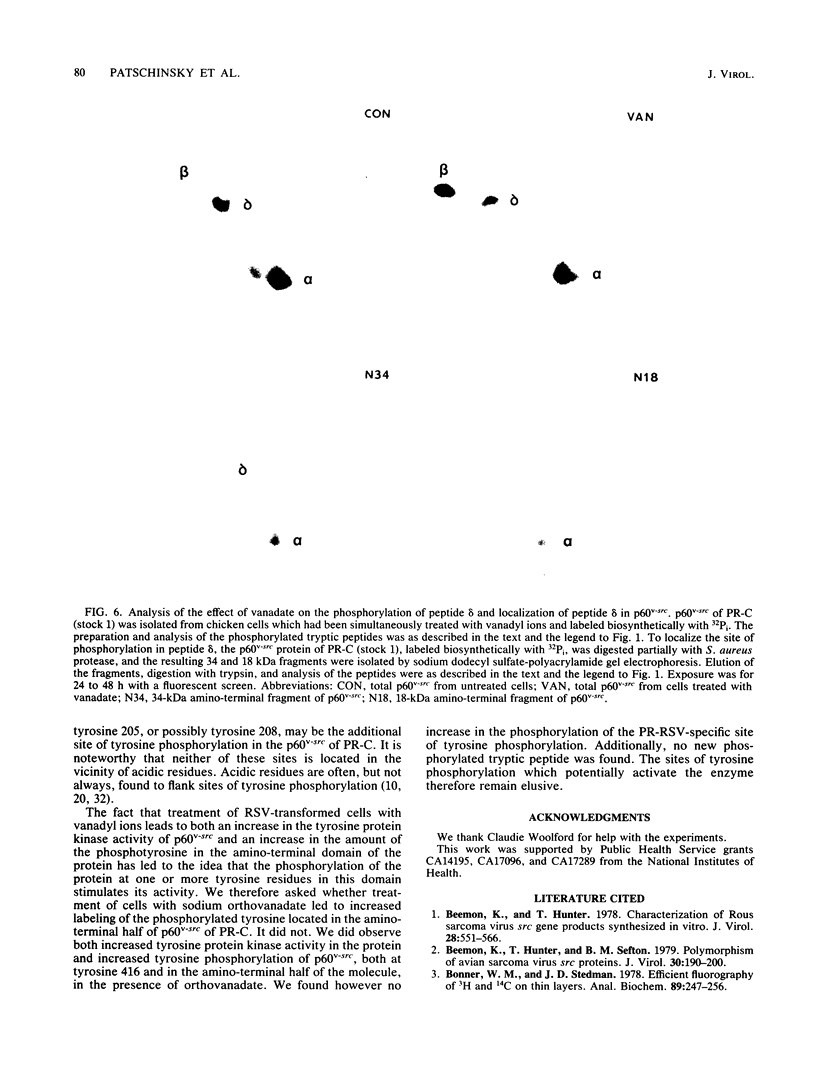
We provide direct evidence that serine 17 is the major site of serine phosphorylation in p60v-src, the transforming protein of Rous sarcoma virus, and in its cellular homolog, p60c-src. The amino acid composition of the tryptic peptide containing the major site of serine phosphorylation in p60v-src was deduced by peptide map analysis of the protein labeled biosynthetically with a variety of radioactive amino acids. Manual Edman degradation revealed that the phosphorylated serine in this peptide was the amino terminal residue. These data are consistent only with the phosphorylation of serine 17. The major site of serine phosphorylation in chicken p60c-src, the cellular homolog of p60v-src, is contained in a tryptic peptide identical to that containing serine 17 in p60v-src of Schmidt Ruppin Rous sarcoma virus of subgroup A. Serine 17 is therefore also phosphorylated in p60c-src. The p60v-src protein encoded by Prague Rous sarcoma virus was found to contain two sites of tyrosine phosphorylation. The previously unrecognized site of tyrosine phosphorylation may be tyrosine 205 or possibly tyrosine 208. Treatment of Prague Rous sarcoma virus-infected cells with vanadyl ions stimulated the protein kinase activity of p60v-src and increased the phosphorylation of tyrosine 416 but not the phosphorylation of the additional site of tyrosine phosphorylation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T., Sefton B. M. Polymorphism of avian sarcoma virus src proteins. J Virol. 1979 Apr;30(1):190–200. doi: 10.1128/jvi.30.1.190-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Brown D. J., Gordon J. A. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem. 1984 Aug 10;259(15):9580–9586. [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. Corrections to the nucleotide sequence of the src gene of Rous sarcoma virus. Nature. 1983 Feb 24;301(5902):736–738. doi: 10.1038/301736b0. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Mardon G., Varmus H. E. Frameshift and intragenic suppressor mutations in a Rous sarcoma provirus suggest src encodes two proteins. Cell. 1983 Mar;32(3):871–879. doi: 10.1016/0092-8674(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Sefton B. M. Evidence that there exist four classes of RNA tumor viruses which encode proteins with associated tyrosine protein kinase activities. J Virol. 1981 Jul;39(1):104–114. doi: 10.1128/jvi.39.1.104-114.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F. Evidence the pp60src, the product of the Rous sarcoma virus src gene, undergoes autophosphorylation. J Virol. 1982 Jan;41(1):1–7. doi: 10.1128/jvi.41.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Shoyab M., Gentry L. E. Site-specific increased phosphorylation of pp60v-src after treatment of RSV-transformed cells with a tumor promoter. Science. 1985 Sep 27;229(4720):1393–1395. doi: 10.1126/science.2994221. [DOI] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Roth C. W., Richert N. D., Pastan I., Gottesman M. M. Cyclic AMP treatment of Rous sarcoma virus-transformed Chinese hamster ovary cells increases phosphorylation of pp60src and increases pp60src kinase activity. J Biol Chem. 1983 Sep 10;258(17):10768–10773. [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Relationship of polypeptide products of the transforming gene of Rous sarcoma virus and the homologous gene of vertebrates. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2059–2063. doi: 10.1073/pnas.77.4.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Patschinsky T., Berdot C., Hunter T., Elliott T. Phosphorylation and metabolism of the transforming protein of Rous sarcoma virus. J Virol. 1982 Mar;41(3):813–820. doi: 10.1128/jvi.41.3.813-820.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Walter G. Antiserum specific for the carboxy terminus of the transforming protein of Rous sarcoma virus. J Virol. 1982 Nov;44(2):467–474. doi: 10.1128/jvi.44.2.467-474.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M. A mutation at the major phosphotyrosine in pp60v-src alters oncogenic potential. Virology. 1984 Jul 30;136(2):375–386. doi: 10.1016/0042-6822(84)90174-0. [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M., Esch F. S. Amino-terminal sequence analysis of alphavirus polypeptides. J Virol. 1981 Jun;38(3):968–972. doi: 10.1128/jvi.38.3.968-972.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. In vitro phosphorylation of angiotensin analogs by tyrosyl protein kinases. J Biol Chem. 1983 Jan 25;258(2):1022–1025. [PubMed] [Google Scholar]
- Woodgett J. R., Davison M. T., Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983 Nov 15;136(3):481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]