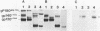Abstract
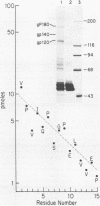
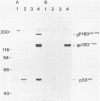
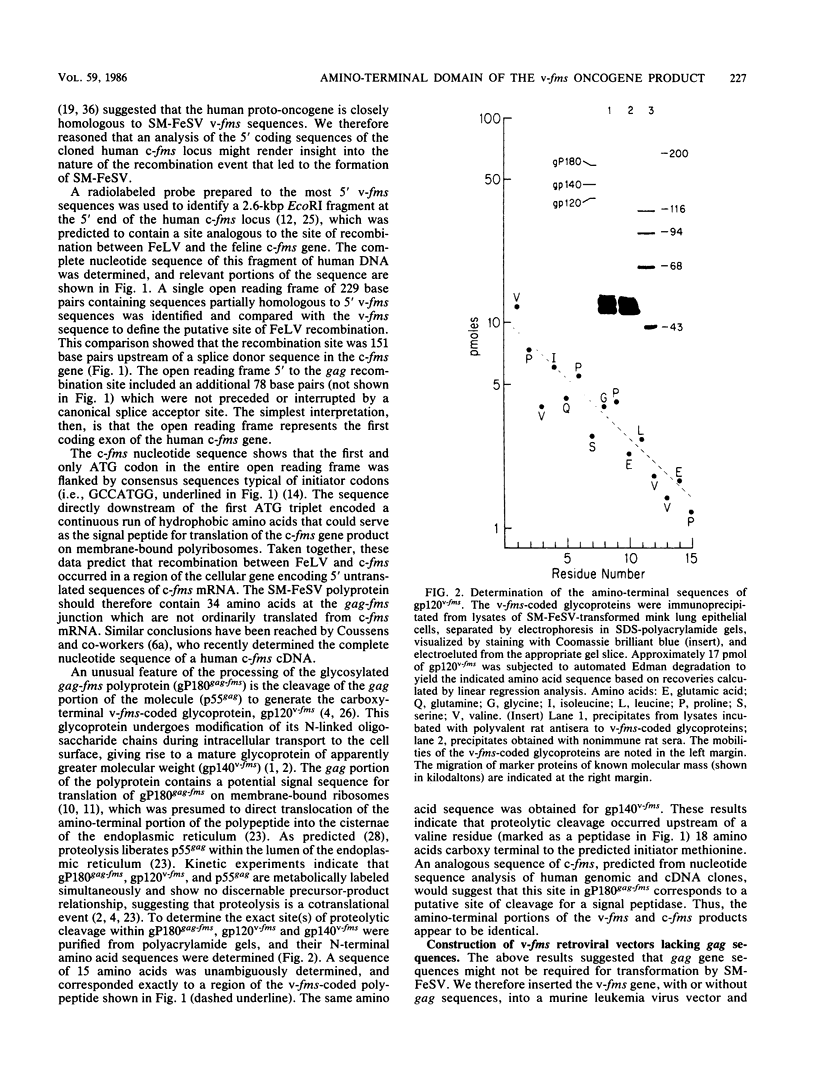
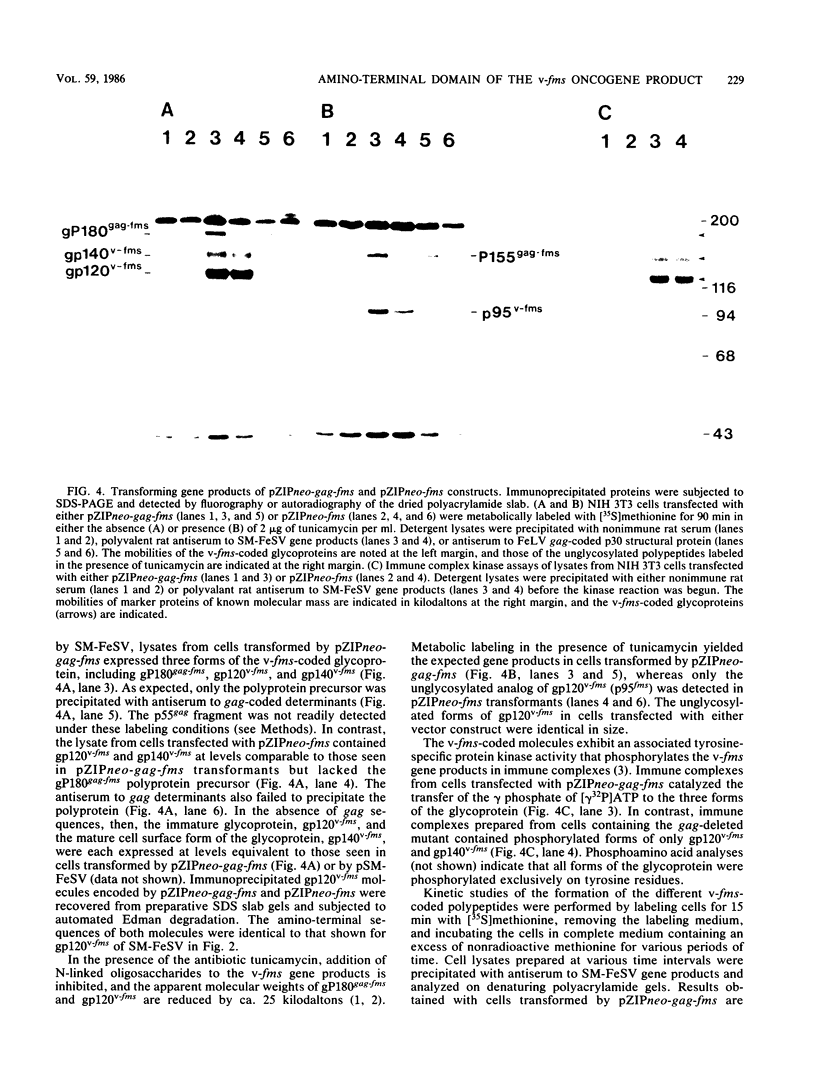
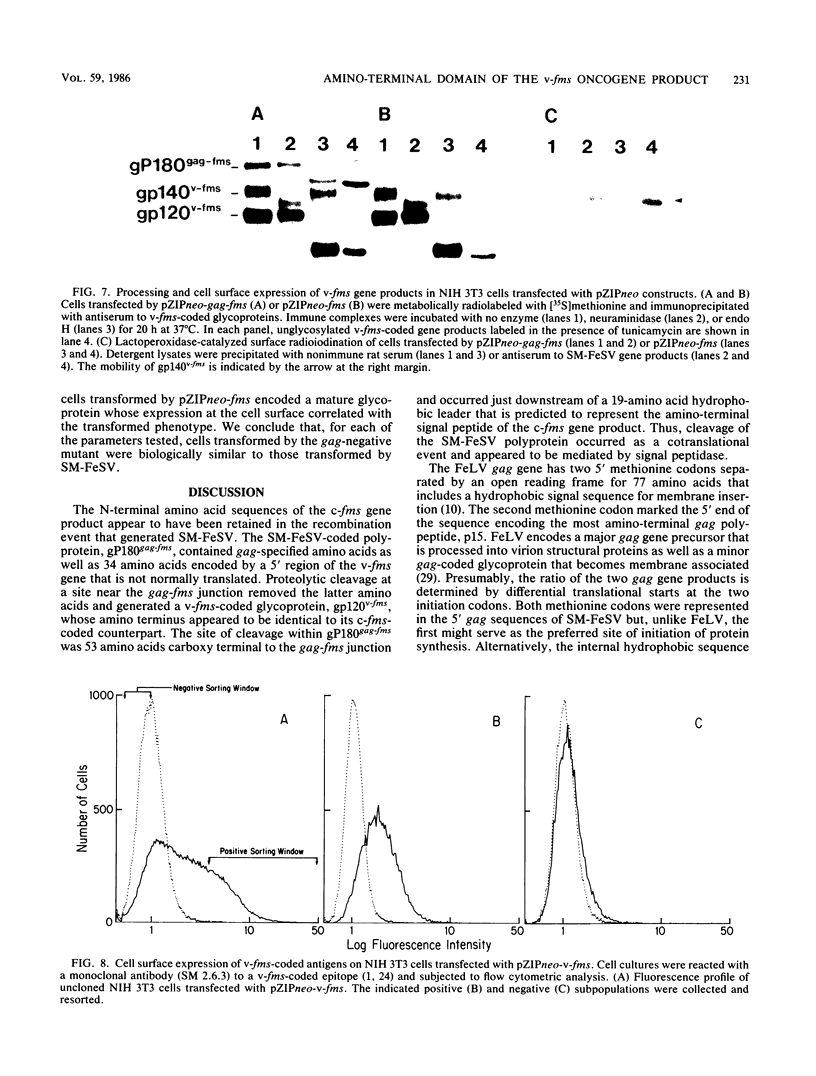
The nucleotide sequence of a 5' segment of the human genomic c-fms proto-oncogene suggested that recombination between feline leukemia virus and feline c-fms sequences might have occurred in a region encoding the 5' untranslated portion of c-fms mRNA. The polyprotein precursor gP180gag-fms encoded by the McDonough strain of feline sarcoma virus was therefore predicted to contain 34 v-fms-coded amino acids derived from sequences of the c-fms gene that are not ordinarily translated from the proto-oncogene mRNA. The (gP180gag-fms) polyprotein was cotranslationally cleaved near the gag-fms junction to remove its gag gene-coded portion. Determination of the amino-terminal sequence of the resulting v-fms-coded glycoprotein, gp120v-fms, showed that the site of proteolysis corresponded to a predicted signal peptidase cleavage site within the c-fms gene product. Together, these analyses suggested that the linked gag sequences may not be necessary for expression of a biologically active v-fms gene product. The gag-fms sequences of feline sarcoma virus strain McDonough and the v-fms sequences alone were inserted into a murine retroviral vector containing a neomycin resistance gene. Both constructs were biologically active when transfected into NIH 3T3 cells and produced morphologically transformed foci at equivalent efficiencies. When transfected into a cell line (psi 2) expressing complementary viral gene functions, G418-resistant (Neor) cells containing either of these vector DNAs produced high titers of transforming viruses. Analysis of proteins produced in cells containing the vector lacking gag gene sequences showed that gP180gag-fms was not synthesized, whereas normal levels of both immature gp120v-fms and mature gp140v-fms were detected. The glycoprotein was efficiently transported to the cell surface, and it retained wild-type tyrosine kinase activity. We conclude that a cryptic hydrophobic signal peptide sequence in v-fms was unmasked by gag deletion, thereby allowing the correct orientation and transport of the v-fms gene product within membranous organelles. It seems likely that the proteolytic cleavage of gP180gag-fms is mediated by signal peptidase and that the amino termini of gp140v-fms and the c-fms gene product are identical.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Furth M., Wolff L., Ruscetti S. K., Sherr C. J. Monoclonal antibodies to the transformation-specific glycoprotein encoded by the feline retroviral oncogene v-fms. J Virol. 1982 Nov;44(2):696–702. doi: 10.1128/jvi.44.2.696-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. J., Gonda M. A., Rettenmier C. W., Sherr C. J. Subcellular localization of glycoproteins encoded by the viral oncogene v-fms. J Virol. 1984 Sep;51(3):730–741. doi: 10.1128/jvi.51.3.730-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V., Devare S. G. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. doi: 10.1128/jvi.33.1.196-207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V. Gene products of McDonough feline sarcoma virus have an in vitro-associated protein kinase that phosphorylates tyrosine residues: lack of detection of this enzymatic activity in vivo. J Virol. 1981 Dec;40(3):812–821. doi: 10.1128/jvi.40.3.812-821.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A., Hanafusa H. A fps gene without gag gene sequences transforms cells in culture and induces tumors in chickens. J Virol. 1983 Dec;48(3):744–751. doi: 10.1128/jvi.48.3.744-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A., Shibuya M., Hanafusa H. Activation of the transformation potential of the cellular fps gene. Cell. 1985 Aug;42(1):105–115. doi: 10.1016/s0092-8674(85)80106-9. [DOI] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Even J., Sherr C. J., Galibert F. Nucleotide sequences of feline sarcoma virus long terminal repeats and 5' leaders show extensive homology to those of other mammalian retroviruses. J Virol. 1983 Jan;45(1):466–472. doi: 10.1128/jvi.45.1.466-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. Isolation of v-fms and its human cellular homolog. Virology. 1983 Apr 15;126(1):248–258. doi: 10.1016/0042-6822(83)90476-2. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Miller A. D., Verma I. M., Curran T. Deletion of the gag region from FBR murine osteosarcoma virus does not affect its enhanced transforming activity. J Virol. 1985 Sep;55(3):521–526. doi: 10.1128/jvi.55.3.521-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Bunn H. F., Turner P. H., Gopal T. V., Nash W. G., O'Brien S. J., Sherr C. J. Expression of the human c-fms proto-oncogene in hematopoietic cells and its deletion in the 5q- syndrome. Cell. 1985 Sep;42(2):421–428. doi: 10.1016/0092-8674(85)90099-6. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Quinn C. O., Kitchingman G. R., Look A. T., Sherr C. J. Transmembrane orientation of glycoproteins encoded by the v-fms oncogene. Cell. 1985 Apr;40(4):971–981. doi: 10.1016/0092-8674(85)90357-5. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Sherr C. J., Barker P. E., Ruddle F. H. Molecular cloning of the c-fms locus and its assignment to human chromosome 5. J Virol. 1983 Dec;48(3):770–773. doi: 10.1128/jvi.48.3.770-773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Turek L. P., Sherr C. J. Three independent isolates of feline sarcoma virus code for three distinct gag-x polyproteins. J Virol. 1980 Jul;35(1):259–264. doi: 10.1128/jvi.35.1.259-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca R., Stanley E. R., Sherr C. J., Rettenmier C. W. Specific binding of the mononuclear phagocyte colony-stimulating factor CSF-1 to the product of the v-fms oncogene. Proc Natl Acad Sci U S A. 1986 May;83(10):3331–3335. doi: 10.1073/pnas.83.10.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Sharar A. L., McDonough S. The SM strain of feline sarcoma virus. Biologic and antigenic characterization of virus. Proc Soc Exp Biol Med. 1972 Sep;140(4):1365–1368. doi: 10.3181/00379727-140-36675. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Nalewaik R. P., Stephenson J. R. Characterization of a 170,000-dalton polyprotein encoded by the McDonough strain of feline sarcoma virus. J Virol. 1980 Jul;35(1):165–175. doi: 10.1128/jvi.35.1.165-175.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek J. S., Roebroek A. J., van den Ouweland A. M., Bloemers H. P., Van de Ven W. J. Human c-fms proto-oncogene: comparative analysis with an abnormal allele. Mol Cell Biol. 1985 Feb;5(2):422–426. doi: 10.1128/mcb.5.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]