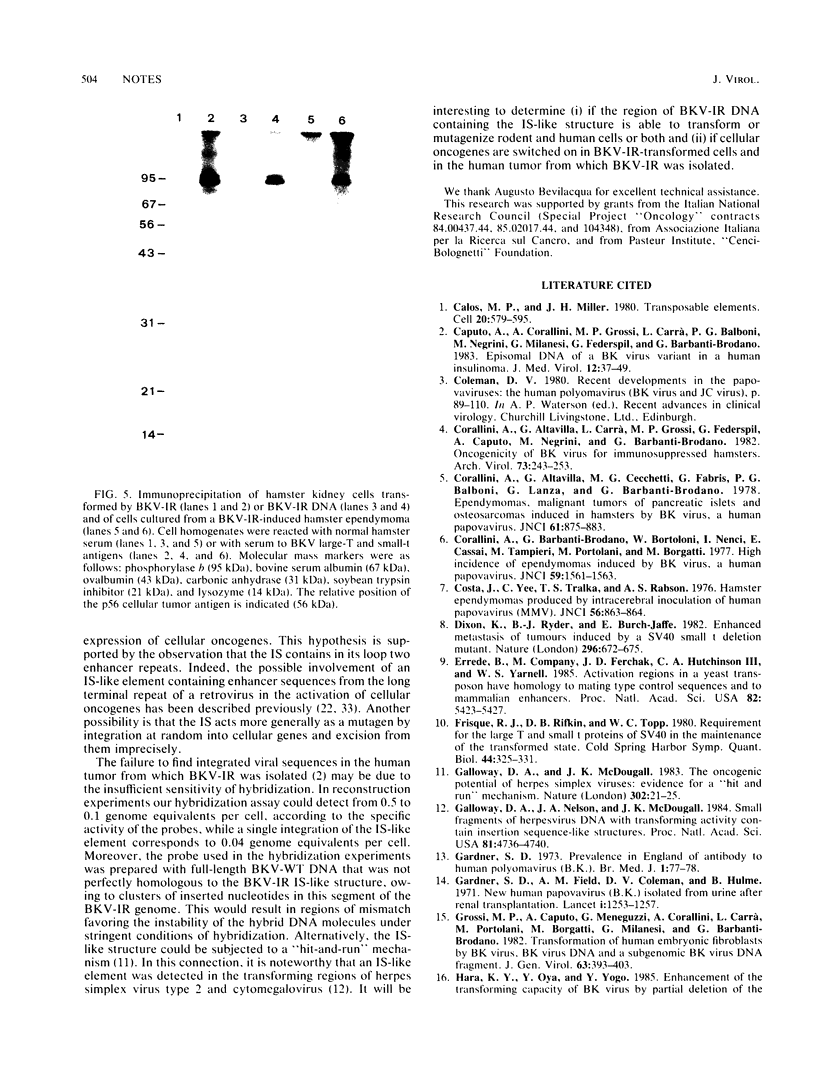
Abstract
We describe the molecular and biological properties of BK virus (BKV)-IR, a new BKV variant isolated from a human tumor of pancreatic islets. BKV-IR bears a 253-base-pair (bp) deletion and an 80-bp insertion in the early region of the genome. The deletion abolishes the expression of small-t antigen. The inserted sequences, grouped in four clusters, produce rearrangements in the first and second enhancer elements. They are bound by 12-bp direct repeats and could form a 217-base stem-loop structure suggestive of an insertion sequence. As compared with wild-type BKV, BKV-IR transformed hamster cells with a reduced efficiency and induced ependymomas in hamsters at a lower frequency and with a longer latency period. Tumors induced by BKV-IR, however, showed features of higher malignancy. The possible role of the insertion sequence-like element in transformation by BKV-IR is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Caputo A., Corallini A., Grossi M. P., Carrà L., Balboni P. G., Negrini M., Milanesi G., Federspil G., Barbanti-Brodano G. Episomal DNA of a BK virus variant in a human insulinoma. J Med Virol. 1983;12(1):37–49. doi: 10.1002/jmv.1890120105. [DOI] [PubMed] [Google Scholar]
- Corallini A., Altavilla G., Carra L., Grossi M. P., Federspil G., Caputo A., Negrini M., Barbanti-Brodano G. Oncogenity of BK virus for immunosuppressed hamsters. Arch Virol. 1982;73(3-4):243–253. doi: 10.1007/BF01318078. [DOI] [PubMed] [Google Scholar]
- Corallini A., Altavilla G., Cecchetti M. G., Fabris G., Grossi M. P., Balboni P. G., Lanza G., Barbanti-Brodano G. Ependymomas, malignant tumors of pancreatic islets, and osteosarcomas induced in hamsters by BK virus, a human papovavirus. J Natl Cancer Inst. 1978 Sep;61(3):875–883. [PubMed] [Google Scholar]
- Corallini A., Barbanti-Brodano G., Bortoloni W., Nenci I., Cassai E., Tampieri M., Portolani M., Borgatti M. High incidence of ependymomas induced by BK virus, a human papovavirus: brief communication. J Natl Cancer Inst. 1977 Nov;59(5):1561–1564. doi: 10.1093/jnci/59.5.1561. [DOI] [PubMed] [Google Scholar]
- Costa J., Yee C., Tralka T. S., Rabson A. S. Hamster ependymomas produced by intracerebral inoculation of a human papovavirus (MMV). J Natl Cancer Inst. 1976 Apr;56(4):863–864. doi: 10.1093/jnci/56.4.863. [DOI] [PubMed] [Google Scholar]
- Dixon K., Ryder B. J., Burch-Jaffe E. Enhanced metastasis of tumours induced by a SV40 small T deletion mutant. Nature. 1982 Apr 15;296(5858):672–675. doi: 10.1038/296672a0. [DOI] [PubMed] [Google Scholar]
- Errede B., Company M., Ferchak J. D., Hutchison C. A., 3rd, Yarnell W. S. Activation regions in a yeast transposon have homology to mating type control sequences and to mammalian enhancers. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5423–5427. doi: 10.1073/pnas.82.16.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Nelson J. A., McDougall J. K. Small fragments of herpesvirus DNA with transforming activity contain insertion sequence-like structures. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4736–4740. doi: 10.1073/pnas.81.15.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Oya Y., Yogo Y. Enhancement of the transforming capacity of BK virus by partial deletion of the 68-base-pair tandem repeats. J Virol. 1985 Sep;55(3):867–869. doi: 10.1128/jvi.55.3.867-869.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M. DNA sequence of human papovavirus BK. Nature. 1980 Mar 13;284(5752):124–125. doi: 10.1038/284124a0. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Physical map of the BK virus genome. J Virol. 1975 Oct;16(4):959–973. doi: 10.1128/jvi.16.4.959-973.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Rechavi G., Givol D., Canaani E. Homology between an endogenous viral LTR and sequences inserted in an activated cellular oncogene. Nature. 1983 Apr 7;302(5908):547–548. doi: 10.1038/302547a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Martin R. G. Oncogenicity of simian virus 40 deletion mutants that induce altered 17-kilodalton t-proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4299–4302. doi: 10.1073/pnas.76.9.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Mason D. H., Jr, Takemoto K. K. Transformation of rabbit kidney cells by BKV(MM) human papovavirus. Int J Cancer. 1977 Mar 15;19(3):391–395. doi: 10.1002/ijc.2910190317. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Portolani M., Marzocchi A., Barbanti-Brodano G., La Placa M. Prevalence in Italy of antibodies to a new human papovavirus (BK virus). J Med Microbiol. 1974 Nov;7(4):543–546. doi: 10.1099/00222615-7-4-543. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Fareed G. C. Transformation of human embryonic kidney cells by human papovarirus BK. J Virol. 1979 Feb;29(2):763–769. doi: 10.1128/jvi.29.2.763-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Rose A. B., Pearlman R. E. Transposable element sequences involved in the enhancement of yeast gene expression. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5428–5432. doi: 10.1073/pnas.82.16.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Warszawski R. M. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis. 1973 Dec;128(6):784–787. doi: 10.1093/infdis/128.6.784. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Characterization of tau antigens isolated from uninfected and simian virus 40-infected monkey cells and papovavirus-transformed cells. J Virol. 1980 Nov;36(2):519–525. doi: 10.1128/jvi.36.2.519-525.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- Topp W. C., Rifkin D. B., Sleigh M. J. SV40 mutants with an altered small-t protein are tumorigenic in newborn hamsters. Virology. 1981 Jun;111(2):341–350. doi: 10.1016/0042-6822(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Furuno A., Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J Natl Cancer Inst. 1979 Jul;63(1):119–126. [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Kato K., Furuno A. Induction of papillary ependymomas and insulinomas in the Syrian golden hamster by BK virus, a human papovavirus. Gan. 1976 Dec;67(6):857–865. [PubMed] [Google Scholar]
- Watanabe S., Soeda E., Uchida S., Yoshiike K. DNA rearrangement affecting expression of the BK virus transforming gene. J Virol. 1984 Jul;51(1):1–6. doi: 10.1128/jvi.51.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Decreasing the number of 68-base-pair tandem repeats in the BK virus transcriptional control region reduces plaque size and enhances transforming capacity. J Virol. 1985 Sep;55(3):823–825. doi: 10.1128/jvi.55.3.823-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Schegget J., Sol C. J., Baan E. W., van der Noordaa J., van Ormondt H. Naturally occurring BK virus variants (JL and Dik) with deletions in the putative early enhancer-promoter sequences. J Virol. 1985 Jan;53(1):302–305. doi: 10.1128/jvi.53.1.302-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]