Abstract
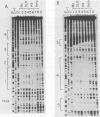
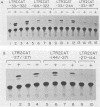
Human T-cell leukemia virus types I (HTLV-I) and II (HTLV-II) are human retroviruses which normally infect T-lymphoid cells. HTLV-I infection is associated with adult T-cell leukemia-lymphoma, and HTLV-II is associated with an indolent form of hairy-cell leukemia. To identify potential transcriptional regulatory elements of these two related human retroviruses, we performed DNase I footprinting of both the HTLV-I and HTLV-II long terminal repeats (LTRs) by using extracts prepared from uninfected T cells, HTLV-I and HTLV-II transformed T cells, and HeLa cells. Five regions of the HTLV-I LTR and three regions of the HTLV-II LTR showed protection by DNase I footprinting. All three of the 21-base-pair repeats previously shown to be important in HTLV transcriptional regulation were protected in the HTLV-I LTR, whereas only one of these repeats was protected in the HTLV-II LTR. Several regions exhibited altered protection in extracts prepared from lymphoid cells as compared with HeLa cells, but there were minimal differences in the protection patterns between HTLV-infected and uninfected lymphoid extracts. A number of HTLV-I and HTLV-II LTR fragments which contained regions showing protection in DNase I footprinting were able to function as inducible enhancer elements in transient CAT gene expression assays in the presence of the HTLV-II tat protein. The alterations in the pattern of the cellular proteins which bind to the HTLV-I and HTLV-II LTRs may in part be responsible for differences in the transcriptional regulation of these two related viruses.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Brady J., Jeang K. T., Duvall J., Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987 Jul;61(7):2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann A. J., Rosenblatt J. D., Wachsman W., Shah N. P., Chen I. S. Identification of the gene responsible for human T-cell leukaemia virus transcriptional regulation. Nature. 1985 Dec 12;318(6046):571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Cann A. J., Shah N. P., Gaynor R. B. Functional relation between HTLV-II x and adenovirus E1A proteins in transcriptional activation. Science. 1985 Nov 1;230(4725):570–573. doi: 10.1126/science.2996140. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Quan S. G., Golde D. W. Human T-cell leukemia virus type II transforms normal human lymphocytes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7006–7009. doi: 10.1073/pnas.80.22.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Slamon D. J., Rosenblatt J. D., Shah N. P., Quan S. G., Wachsman W. The x gene is essential for HTLV replication. Science. 1985 Jul 5;229(4708):54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Nucleotide sequences of the murine retrovirus Friend SFFVp long terminal repeats: identification of a structure with extensive dyad symmetry 5' to the TATA box. Nucleic Acids Res. 1982 May 25;10(10):3315–3330. doi: 10.1093/nar/10.10.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., DeLorbe W., Swanstrom R., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. The nucleotide sequence of an untranslated but conserved domain at the 3' end of the avian sarcoma virus genome. Nucleic Acids Res. 1980 Jul 11;8(13):2967–2984. doi: 10.1093/nar/8.13.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson I., Fromental C., Augereau P., Wildeman A., Zenke M., Chambon P. Cell-type specific protein binding to the enhancer of simian virus 40 in nuclear extracts. Nature. 1986 Oct 9;323(6088):544–548. doi: 10.1038/323544a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Sato M., Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40 chi HTLV-I. EMBO J. 1986 Apr;5(4):713–718. doi: 10.1002/j.1460-2075.1986.tb04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Kalyanaraman V. S., Sarngadharan M. G., Sliski A., Vonderheid E. C., Maeda M., Nakao Y., Yamada K., Ito Y., Gutensohn N. Association of the human type C retrovirus with a subset of adult T-cell cancers. Cancer Res. 1983 Aug;43(8):3892–3899. [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Garcia J., Wu F., Gaynor R. Upstream regulatory regions required to stabilize binding to the TATA sequence in an adenovirus early promoter. Nucleic Acids Res. 1987 Oct 26;15(20):8367–8385. doi: 10.1093/nar/15.20.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giam C. Z., Nerenberg M., Khoury G., Jay G. Expression of the complete human T-cell leukemia virus type I pX coding sequence as a functional protein in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7192–7196. doi: 10.1073/pnas.83.19.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. C., Sodroski J., Rosen C., Essex M., Haseltine W. A. Subcellular localization of the product of the long open reading frame of human T-cell leukemia virus type I. Science. 1985 Mar 8;227(4691):1227–1228. doi: 10.1126/science.2983419. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Sodroski J., Patarca R., Briggs D., Perkins D., Wong-Staal F. Structure of 3' terminal region of type II human T lymphotropic virus: evidence for new coding region. Science. 1984 Jul 27;225(4660):419–421. doi: 10.1126/science.6330894. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A enhancer contains two functionally distinct domains: one is specific for E1A and the other modulates all early units in cis. Cell. 1986 Apr 25;45(2):229–236. doi: 10.1016/0092-8674(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Bunn P. A., Minna J. D., Gallo R. C. Antibodies in human sera reactive against an internal structural protein of human T-cell lymphoma virus. Nature. 1981 Nov 19;294(5838):271–273. doi: 10.1038/294271a0. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Iwashita S., Imagawa K., Shimizu F., Yoshida M. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Chen I. S., Golde D. W. Characterization of a novel HTLV-infected cell line. Blood. 1984 Aug;64(2):482–490. [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Green M. R. A cellular transcription factor E4F1 interacts with an E1a-inducible enhancer and mediates constitutive enhancer function in vitro. EMBO J. 1987 May;6(5):1345–1353. doi: 10.1002/j.1460-2075.1987.tb02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Sodroski J. G., Haseltine W. A., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Essex M. Antigens encoded by the 3'-terminal region of human T-cell leukemia virus: evidence for a functional gene. Science. 1984 Oct 5;226(4670):57–61. doi: 10.1126/science.6089350. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Ohtani K., Nakamura M., Saito S., Noda T., Ito Y., Sugamura K., Hinuma Y. Identification of two distinct elements in the long terminal repeat of HTLV-I responsible for maximum gene expression. EMBO J. 1987 Feb;6(2):389–395. doi: 10.1002/j.1460-2075.1987.tb04767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskalis H., Felber B. K., Pavlakis G. N. Cis-acting sequences responsible for the transcriptional activation of human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. Cell-type preference of immunoglobulin kappa and lambda gene promoters. EMBO J. 1985 Nov;4(11):2831–2838. doi: 10.1002/j.1460-2075.1985.tb04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Park R., Sodroski J. G., Haseltine W. A. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4919–4923. doi: 10.1073/pnas.84.14.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. Location of cis-acting regulatory sequences in the human T-cell leukemia virus type I long terminal repeat. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6502–6506. doi: 10.1073/pnas.82.19.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Haseltine W. A. Activation of enhancer sequences in type II human T-cell leukemia virus and bovine leukemia virus long terminal repeats by virus-associated trans-acting regulatory factors. J Virol. 1986 Mar;57(3):738–744. doi: 10.1128/jvi.57.3.738-744.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. D., Golde D. W., Wachsman W., Giorgi J. V., Jacobs A., Schmidt G. M., Quan S., Gasson J. C., Chen I. S. A second isolate of HTLV-II associated with atypical hairy-cell leukemia. N Engl J Med. 1986 Aug 7;315(6):372–377. doi: 10.1056/NEJM198608073150606. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Golde D. W. T-lymphocyte variant of hairy-cell leukemia. Ann Intern Med. 1978 Mar;88(3):323–326. doi: 10.7326/0003-4819-88-3-323. [DOI] [PubMed] [Google Scholar]
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N. P., Wachsman W., Cann A. J., Souza L., Slamon D. J., Chen I. S. Comparison of the trans-activation capabilities of the human T-cell leukemia virus type I and II chi proteins. Mol Cell Biol. 1986 Nov;6(11):3626–3631. doi: 10.1128/mcb.6.11.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takano M., Teruuchi T., Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Shimotohno K., Cline M. J., Golde D. W., Chen I. S. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science. 1984 Oct 5;226(4670):61–65. doi: 10.1126/science.6089351. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Goh W. C., Rosen C. A., Salahuddin S. Z., Aldovini A., Franchini G., Wong-Staal F., Gallo R. C., Sugamura K., Hinuma Y. trans-Activation of the human T-cell leukemia virus long terminal repeat correlates with expression of the x-lor protein. J Virol. 1985 Sep;55(3):831–835. doi: 10.1128/jvi.55.3.831-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Wachsman W., Shimotohno K., Clark S. C., Golde D. W., Chen I. S. Expression of the 3' terminal region of human T-cell leukemia viruses. Science. 1984 Oct 12;226(4671):177–179. doi: 10.1126/science.6091270. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Garcia J., Mitsuyasu R., Gaynor R. Alterations in binding characteristics of the human immunodeficiency virus enhancer factor. J Virol. 1988 Jan;62(1):218–225. doi: 10.1128/jvi.62.1.218-225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S., Dean N., Han M., Berk A. J. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]