Abstract
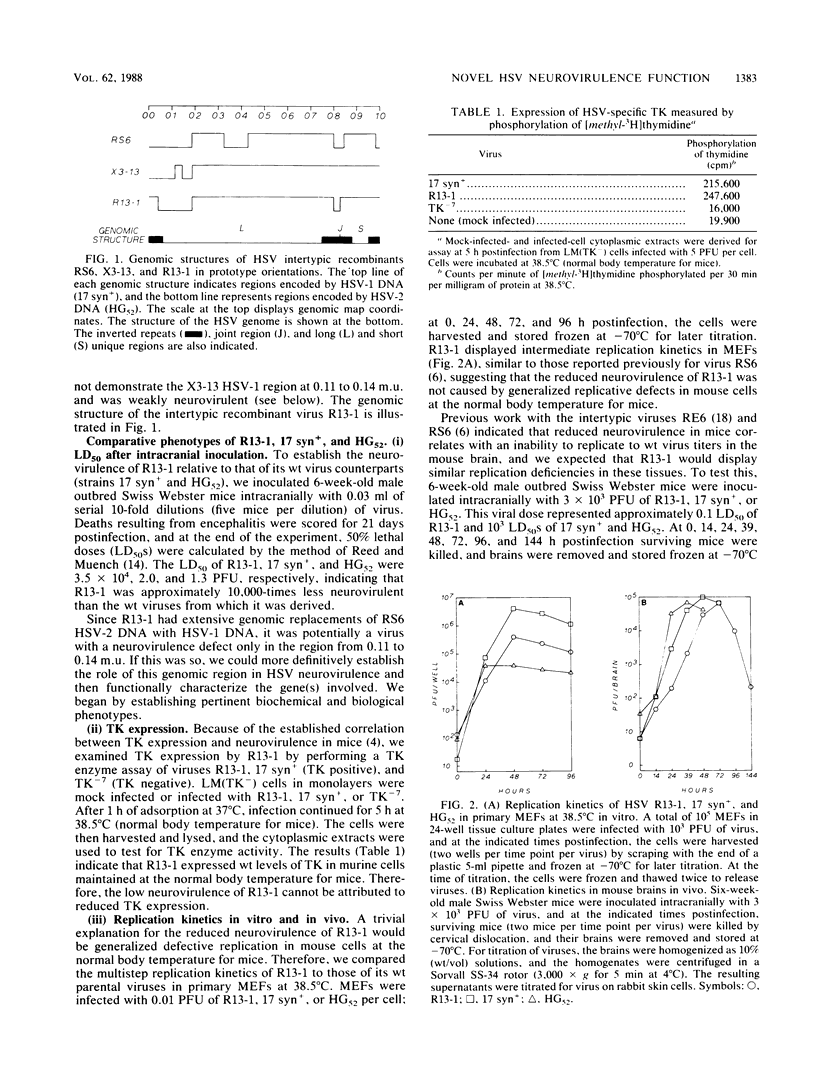
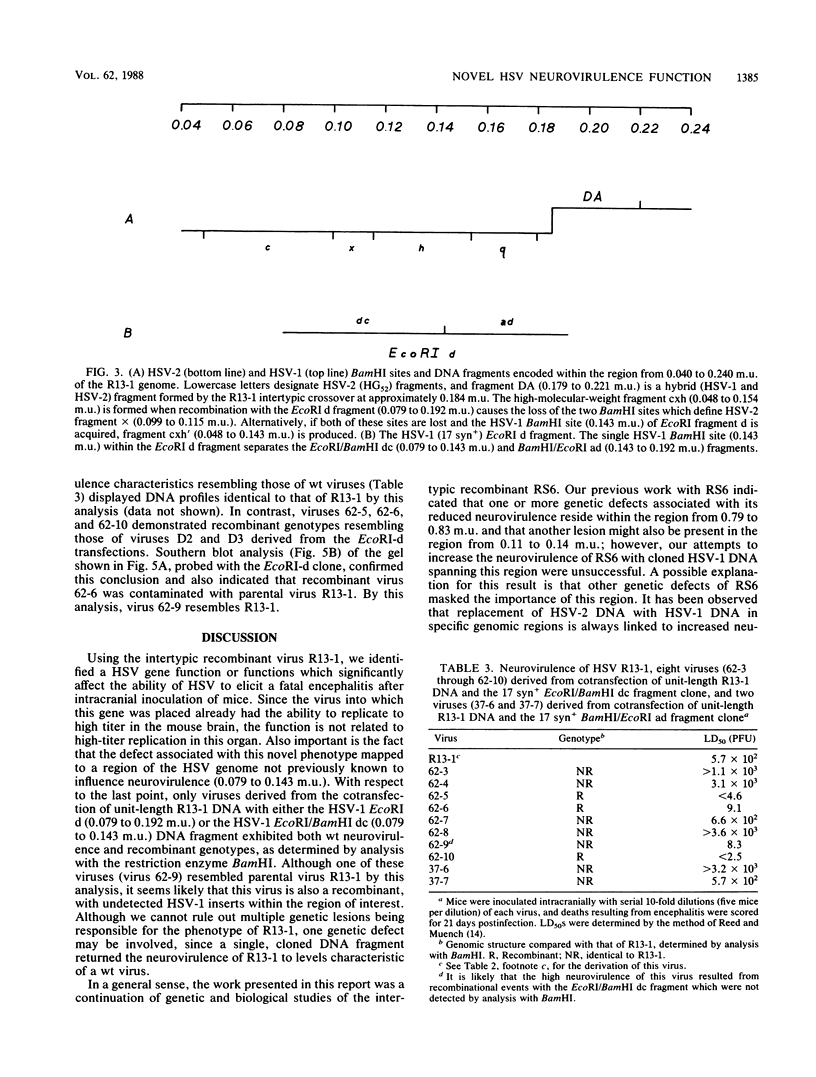
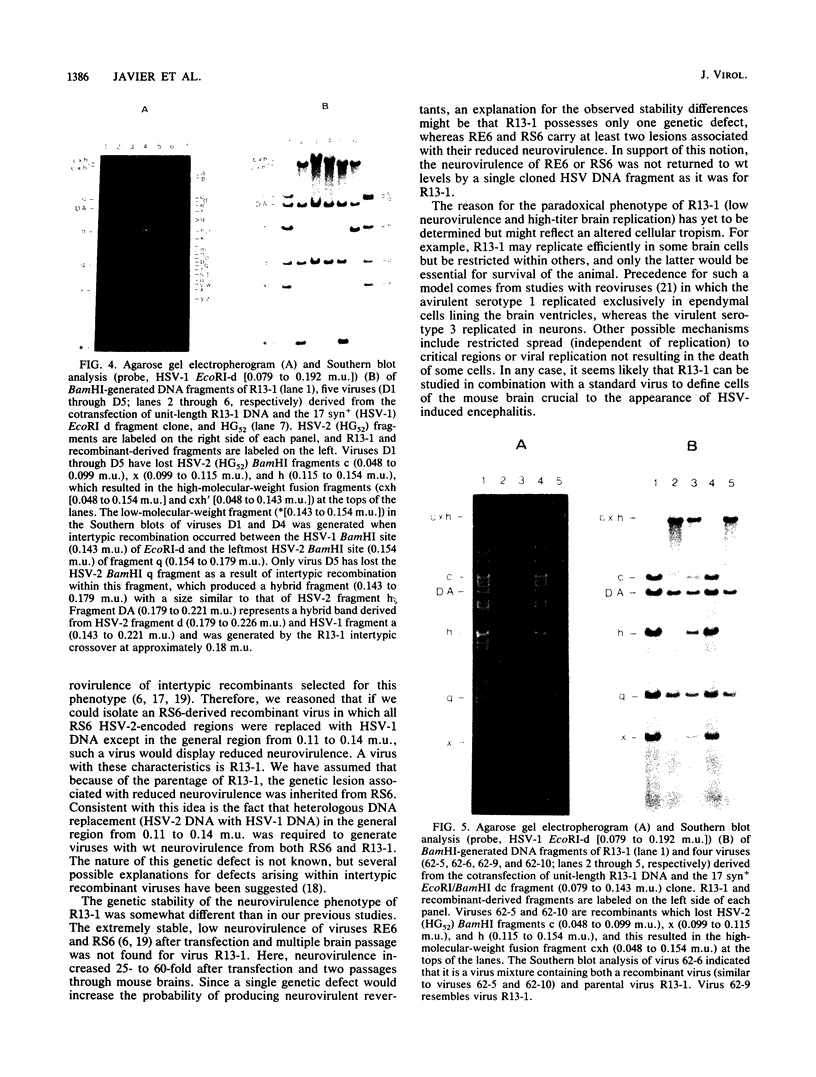
Previous studies with the herpes simplex virus type 1 X type 2 intertypic recombinant RS6 suggested that the genomic region from 0.11 to 0.14 map units is involved in neurovirulence (R. T. Javier, R. L. Thompson, and J. G. Stevens, J. Virol. 61:1978-1984, 1987). To study this further, we isolated an RS6-derived herpes simplex virus intertypic recombinant (R13-1) which has a genetic defect within this area. After inoculation into mouse brains, R13-1 was found to be approximately 10,000-fold less neurovirulent than either the wild-type type 1 or type 2 parental virus. However, R13-1 replicated in the mouse brain to titers resembling those of the wild-type parents. Further comparisons with wild-type counterparts indicated that R13-1 expressed equivalent levels of the enzyme thymidine kinase and replicated to intermediate levels in primary mouse embryo fibroblasts maintained at the normal body temperature for mice. Using marker rescue techniques combined with in vivo selection, we found that recombination between unit-length R13-1 DNA and a cloned type 1 DNA fragment spanning the region from 0.11 to 0.14 map units (EcoRI-d, 0.079 to 0.192 map units) generated viruses with a wild-type neurovirulence phenotype. To further refine the genomic region of interest, we performed marker rescue experiments using two EcoRI-d subclones, EcoRI/BamHI dc (0.079 to 0.143 map units) and BamHI/EcoRI and (0.143 to 0.192 map units), representing the left and right halves of the EcoRI d fragment, respectively. In these experiments the EcoRI/BamHI dc clone, but not the BamHI/EcoRI ad clone, yielded recombinant viruses exhibiting wild-type neurovirulence. These results show that at least one herpes simplex virus gene function associated with neurovirulence is located within a 9.1-kilobase region at 0.079 to 0.143 map units of the viral genome. Perhaps more significantly, the results indicate that this neurovirulence property functions independently of high-titer virus replication in the brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. T., Parris D. S., Dixon R. A., Farber F. E., Schaffer P. A. Hydroxylamine mutagenesis of HSV DNA and DNA fragments: introduction of mutations into selected regions of the viral genome. Virology. 1979 Oct 15;98(1):168–181. doi: 10.1016/0042-6822(79)90535-x. [DOI] [PubMed] [Google Scholar]
- Field H. J., Coen D. M. Pathogenicity of herpes simplex virus mutants containing drug resistance mutations in the viral DNA polymerase gene. J Virol. 1986 Oct;60(1):286–289. doi: 10.1128/jvi.60.1.286-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Javier R. T., Thompson R. L., Stevens J. G. Genetic and biological analyses of a herpes simplex virus intertypic recombinant reduced specifically for neurovirulence. J Virol. 1987 Jun;61(6):1978–1984. doi: 10.1128/jvi.61.6.1978-1984.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kaerner H. C., Schröder C. H., Ott-Hartmann A., Kümel G., Kirchner H. Genetic variability of herpes simplex virus: development of a pathogenic variant during passaging of a nonpathogenic herpes simplex virus type 1 virus strain in mouse brain. J Virol. 1983 Apr;46(1):83–93. doi: 10.1128/jvi.46.1.83-93.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980 Sep;35(3):798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz B., Subak-Sharpe J. H., Preston V. G. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 using cloned restriction endonuclease fragments. J Gen Virol. 1983 Oct;64(Pt 10):2261–2270. doi: 10.1099/0022-1317-64-10-2261. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Dixon R. A., Schaffer P. A. Physical mapping of herpes simplex virus type 1 ts mutants by marker rescue: correlation of the physical and genetic maps. Virology. 1980 Jan 30;100(2):275–287. doi: 10.1016/0042-6822(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. DNA processing in temperature-sensitive morphogenic mutants of HSV-1. Virology. 1987 Jun;158(2):427–430. doi: 10.1016/0042-6822(87)90214-5. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Devi-Rao G. V., Stevens J. G., Wagner E. K. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J Virol. 1985 Aug;55(2):504–508. doi: 10.1128/jvi.55.2.504-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Stevens J. G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983 Nov;131(1):171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]