Abstract
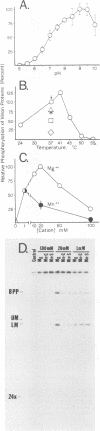
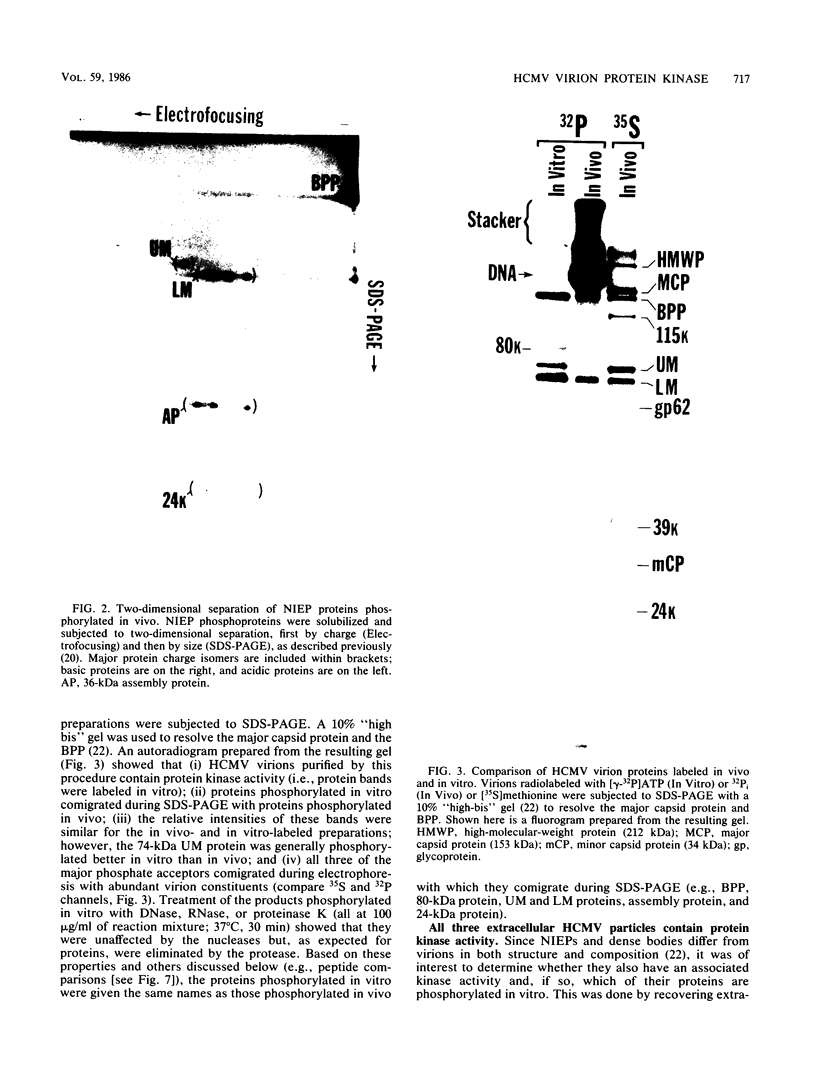
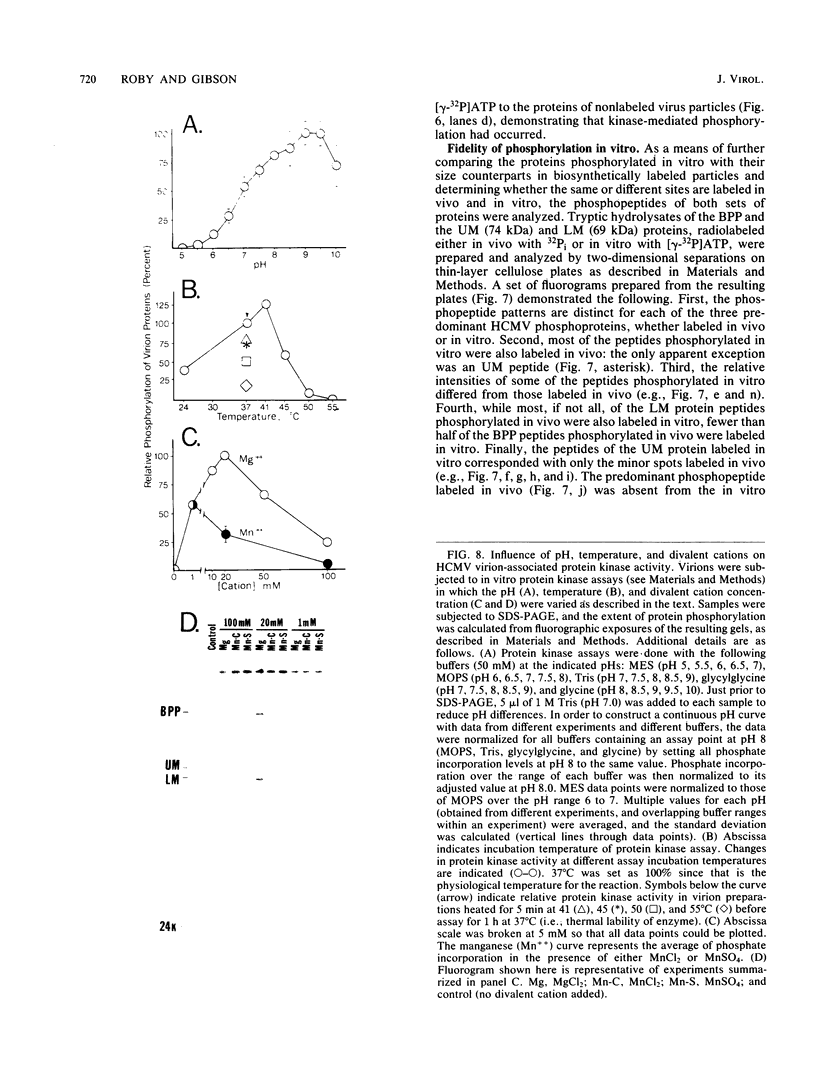
Phosphorylation of the proteins of human cytomegalovirus (CMV) virions, noninfectious enveloped particles (NIEPs), and dense bodies was investigated. Analyses of particles phosphorylated in vivo showed the following. Virions contain three predominant phosphoproteins (i.e., basic phosphoprotein and upper and lower matrix proteins) and at least nine minor phosphorylated species. NIEPs contain all of these and one additional major species, the assembly protein. Dense bodies contain only one (i.e., lower matrix) of the predominant and four of the minor virion phosphoproteins. Two-dimensional (charge-size) separations in denaturing polyacrylamide gels showed that the relative net charges of the predominant phosphorylated species ranged from the basic phosphoprotein to the more neutral upper matrix protein. In vitro assays showed that purified virions of human CMV have an associated protein kinase activity. The activity was detected only after disrupting the envelope; it had a pH optimum of approximately 9 to 9.5 and required a divalent cation, preferring magnesium to manganese. In vitro, this activity catalyzed phosphorylation of the virion proteins observed to be phosphorylated in vivo. Peptide comparisons indicated that the sites phosphorylated in vitro are a subset of those phosphorylated in vivo, underscoring the probable biological relevance of the kinase activity. Casein, phosvitin, and to a minor extent lysine-rich histones served as exogenous phosphate acceptors. Arginine-rich and lysine-rich histones and protamine sulfate, as well as the polyamines spermine and spermidine, stimulated incorporation of phosphate into the endogenous viral proteins. Virions of all human and simian CMV strains tested showed this activity. Analyses of other virus particles, including three intracellular capsid forms (i.e., A, B, and C capsids), NIEPs, and dense bodies, indicated that the active enzyme was not present in the capsid. Rate-velocity sedimentation of disrupted virions separated the protein kinase activity into two fractions: one that phosphorylated exogenous casein and another that phosphorylated primarily the endogenous virion proteins.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin C., Robinson W. S. Protein kinase activity in hepatitis B virus. J Virol. 1980 Apr;34(1):297–302. doi: 10.1128/jvi.34.1.297-302.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai R., Lazarus L. H., Goldblum N. Viscosity-density gradient for purification of foot-and-mouth disease virus. Arch Gesamte Virusforsch. 1972;36(1):141–146. doi: 10.1007/BF01250304. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Davis M. G., Huang E. S. Nucleotide sequence of a human cytomegalovirus DNA fragment encoding a 67-kilodalton phosphorylated viral protein. J Virol. 1985 Oct;56(1):7–11. doi: 10.1128/jvi.56.1.7-11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Mar E. C., Wu Y. M., Huang E. S. Mapping and expression of a human cytomegalovirus major viral protein. J Virol. 1984 Oct;52(1):129–135. doi: 10.1128/jvi.52.1.129-135.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Flügel R. M., Darai G. Protein kinase and specific phosphate acceptor proteins associated with tupaia herpesvirus. J Virol. 1982 Aug;43(2):410–415. doi: 10.1128/jvi.43.2.410-415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs W. E., Grose C. Varicella-zoster virus p32/p36 complex is present in both the viral capsid and the nuclear matrix of the infected cell. J Virol. 1986 Jan;57(1):155–164. doi: 10.1128/jvi.57.1.155-164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Irmiere A. Selection of particles and proteins for use as human cytomegalovirus subunit vaccines. Birth Defects Orig Artic Ser. 1984;20(1):305–324. [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Gibson W. Replica images of silver-stained gels using direct duplicating film. Anal Biochem. 1983 Jul 1;132(1):171–173. doi: 10.1016/0003-2697(83)90443-8. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Gibson W., van Breemen R., Fields A., LaFemina R., Irmiere A. D,L-alpha-difluoromethylornithine inhibits human cytomegalovirus replication. J Virol. 1984 Apr;50(1):145–154. doi: 10.1128/jvi.50.1.145-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Viron-associated protein kinase and its involvement in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus. Virology. 1972 Jun;48(3):847–851. doi: 10.1016/0042-6822(72)90167-5. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Gibson W. Characterization of the mRNA's for the polyoma virus capsid proteins VP1, VP2, and VP3. J Virol. 1978 Oct;28(1):240–253. doi: 10.1128/jvi.28.1.240-253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation of human cytomegalovirus intranuclear capsids, characterization of their protein constituents, and demonstration that the B-capsid assembly protein is also abundant in noninfectious enveloped particles. J Virol. 1985 Oct;56(1):277–283. doi: 10.1128/jvi.56.1.277-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanich R. E., Craighead J. E. Human cytomegalovirus infection of cultured fibroblasts. II. Viral replicative sequence of a wild and an adapted strain. Lab Invest. 1972 Sep;27(3):273–282. [PubMed] [Google Scholar]
- Knopf K. W., Kaerner H. C. Virus-specific basic phosphoproteins associated with herpes simplex virus type a (HSV-1) particles and the chromatin of HSV-1-infected cells. J Gen Virol. 1980 Feb;46(2):405–414. doi: 10.1099/0022-1317-46-2-405. [DOI] [PubMed] [Google Scholar]
- Ladin B. F., Ihara S., Hampl H., Ben-Porat T. Pathway of assembly of herpesvirus capsids: an analysis using DNA+ temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Jan 30;116(2):544–561. doi: 10.1016/0042-6822(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980 Sep;35(3):798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGAVRAN M. H., SMITH M. G. ULTRASTRUCTURAL, CYTOCHEMICAL, AND MICROCHEMICAL OBSERVATIONS ON CYTOMEGALOVIRUS (SALIVARY GLAND VIRUS) INFECTION OF HUMAN CELLS IN TISSUE CULTURE. Exp Mol Pathol. 1965 Feb;76:1–10. doi: 10.1016/0014-4800(65)90019-5. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Patel P. C., Huang E. S. Human cytomegalovirus-associated DNA polymerase and protein kinase activities. J Gen Virol. 1981 Nov;57(Pt 1):149–156. doi: 10.1099/0022-1317-57-1-149. [DOI] [PubMed] [Google Scholar]
- Michelson S., Tardy-Panit M., Bârzu O. Catalytic properties of a human cytomegalovirus-induced protein kinase. Eur J Biochem. 1985 Jun 3;149(2):393–399. doi: 10.1111/j.1432-1033.1985.tb08938.x. [DOI] [PubMed] [Google Scholar]
- Michelson S., Tardy-Panit M., Bârzu O. Properties of a human cytomegalovirus-induced protein kinase. Virology. 1984 Apr 30;134(2):259–268. doi: 10.1016/0042-6822(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Karshin W. L., Wu Y. H., Arlinghaus R. B. Characterization of 40,000- and 25,000-dalton intermediate precursors to Rauscher murine leukemia virus gag gene products. J Virol. 1979 Oct;32(1):187–198. doi: 10.1128/jvi.32.1.187-198.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- Randall C. C., Rogers H. W., Downer D. N., Gentry G. A. Protein kinase activity in equine herpesvirus. J Virol. 1972 Feb;9(2):216–222. doi: 10.1128/jvi.9.2.216-222.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Kolakofsky D. Protein kinase associated with Sendai virions. J Virol. 1974 Feb;13(2):545–547. doi: 10.1128/jvi.13.2.545-547.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Gravell M., Darlington R. Protein kinase in enveloped herpes simplex virions. Virology. 1972 Oct;50(1):287–290. doi: 10.1016/0042-6822(72)90374-1. [DOI] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Phosphorylation of animal virus proteins by a virion protein kinase. J Virol. 1973 Sep;12(3):511–522. doi: 10.1128/jvi.12.3.511-522.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S., Katan M., Stirling V., Smith G., Leader D. P. Protein kinase activities associated with the virions of pseudorabies and herpes simplex virus. J Gen Virol. 1985 Apr;66(Pt 4):661–673. doi: 10.1099/0022-1317-66-4-661. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Talbot P., Almeida J. D. Human cytomegalovirus: purification of enveloped virions and dense bodies. J Gen Virol. 1977 Aug;36(2):345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- Tan K. B. Comparative study of the protein kinase associated with animal viruses. Virology. 1975 Apr;64(2):566–570. doi: 10.1016/0042-6822(75)90135-x. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Virion-bound protein kinase in Semliki forest and Sindbis viruses. J Virol. 1974 Jun;13(6):1245–1253. doi: 10.1128/jvi.13.6.1245-1253.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D., Gibson W., Fields K. L. Anti-complement immunofluorescence establishes nuclear localization of human cytomegalovirus matrix protein. Virology. 1985 Nov;147(1):19–28. doi: 10.1016/0042-6822(85)90223-5. [DOI] [PubMed] [Google Scholar]
- Weiner D., Gibson W. Phosphorylation, maturational processing, and relatedness of strain Colburn matrix proteins. Virology. 1983 Aug;129(1):155–169. doi: 10.1016/0042-6822(83)90403-8. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. In vitro phosphorylation of murine leukemia virus proteins: specific phosphorylation of Pr65gag, the precursor of the internal core antigens. Virology. 1982 Jan 15;116(1):181–195. doi: 10.1016/0042-6822(82)90412-3. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Shames R., Luftig R. B. Separation of a murine leukaemia virus protein kinase activity from its Pr65gag polyprotein substrate after DNA--cellulose chromatography. J Gen Virol. 1983 Jan;64(Pt 1):95–102. doi: 10.1099/0022-1317-64-1-95. [DOI] [PubMed] [Google Scholar]