Abstract
The receptor-binding domain of Plasmodium vivax Duffy binding protein, region II (PvRII), is an attractive candidate for a vaccine against P. vivax malaria. Here, we have studied the safety and immunogenicity of recombinant PvRII in Macaca mulatta (rhesus monkeys). Recombinant PvRII with a C-terminal 6-histidine tag was expressed in E. coli, recovered from inclusion bodies, refolded into its functional conformation, purified to homogeneity and formulated with three adjuvants, namely, Alhydrogel, Montanide ISA 720 and the GSK proprietary Adjuvant System AS02A for use in immunogenicity studies. All the PvRII vaccine formulations tested were safe and highly immunogenic. The overall magnitude of the antibody response was significantly higher for both Montanide ISA 720 and AS02A formulations in comparison with Alhydrogel. Furthermore, there was a significant correlation between antibody recognition titers by ELISA and binding inhibition titers in in vitro binding assays. The PvRII vaccine formulations also induced IFN-γ recall responses that were identified using ex vivo ELISPOT assays. These results provide support for further clinical development of a vaccine for P. vivax malaria based on recombinant PvRII.
Keywords: malaria vaccine, Duffy Binding Protein, Plasmodium vivax, adjuvant formulation
1. Introduction
The annual number of clinical cases of malaria is estimated to be over 500 million with the number of fatal malaria cases being greater than one million [1]. These numbers place malaria at the top of the list of transmissible diseases with significant global health impact. The epidemiology of malaria is characterized by increased prevalence of insecticide-resistant vectors and drug-resistant parasites resulting in the failure of vector and parasite control measures [2]. Plasmodium falciparum and P. vivax are the two species of malaria parasites that are the primary cause of human malaria. Epidemiological evidence has indicated that following repeated exposure, clinical immunity to malaria is acquired by residents of endemic areas suggesting that the development of a malaria vaccine should be feasible [3].
Merozoite proteins that are involved in erythrocyte invasion are important candidates for the development of vaccines aimed at neutralizing blood-stage growth by modifying the kinetics of erythrocyte invasion. Extensive research has shown that the invasion of human erythrocytes by P. vivax and the related simian species P. knowlesi is completely dependent on merozoite interaction with the Duffy antigen receptor for chemokines (DARC) [4-7]. The P. vivax and P. knowlesi Duffy binding proteins (PvDBP and PkDBP), which mediate this interaction, belong to a family of erythrocyte binding proteins (EBP) that also includes the 175 kD P. falciparum erythrocyte binding antigen (EBA-175) [8]. The binding domains of EBPs reside in conserved, extracellular, cysteine-rich regions known as region II [9]. Antibodies raised against region II, the receptor-binding domain of PkDBP, have been shown to block erythrocyte invasion by P. knowlesi [10]. This result provides support for the development of a vaccine for P. vivax malaria based on the homologous receptor-binding domain, region II (PvRII), of PvDBP. It has been demonstrated that naturally acquired antibodies elicited against PvDBP can block the binding of PvRII to Duffy positive human erythrocytes although the binding inhibitory activity is poor [11, 12]. Interestingly, structural analysis has demonstrated that clusters of polymorphic amino acid residues in PvRII from P. vivax field isolates lie in regions that are distant from the binding site [13]. The DARC binding site within PvRII thus does not appear to be under significant immune pressure. Although high titer binding inhibitory antibodies against PvRII do not develop upon natural exposure to P. vivax [14], it is possible to raise high titer binding inhibitory antibodies by immunization with recombinant PvRII [15]. Importantly, since the polymorphism clusters are distal to the binding site, anti-PvRII, binding inhibitory antibodies elicited by immunization should be effective against diverse P. vivax isolates [16].
We have previously described the production of recombinant PvRII in its functional, correctly folded form [17, 18]. Immunization with recombinant PvRII formulated with Freund’s adjuvant has been shown to provide partial protection to Aotus lemurinus griseimembra monkeys against P. vivax blood stage challenge [19]. The immunogenicity of recombinant PvRII formulated with human compatible adjuvants has also been studied in small animals [15]. Of the five adjuvants tested, namely, Montanide ISA 720, AS02A, MF59, QS21 and Alhydrogel, formulations made with Montanide ISA 720 and AS02A elicited the highest titer binding inhibitory antibodies [15]. Recombinant PvRII formulated with Alhydrogel also yielded antibodies with significant binding inhibitory activity. Based on these observations, we decided to test the safety and immunogenicity of recombinant PvRII formulated in Montanide ISA 720, AS02A and Alhydrogel in rhesus monkeys. Safety of these PvRII vaccine formulations was assessed by characterization of several clinical, haematological and biochemical parameters at different time points after immunization. The immunogenicity of PvRII in rhesus monkeys was determined by measuring end point titers for recognition of PvRII by total IgG using ELISA, measuring 50% binding inhibition titers using in vitro PvRII-DARC binding assays and by characterizing the prevalence of protein-specific IFN-γ secreting cells by ex vivo ELISPOT assays. We report that all three adjuvant formulations were found to be safe and highly immunogenic in rhesus monkeys. All three formulations tested yielded high titer antibodies with significant binding inhibitory activity. Montanide ISA 720 and AS02A formulations had higher binding inhibitory activity than the Alhydrogel formulation. These results provide support for further development of a vaccine for P. vivax malaria based on PvRII.
2. Materials and Methods
2.1. Animals
A group of 60 rhesus macaques of Chinese origin from the Yerkes National Primate Research Center facility were initially included in the study. The monkeys were screened for antibody reactivity against PvRII by ELISA and to simian malaria parasites by immunofluorescence using P. cynomolgi-infected red blood cells. From this group, thirty-six healthy, malaria naïve rhesus macaques were included in the immunization protocol (Table 1). Selected animals were matched by age, sex and weight, housed in social settings and randomly assigned to six experimental groups of 5 individuals each that received different vaccine formulations (Groups 1-6) and three control groups of two individuals each that received adjuvant alone (Groups 7-9). The procedures were approved by Emory University’s Institutional Animal Care and Use Committee.
Table 1.
Groups of rhesus macaques immunized with rPvRII using several adjuvant formulations.
| Group code | rPvRII dosea | Adjuvant formulationb |
|---|---|---|
| 1 | 50 μg | Alhydrogel |
| 2 | 10 μg | Alhydrogel |
| 3 | 50 μg | Montanide ISA 720 |
| 4 | 10 μg | Montanide ISA 720 |
| 5 | 50 μg | AS02A |
| 6 | 10 μg | AS02A |
| 7 | Saline | Alhydrogel |
| 8 | Saline | Montanide ISA 720 |
| 9 | Saline | AS02A |
Administered intramuscularly in a final volume of 500 μl.
Rhesus macaques per experimental groups N=5 and placebo groups N=2.
2.2. Vaccine formulation and immunization schedule
The production and characterization of recombinant PvRII has been described previously [17, 20]. Briefly, a gene coding for PvRII from P. vivax Salvador I strain (aminoacid D194-T521; GenBank accession number M61095) was cloned as a NcoI-SalI fragment in the E. coli expression vector pET28a(+) as described [17]. Bacterial transformation for expression of the recombinant construct was performed using E. coli BL21 (DE3) cells (Novagen, Madison, WI) and kanamycin selection. Protein expression of the recombinant 6-His tag PvRII was induced with 1 mM IPTG for 4 hours, purified by metal affinity chromatography under denaturing conditions, refolded by rapid dilution and purified further to homogeneity by ion exchange and gel filtration chromatography resulting in a protein of apparent molecular mass of ~39 kDa. The molecular mass determined by electron spray ionization mass spectrometry was 39,802 Da. Refolded PvRII was characterized for purity, homogeneity, identity and functional activity by SDS-PAGE, western blotting, reverse phase chromatography and erythrocyte binding assays as described previously [17]. Using reverse phase chromatography refolded PvRII elutes as a single, homogeneous product. The endotoxin content of purified PvRII was less than 25 EU per 25 μg using standard LAL assay [15, 21]. Recombinant PvRII was formulated with either Alhydrogel [15], Montanide ISA 720 (Seppic, France) or AS02A Adjuvant System (GlaxoSmithKline Biologicals, Belgium) prior to immunization following manufacturers’ protocols. The final formulation was adjusted to 0.5 ml containing 50μg or 10 μg of PvRII (see Table 1). Groups 1 and 2 were immunized with 50 μg and 10 μg of PvRII adsorbed to Alhydrogel, respectively. Groups 3 and 4 received 50 μg and 10 μg of PvRII emulsified in Montanide ISA 720, respectively. Groups 5 and 6 were immunized with 50 μg and 10 μg of PvRII formulated in AS02A, respectively. Six control animals were distributed in three groups (Groups 7 to 9) of two individuals each. Groups 7, 8 and 9 received saline solution adsorbed to Alhydrogel, Montanide ISA 720 and AS02A, respectively. The immunizations were given intramuscularly (IM) using the following schedule: priming into the right quadriceps femoris on day 0, first boost into the right musculus deltoideus on day 60 and the last boost into the left musculus deltoideus on day 150. Immunizations were given using a tuberculin syringe and a 22 gauge needle. Blood samples were obtained by femoral phlebotomy at seven time points before and during the study on days 0, 30, 60, 90, 120, 150 and 180. Serum and plasma were separated and either used for clinical chemistry assays immediately or frozen in aliquots at -80 °C for immunological studies. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradients and frozen in aliquots at -80 °C for use in ELISPOT assays.
2.3. Clinical characterization and safety assays
Animals were observed for general behavior and visible side effects during the entire study by two veterinary clinicians and their staff. Observations included analysis of skin for warmth, erythema and edema/swelling, and for muscle induration or necrosis. The clinical veterinary staff examining the animals and injection sites was blinded to which vaccine formulation had been given. At the specified time points, the animals were sedated for bleedings and examination of the injection sites by the veterinary clinician. The total observation period of the animals was 240 days. Analysis of hematology and clinical chemistry of the different animals was performed on days 0, 30, 60, 90, 120, 150 and 180. Hematological analysis consisted of determination of number of erythrocytes (RBC), leukocytes (WBC), hemoglobin (HGB), hematocrit (HCT), mean cell volume (MCV) and platelets (PLT). Analysis of clinical chemistry consisted of glucose, blood urea nitrogen (BUN), creatinine, protein, albumin, alkaline phosphatase, serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), amylase and creatine phosphokinase (CPK) determinations.
2.4. ELISA
Sera were tested for recognition of PvRII by ELISA using Immulon-2 plates (Dynatech Laboratories, Chantilly, VA) coated with 1 μg/ml of the recombinant protein. Antigen specificity was confirmed by testing sera samples with a P. yoelii his-tag recombinant protein and synthetic peptides containing a (His)5 sequence (VDKLAAALEHHHHH and HHHHHLESTSLYKKAG). After blocking with 5% bovine serum albumin (BSA), the plates were incubated with individual sera diluted in PBS with 2.5% BSA for 1 h at 37°C. Bound antibodies were detected using peroxidase-labeled goat anti-monkey IgG (Kirkegaard & Perry Laboratories, Gaithersburg, MD) and H2O2/2,2’-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Optical densities (OD) were determined using a VERSAmax ELISA reader (Molecular Device Corporation, Sunnyvale, CA) with a 405 nm filter. The reciprocal end point antibody titers, defined as the last serum dilution that yielded an OD greater than mean OD plus two standard deviations (SD) obtained with malaria naïve rhesus macaques at 1:200 dilution was considered as the endpoint ELISA titer.
2.5 Blocking of binding of PvRII to DARC-Fc by rhesus sera
Inhibition of binding of PvRII to DARC by sera from immunized rhesus macaques was determined using an ELISA-based binding assay. The N-terminal extracellular region of DARC was expressed as a fusion to Fc of human IgG (DARC-Fc) in a mammalian cell culture system as previously described [22]. ELISA plates were coated with 1 μg/ml DARC-Fc using sodium bicarbonate buffer pH 9.6 and the plates incubated overnight at 4°C. The plates were then washed three times with PBS pH 7.4 containing 0.05% Tween 20. Plates were then blocked with 1% skimmed milk in PBS for 2 hours at 37°C. Pooled rhesus sera from groups 1 to 6 collected on days 30, 90, 150 or 180 were used as test sera. Pre-immune sera and adjuvant alone sera from each group were used as controls. PvRII (0.1μg/ml) was pre-incubated with pooled sera at final dilutions of 1:10, 1:50, 1:250, 1:1,250 and 1:6,250 for 1 hour at room temperature prior to addition to DARC-Fc coated plates. Sera from pre-immune and adjuvant control groups were used at a dilution of 1:10. PvRII (0.1 μg/ml) without sera was used as positive control for binding to DARC-Fc. PvRII with/without sera was allowed to bind DARC-Fc coated wells for 1 hour at 37°C. Bound PvRII was then detected with anti-PvRII rabbit polyclonal sera (1:1,500), followed by mouse anti-rabbit IgG conjugated to horseradish peroxidase (Sigma Aldrich Corp., St Louis, MO) at a dilution of 1:2,500. Plates were developed using o-phenelenediamine (Sigma Aldrich Corp., St Louis, MO). Mean OD (A490 nm) values from test and control groups were determined. Mean OD values of binding in presence of sera were normalized as %OD compared to OD values of PvRII binding in absence of sera. A standard curve for PvRII binding to DARC was used to estimate bound PvRII in presence of sera using the %OD values. The results are expressed as the dilution of sera at which 50% binding inhibition was observed.
2.6. ELISPOT
The frequency of peptide-specific T lymphocytes was determined by IFN-γ–specific ELISPOT as described [23]. Briefly, 96-well nitrocellulose-bottom plates (Multiscreen-HA, Millipore, Molsheim, France) were coated with 100 μl/well of GZ-4 monoclonal antibody (mAb) at 15 μg/ml (Mabtech Inc., Mariemont, OH) and incubated overnight at 4 °C. The following day, plates were washed five times in PBS and blocked with RPMI medium containing 10% FCS. A cytokine cocktail composed of 25 ng/ml each of IL-7 and IL-15 (R&D Systems, Minneapolis, MN, USA) was added in 100 μl followed by 50 μl of PvRII or negative control recombinant protein to a final concentration of 10 μg/ml. Cryopreserved PBMC were thawed, washed twice with RPMI and the cell viability evaluated by Trypan blue exclusion. 2×105 PBMCs were then added in a volume of 50 μl, bringing the total volume in each well to 200 μl. Incubation was continued for 24 hours at 37 °C, 5% CO2. After incubation the plates were washed and incubated with biotinylated mAb 7-B6-1 (Mabtech Inc., Mariemont, OH) followed by incubation with Streptavidin-HRP. The reaction was developed using 3-amino-9-ethylcarbazole (AEC) (BD Biosciences Pharmingen, San Jose, CA) and evaluated in an Immunospot Analyzer (Cellular Technology-Becton Dickinson, San Diego, CA). Staphylococcal enterotoxin B (SEB) (Sigma Aldrich Corp., St Louis, MO) was used as positive control for activation. The results are expressed as total number of spot forming cells (SFC) per 106 splenocytes. Total number for duplicate wells were averaged and normalized to numbers of IFN-γ spot forming cells per 1×106 PBMC. Average values for negative medium control wells in the presence of cytokines were subtracted from the average values from antigen-stimulated wells. To take in to consideration individual variability, recall PvRII-reactive T cells were calculated by subtracting the spot forming cells obtained with pre-immune samples.
2.7. Statistical analysis
Antibody levels were log-transformed and multiple comparisons were conducted using Student’s t-test. To evaluate the correlation between antibody titers and inhibition of binding of PvRII to DARC-Fc by anti-PvRII rhesus sera, a Spearman rank correlation was used. For hematology and clinical laboratory values repeated measures of analysis of variance (ANOVA) were conducted to evaluate overall differences at a particular time point. For the ELISPOT assays, we tested the significance of the differences between doses using the one-tailed paired Student’s t-test after subtracting the background spots and using data log transformation. P-values of 0.05 or less were considered significant.
3. Results
3.1 Safety assessment
Animals were evaluated daily for the presence of clinical abnormalities and general behavior. They were also examined closely under general anesthesia at days 30, 60, 90, 120, 150 and 180 to identify signs of local reactogenicity. No local abnormalities were identified in the inoculation sites upon close examination. Hematology and clinical chemistry values determined at days 0, 30, 60, 90, 120, 150 and 180 remained within the normal range. In some animals, in both experimental or control groups at different time points, high levels of CPK were detected randomly without clinical expression of tissue damage. These changes were considered within a normal range for rhesus macaques housed in social settings where minor sub-clinical traumas are expected.
3.2 Antibody mediated immune response
We have evaluated six formulations of PvRII using three different clinically accepted adjuvants. Figure 1 summarizes the kinetics of the anti-rPvRII antibody response determined by ELISA. Pre-immune geometric mean antibody titers ranged between 1:200 and 1:3200. Thirty days after the first immunization all rhesus macaques seroconverted (defined by fourfold or greater increase over baseline) after receiving PvRII with either formulation with five to eight-fold increase in titers from the pre-immune values. After a single immunization, differences were significant only between Montanide and Alhydrogel groups for the 10 μg protein concentration (P=0.004). This is in contrast with antibody titers evaluated 60 days after the second immunization where both Montanide and AS02A groups were significantly greater than Alhydrogel groups (P values: Montanide 50 -Alhydrogel 50 = 0.01; AS02A 50-Alhydrogel 50 = 0.0009; Montanide 10-Alhydrogel 10 = 0.000277 and AS02A 10-Alhydrogel 10 = 0.015). Differences in antibody titers between animals that received PvRII formulated in Montanide or AS02A were not significant. These trends were maintained after second boosting conducted 150 days after the first immunization. Differences in antibody titers obtained with 10 and 50 μg immunization regimes at different time points were only statistically significant for the PvRII formulated with Alhydrogel (P-values ranged from 0.005 to 0.019). There were no differences in antibody titers in rhesus macaques that received placebo in comparison to the pre-immune levels.
Figure 1.
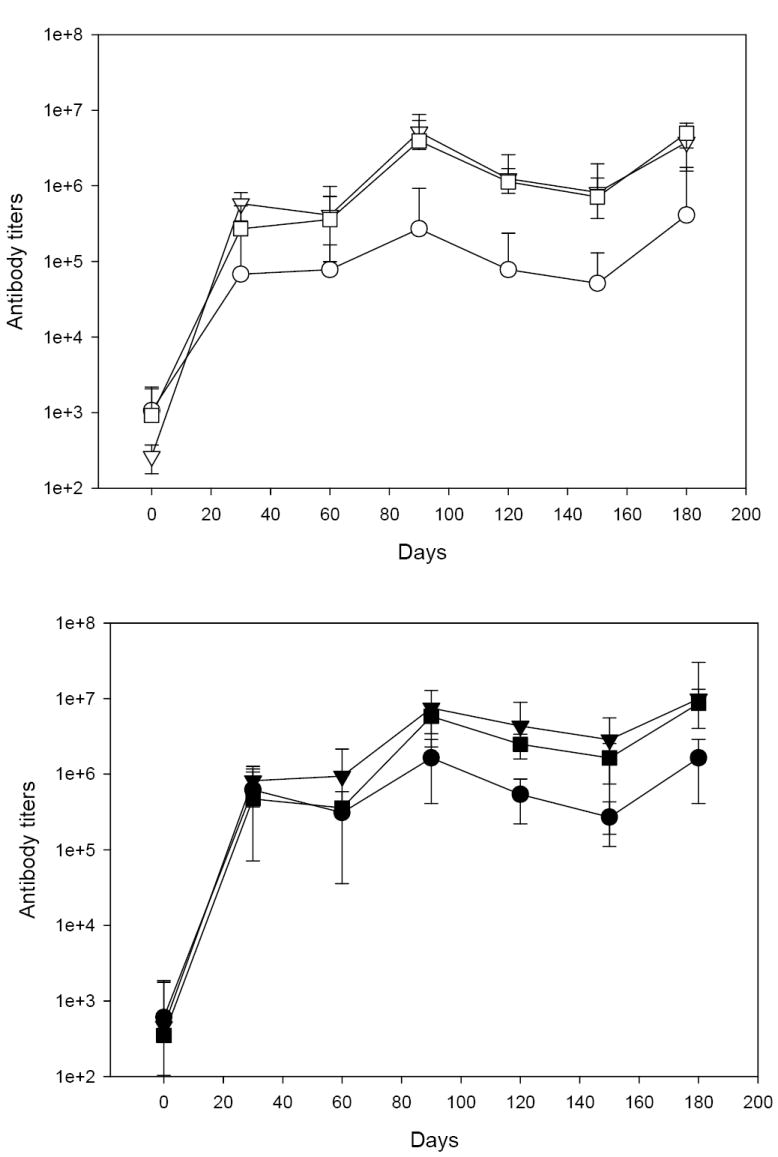
PvRII formulated with different adjuvants induces high antibody titers in rhesus macaques. The figure represents the kinetics of the antibody response to PvRII determined by ELISA using geometric mean antibody titers for individual formulations (± standard deviation). Open symbols, rhesus macaques immunized with 10 μg PvRII. Closed symbols, rhesus macaques immunized with 50 μg PvRII. Alhydrogel group (○ ●), Montanide ISA 720 group (▽ ▼) and AS02A group (□ ■).
3.3. Functional characterization of antibodies elicited by immunization
The ability of the antibodies to block binding of PvRII to DARC was characterized by binding inhibition assays. Figure 2 summarizes the kinetics of functional antibodies determined at four different time points expressed as the dilution of sera at which 50% binding inhibition was observed. Functional antibodies were detected in pools of sera obtained from animals 60 days after the second immunization with each formulation. When the inhibition of binding profiles were compared between groups that received 10 or 50 μg with the same adjuvant, differences were only significant for Alhydrogel (P=0.05). Significant correlations were found between the antibody titers determined by ELISA and inhibition of binding assays. For statistical analysis antibody titers determined on days 0, 90, 150 and 180 were used to rank antibody levels for both protein concentrations and adjuvants used for immunization, and the data were compared with inhibition of binding using a Spearman rank correlation test. The correlation between ELISA and inhibition of binding ranked from 0.9048 to 0.9461, all with P values of <0.05. For overall data the Spearman rank-order correlation between ELISA titers obtained with adjuvants Alhydrogel, Montanide and AS02A and inhibition of binding coefficient was 0.9097 (P<0.000001).
Figure 2.
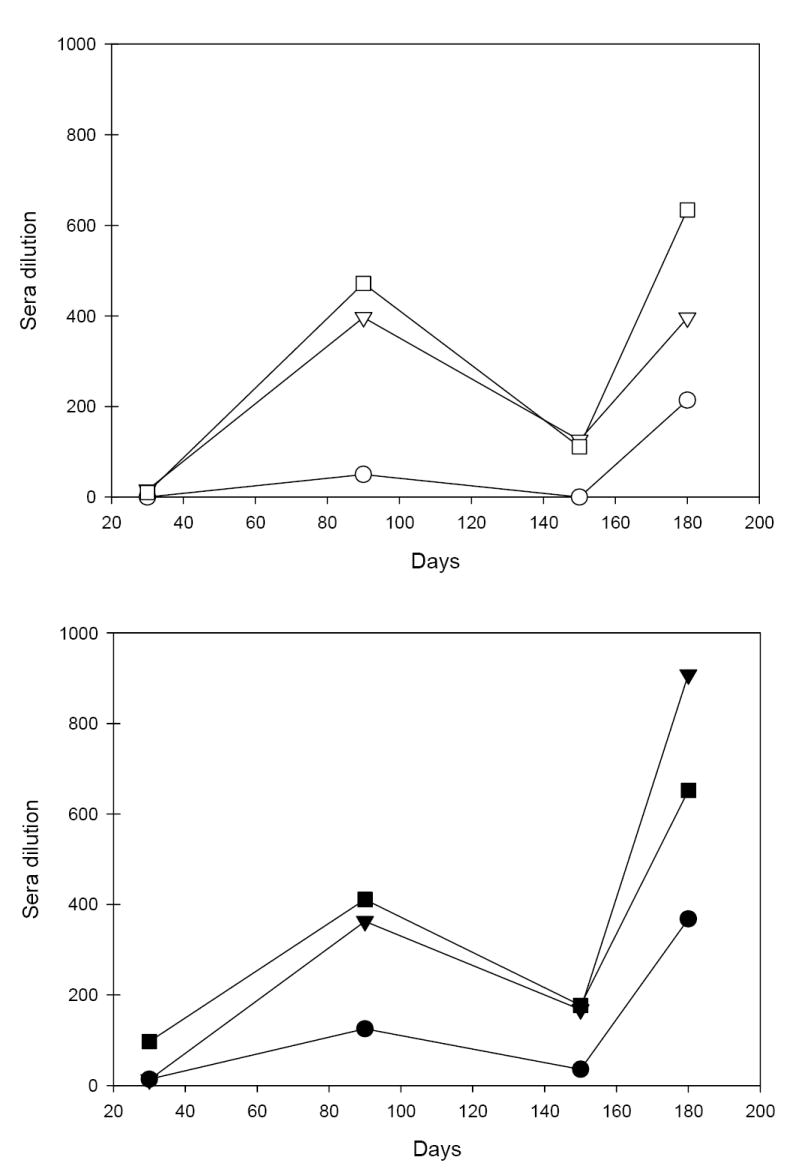
Binding inhibitory activity of anti-PvRII sera induced by immunization of rhesus macaques with PvRII formulated with different adjuvants. Sera samples were obtained at different time points after immunization and tested for inhibition of PvRII binding to DARC-Fc as described [22]. Functional activity is expressed as sera dilution at which 50% binding inhibition was observed. Open symbols, rhesus macaques immunized with 10 μg rPvRII. Closed symbols, rhesus macaques immunized with 50 μg P. vivax DBP RII. Alhydrogel group (○ ●), Montanide ISA 720 group (▽ ▼) and AS02A group (□ ■).
3.4. T cell recall response
PvRII-specific IFN-γ producing T cells were identified by ELISPOT at different time points. Broad individual variability was observed in the magnitude of IFN-γ ELISPOT responses. Normalized results were expressed as IFN-γ Spot Forming Cells (SFC) per million PBMC after subtracting pre-immune values (Figure 3). For statistical analysis, comparisons between groups that received 10 μg or 50 μg of PvRII formulated with the same adjuvant were conducted. The differences observed between 10 μg or 50 μg doses were significant for Montanide ISA 720 (P=0.0018) and Alhydrogel (P<0.0001).
Figure 3.
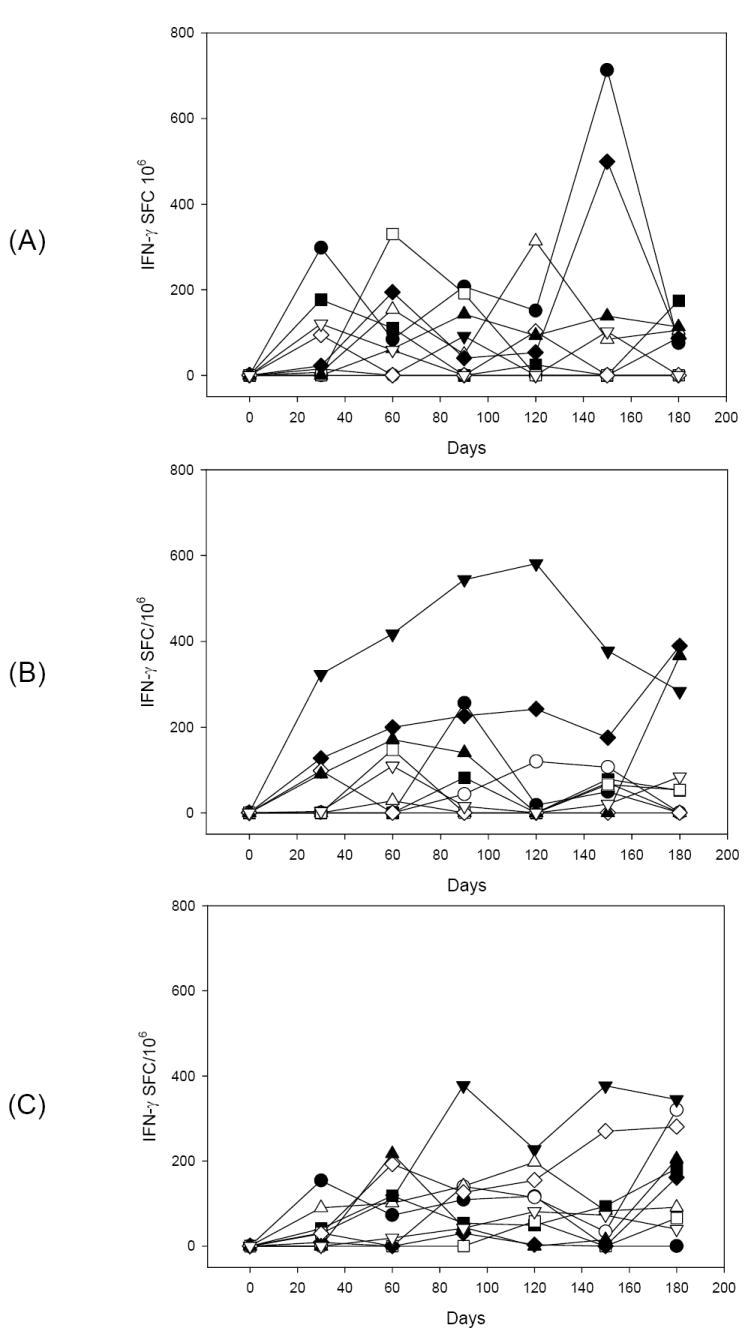
Kinetics of the prevalence of IFN-γ secreting cells determined by ELISPOT in PBMC obtained from animals immunized with different formulations of rPvRII after ex vivo stimulation with the recombinant protein. Data are presented for individual animal at different time points and expressed as IFN-γ spot forming cells per 1×106 PBMC. Average values for medium control wells in the presence of cytokines were initially subtracted from the average values obtained from antigen-stimulated wells. To take in consideration individual variability, recall PvRII-reactive T cells were calculated by subtracting the spot forming cells obtained with pre-immune samples. Rhesus macaques were immunized with 50 μg P. vivax DBP RII (closed symbols) or 10 μg P. vivax DBP RII (open symbols) formulated with Alhydrogel (A), Montanide ISA 720 (B) or AS02A (C).
4. Discussion
The Duffy binding protein is a leading vaccine candidate for protection against P. vivax malaria. The functional DARC-binding domain is located in region II (PvRII) of P. vivax DBP [9]. PvRII is the prototypical Duffy-binding-like (DBL) domain, which shares structural features with other receptor-binding domains belonging to the DBL family [8, 24, 25]. Antibodies directed against the homologous DBL domains of P. knowlesi DBP and P. falciparum EBA-175 block red cell invasion by P. knowlesi and P. falciparum respectively providing a rationale for development of a vaccine for P. vivax malaria based on PvRII [6, 10, 26, 27]. Rhesus macaques were used to demonstrate safety and immunogenicity of recombinant PvRII. We have shown that recombinant PvRII induced comparable levels of antibodies when it is formulated either with Montanide 720 or AS02A. Titers of antibodies significantly correlate with their ability to inhibit binding of PvRII to DARC. Antibody levels induced by immunization with PvRII adsorbed to Alhydrogel were lower than the titers induced by immunization with PvRII formulated in Montanide ISA 720 and AS02A. Despite these low antibody titers, sera samples obtained from animals immunized with the Alhydrogel formulation also had an effect on neutralizing the binding of PvRII to DARC-Fc in vitro. Functional antibodies that block PvRII-DARC-Fc interaction are likely to inhibit erythrocyte invasion by P. vivax merozoites. The binding inhibitory activity of such antibodies may serve as an important in vitro correlate for protection in efficacy trials of a PvRII-based malaria vaccine [28].
All three PvRII formulations tested in rhesus monkeys were well tolerated. To facilitate the identification of local reactivity, immunization sites were alternated between right quadriceps femoris and right and left musculus deltoideus. Systemic reactogenicity was evaluated by periodic evaluation of hematological and clinical chemistry parameters. Although we found individual variability, clinical laboratory values remained within normal reference ranges. These results are comparable to recent reports testing a malaria vaccine candidate in rhesus macaques using similar adjuvant formulations [29]. The use of rhesus macaques in preclinical trials of malaria vaccine candidates has recently gained attention [29, 30]. Rhesus macaques are phylogenetically closely related to humans. The broad use of this animal model in biomedical research has stimulated and facilitated the development of species-specific reagents [31]. The substantial body size of rhesus macaques, compared with small New World monkeys [32], also facilitates the routine assessment of clinical laboratory chemistry and hematological parameters using standard phlebotomy procedures in the course of the trial. Future investigations using this model can evaluate the efficacy of vaccine candidates based on P. cynomolgi antigens for proof of principle, with the challenge of different strains of P. cynomolgi parasites. Animals immunized with P. vivax antigens, as reported here, can also be challenged with P. cynomolgi parasites as a rigorous test for efficacy, to show and predict the likelihood of achieving heterologous protection as would be desired in field trials [33].
The prevalence of naturally acquired antibodies to P. vivax DBP increases with age in endemic areas. This age-dependent pattern of immune recognition also correlates with acquisition of protection against P. vivax malaria [12, 14, 34, 35]. Acquired anti-DBP antibodies are also correlated with levels of exposure [36, 37]. Relevant for malaria vaccine development, anti-DBP antibodies are higher in asymptomatic individuals suggesting that the prevalence of P. vivax DBP antibodies may modify the clinical outcome, reducing the severity of the disease state [14, 36]. The immunological relevance of PvRII has been confirmed by genotypic characterization of wild isolates, which indicates a decline in the proportion of individuals infected with multiple PvRII haplotypes with increasing age [38]. We show here that immunization of rhesus macaques with a refolded PvRII induced functional antibodies with the potential to inhibit parasite invasion. These results are consistent with the high immunogenicity and partial protection induced in Aotus monkeys in previously described experiments [19].
PvRII also contains a cluster of T cell epitopes that are recognized by individuals living in endemic areas of P. vivax malaria [14, 39]. PvRII-specific IFN-γ secreting cells were identified here at different time points after immunization. Surprisingly, statistically significant differences were defined in animals immunized with Alhydrogel and Montanide ISA 720 formulations but not with AS02A. Recent clinical trials of malaria vaccine candidates have defined the complexity of the T cell reactivity and the relevance of using cultured ELISPOT assays to characterize protective T cells [40]. In contrast with ex vivo ELISPOT assays, as used here, cultured ELISPOT assays involve the in vitro culture of PBMC in the presence of relevant antigens and IL-2 for several days to identify resting memory cells. This T cell subset persists for several months after immunization and correlates with protection [41]. We have evaluated T cell reactivity using ELISPOT after ex vivo stimulation with PvRII. The evidence that PvRII formulated with different adjuvants induces heterogeneous T cell reactivity in rhesus macaques requires further characterization and the use of a panel of synthetic peptides for ex vivo stimulation. It has been described that several assays to test T cell reactivity are required to have a clear picture of memory cells in clinical trials of malaria vaccine candidates [42].
In conclusion we report that PvRII formulated in the human compatible adjuvants Alhydrogel, Montanide ISA 720 and AS02A is safe and highly immunogenic in rhesus monkeys. Each formulation tested elicited high titer binding inhibitory antibodies. These results support further clinical development of this promising candidate as a vaccine for P. vivax.
Acknowledgments
This research was supported by the Yerkes National Primate Research Center Base Grant No RR00165 awarded by the National Center for Research Resources of the National Institutes of Health., Malaria Vaccine Initiative at PATH (Program for Appropriate Technology in Health) and the Indo-US Vaccine Action Programme (VAP). CEC is a recipient of TATA Innovation Fellowship for Translational Research from the Department of Biotechnology, Government of India. We thank Joe Cohen and Sylvie Cayphas, GlaxoSmithKline Biologicals, Rixensart, Belgium for providing AS02A and Vincent Gannet, Seppic Inc., France for providing Montanide ISA 720. We also thank Lakshmi Chennareddi for her expertise with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guinovart C, Navia MM, Tanner M, Alonso PL. Malaria: burden of disease. Curr Mol Med. 2006;6(2):137–40. doi: 10.2174/156652406776055131. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood B. Malaria vaccines. Evaluation and implementation. Acta Trop. 2005;95(3):298–304. doi: 10.1016/j.actatropica.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189(4202):561–3. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 5.Barnwell JW, Nichols ME, Rubinstein P. In vitro evaluation of the role of the Duffy blood group in erythrocyte invasion by Plasmodium vivax. J Exp Med. 1989;169(5):1795–802. doi: 10.1084/jem.169.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261(5125):1182–4. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 7.Wertheimer SP, Barnwell JW. Plasmodium vivax interaction with the human Duffy blood group glycoprotein: identification of a parasite receptor-like protein. Exp Parasitol. 1989;69(4):340–50. doi: 10.1016/0014-4894(89)90083-0. [DOI] [PubMed] [Google Scholar]
- 8.Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89(15):7085–9. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitnis CE, Chaudhuri A, Horuk R, Pogo AO, Miller LH. The domain on the Duffy blood group antigen for binding Plasmodium vivax and P. knowlesi malarial parasites to erythrocytes. J Exp Med. 1996;184(4):1531–6. doi: 10.1084/jem.184.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh AP, Puri SK, Chitnis CE. Antibodies raised against receptor-binding domain of Plasmodium knowlesi Duffy binding protein inhibit erythrocyte invasion. Mol Biochem Parasitol. 2002;121(1):21–31. doi: 10.1016/s0166-6851(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 11.Dutta S, Daugherty JR, Ware LA, Lanar DE, Ockenhouse CF. Expression, purification and characterization of a functional region of the Plasmodium vivax Duffy binding protein. Mol Biochem Parasitol. 2000;109(2):179–84. doi: 10.1016/s0166-6851(00)00244-9. [DOI] [PubMed] [Google Scholar]
- 12.Michon P, Fraser T, Adams JH. Naturally acquired and vaccine-elicited antibodies block erythrocyte cytoadherence of the Plasmodium vivax Duffy binding protein. Infect Immun. 2000;68(6):3164–71. doi: 10.1128/iai.68.6.3164-3171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SK, Hora R, Belrhali H, Chitnis CE, Sharma A. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature. 2006;439(7077):741–4. doi: 10.1038/nature04443. [DOI] [PubMed] [Google Scholar]
- 14.Xainli J, Cole-Tobian JL, Baisor M, Kastens W, Bockarie M, Yazdani SS, Chitnis CE, Adams JH, King CL. Epitope-specific humoral immunity to Plasmodium vivax Duffy binding protein. Infect Immun. 2003;71(5):2508–15. doi: 10.1128/IAI.71.5.2508-2515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazdani SS, Shakri AR, Mukherjee P, Baniwal SK, Chitnis CE. Evaluation of immune responses elicited in mice against a recombinant malaria vaccine based on Plasmodium vivax Duffy binding protein. Vaccine. 2004;22(2728):3727–37. doi: 10.1016/j.vaccine.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180(2):497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh S, Pandey K, Chattopadhayay R, Yazdani SS, Lynn A, Bharadwaj A, Ranjan A, Chitnis C. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax duffy-binding protein. J Biol Chem. 2001;276(20):17111–6. doi: 10.1074/jbc.M101531200. [DOI] [PubMed] [Google Scholar]
- 18.Yazdani SS, Shakri AR, Pattnaik P, Rizvi MM, Chitnis CE. Improvement in Yield and Purity of a Recombinant Malaria Vaccine Candidate Based on the Receptor-Binding Domain of Plasmodium vivax Duffy Binding Protein by Codon Optimization. Biotechnol Lett. 2006;28(14):1109–14. doi: 10.1007/s10529-006-9061-3. [DOI] [PubMed] [Google Scholar]
- 19.Arevalo-Herrera M, Castellanos A, Yazdani SS, Shakri AR, Chitnis CE, Dominik R, Herrera S. Immunogenicity and protective efficacy of recombinant vaccine based on the receptor-binding domain of the Plasmodium vivax Duffy binding protein in Aotus monkeys. Am J Trop Med Hyg. 2005;73(5 Suppl):25–31. doi: 10.4269/ajtmh.2005.73.5_suppl.0730025. [DOI] [PubMed] [Google Scholar]
- 20.Yazdani SS, Shakri AR, Chitnis CE. A high cell density fermentation strategy to produce recombinant malarial antigen in E. coli. Biotechnol Lett. 2004;26(24):1891–5. doi: 10.1007/s10529-004-6040-4. [DOI] [PubMed] [Google Scholar]
- 21.Devi YS, Mukherjee P, Yazdani SS, Shakri AR, Mazumdar S, Pandey S, Chitnis CE, Chauhan VS. Immunogenicity of Plasmodium vivax combination subunit vaccine formulated with human compatible adjuvants in mice. Vaccine. 2007;25(28):5166–74. doi: 10.1016/j.vaccine.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 22.Choe H, Moore MJ, Owens CM, Wright PL, Vasilieva N, Li W, Singh AP, Shakri R, Chitnis CE, Farzan M. Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC) Mol Microbiol. 2005;55(5):1413–22. doi: 10.1111/j.1365-2958.2004.04478.x. [DOI] [PubMed] [Google Scholar]
- 23.Jennes W, Kestens L, Nixon DF, Shacklett BL. Enhanced ELISPOT detection of antigen-specific T cell responses from cryopreserved specimens with addition of both IL-7 and IL-15--the Amplispot assay. J Immunol Methods. 2002;270(1):99–108. doi: 10.1016/s0022-1759(02)00275-2. [DOI] [PubMed] [Google Scholar]
- 24.Gilberger TW, Thompson JK, Triglia T, Good RT, Duraisingh MT, Cowman AF. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem. 2003;278(16):14480–6. doi: 10.1074/jbc.M211446200. [DOI] [PubMed] [Google Scholar]
- 25.Mayer DC, Kaneko O, Hudson-Taylor DE, Reid ME, Miller LH. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A. 2001;98(9):5222–7. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey KC, Singh S, Pattnaik P, Pillai CR, Pillai U, Lynn A, Jain SK, Chitnis CE. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol. 2002;123(1):23–33. doi: 10.1016/s0166-6851(02)00122-6. [DOI] [PubMed] [Google Scholar]
- 27.Narum DL, Haynes JD, Fuhrmann S, Moch K, Liang H, Hoffman SL, Sim BKL. Antibodies against the Plasmodium falciparum Receptor Binding Domain of EBA-175 Block Invasion Pathways That Do Not Involve Sialic Acids. Infect Immun. 2000;68(4):1964–6. doi: 10.1128/iai.68.4.1964-1966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimberg BT, Udomsangpetch R, Xainli J, McHenry A, Panichakul T, Sattabongkot J, Cui L, Bockarie M, Chitnis C, Adams J, Zimmerman PA, King CL. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PloS medicine. 2007;4(12):e337. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware LA, Kester KE, Cummings JF, Burge JR, Voss G, Delchambre M, Garcon N, Tang DB, Cohen JD, Heppner DG., Jr Preclinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS,S/AS02A. Vaccine. 2006;24(4243):6483–92. doi: 10.1016/j.vaccine.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Pichyangkul S, Kum-Arb U, Yongvanitchit K, Limsalakpetch A, Gettayacamin M, Lanar DE, Ware LA, Stewart VA, Heppner DG, Mettens P, Cohen JD, Ballou WR, Fukuda MM. Preclinical evaluation of the safety and immunogenicity of a vaccine consisting of Plasmodium falciparum liver-stage antigen 1 with adjuvant AS01B administered alone or concurrently with the RTS,S/AS01B vaccine in rhesus primates. Infect Immun. 2008;76(1):229–38. doi: 10.1128/IAI.00977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316(5822):222–34. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 32.Collins WE. South American monkeys in the development and testing of malarial vaccines--a review. Mem Inst Oswaldo Cruz. 1992;87(Suppl 3):401–6. doi: 10.1590/s0074-02761992000700068. [DOI] [PubMed] [Google Scholar]
- 33.Dutta S, Kaushal DC, Ware LA, Puri SK, Kaushal NA, Narula A, Upadhyaya DS, Lanar DE. Merozoite surface protein 1 of Plasmodium vivax induces a protective response against Plasmodium cynomolgi challenge in rhesus monkeys. Infect Immun. 2005;73(9):5936–44. doi: 10.1128/IAI.73.9.5936-5944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser T, Michon P, Barnwell JW, Noe AR, Al-Yaman F, Kaslow DC, Adams JH. Expression and serologic activity of a soluble recombinant Plasmodium vivax Duffy binding protein. Infect Immun. 1997;65(7):2772–7. doi: 10.1128/iai.65.7.2772-2777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michon PA, Arevalo-Herrera M, Fraser T, Herrera S, Adams JH. Serologic responses to recombinant Plasmodium vivax Duffy binding protein in a Colombian village. Am J Trop Med Hyg. 1998;59(4):597–9. doi: 10.4269/ajtmh.1998.59.597. [DOI] [PubMed] [Google Scholar]
- 36.Ceravolo IP, Bruna-Romero O, Braga EM, Fontes CJ, Brito CF, Souza JM, Krettli AU, Adams JH, Carvalho LH. Anti-Plasmodium vivax duffy binding protein antibodies measure exposure to malaria in the Brazilian Amazon. Am J Trop Med Hyg. 2005;72(6):675–81. [PubMed] [Google Scholar]
- 37.Tran TM, Oliveira-Ferreira J, Moreno A, Santos F, Yazdani SS, Chitnis CE, Altman JD, Meyer EV, Barnwell JW, Galinski MR. Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population. Am J Trop Med Hyg. 2005;73(2):244–55. [PubMed] [Google Scholar]
- 38.Cole-Tobian JL, Cortes A, Baisor M, Kastens W, Xainli J, Bockarie M, Adams JH, King CL. Age-acquired immunity to a Plasmodium vivax invasion ligand, the duffy binding protein. J Infect Dis. 2002;186(4):531–9. doi: 10.1086/341776. [DOI] [PubMed] [Google Scholar]
- 39.Xainli J, Baisor M, Kastens W, Bockarie M, Adams JH, King CL. Age-dependent cellular immune responses to Plasmodium vivax Duffy binding protein in humans. J Immunol. 2002;169(6):3200–7. doi: 10.4049/jimmunol.169.6.3200. [DOI] [PubMed] [Google Scholar]
- 40.Bejon P, Kai OK, Mwacharo J, Keating S, Lang T, Gilbert SC, Peshu N, Marsh K, Hill AV. Alternating vector immunizations encoding pre-erythrocytic malaria antigens enhance memory responses in a malaria endemic area. Eur J Immunol. 2006;36(8):2264–72. doi: 10.1002/eji.200636187. [DOI] [PubMed] [Google Scholar]
- 41.Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, Webster DP, Dunachie SJ, Moorthy VS, McConkey SJ, Gilbert SC, Hill AV. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175(9):5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 42.Flanagan KL, Lee EA, Gravenor MB, Reece WH, Urban BC, Doherty T, Bojang KA, Pinder M, Hill AV, Plebanski M. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J Immunol. 2001;167(8):4729–37. doi: 10.4049/jimmunol.167.8.4729. [DOI] [PubMed] [Google Scholar]


