Abstract
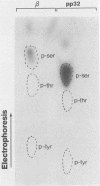
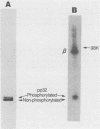
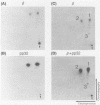
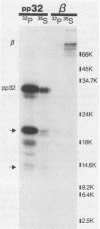
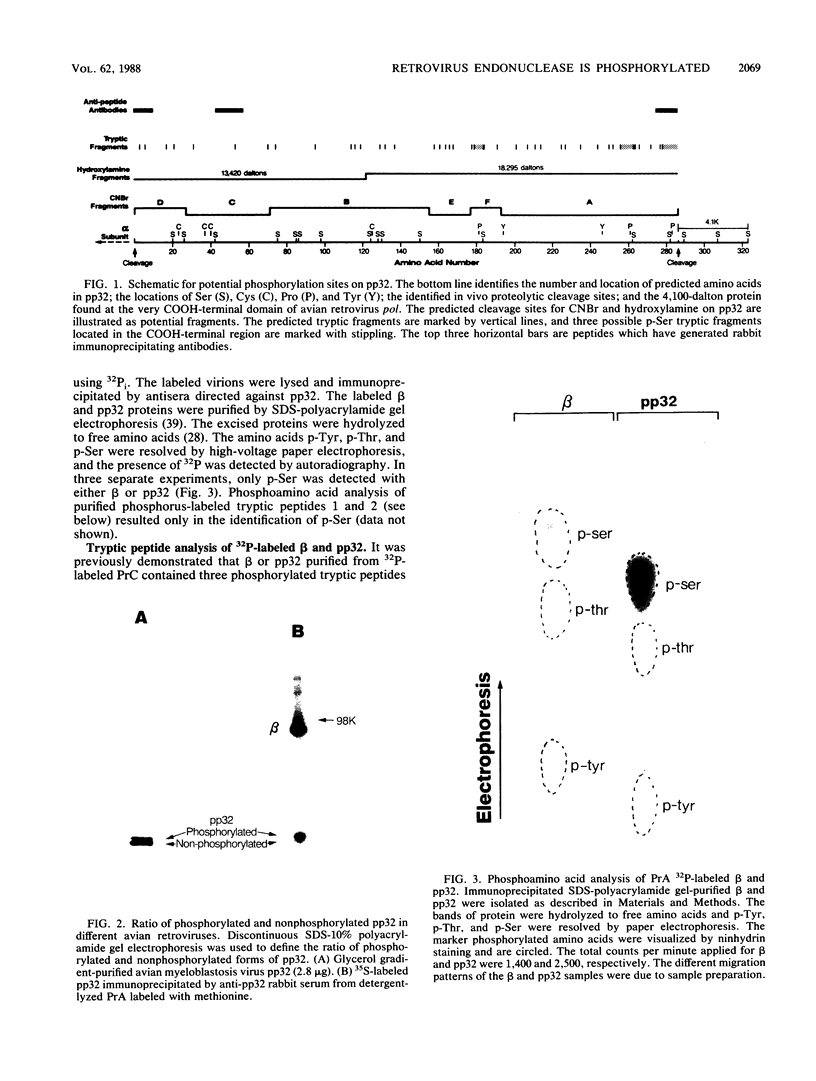
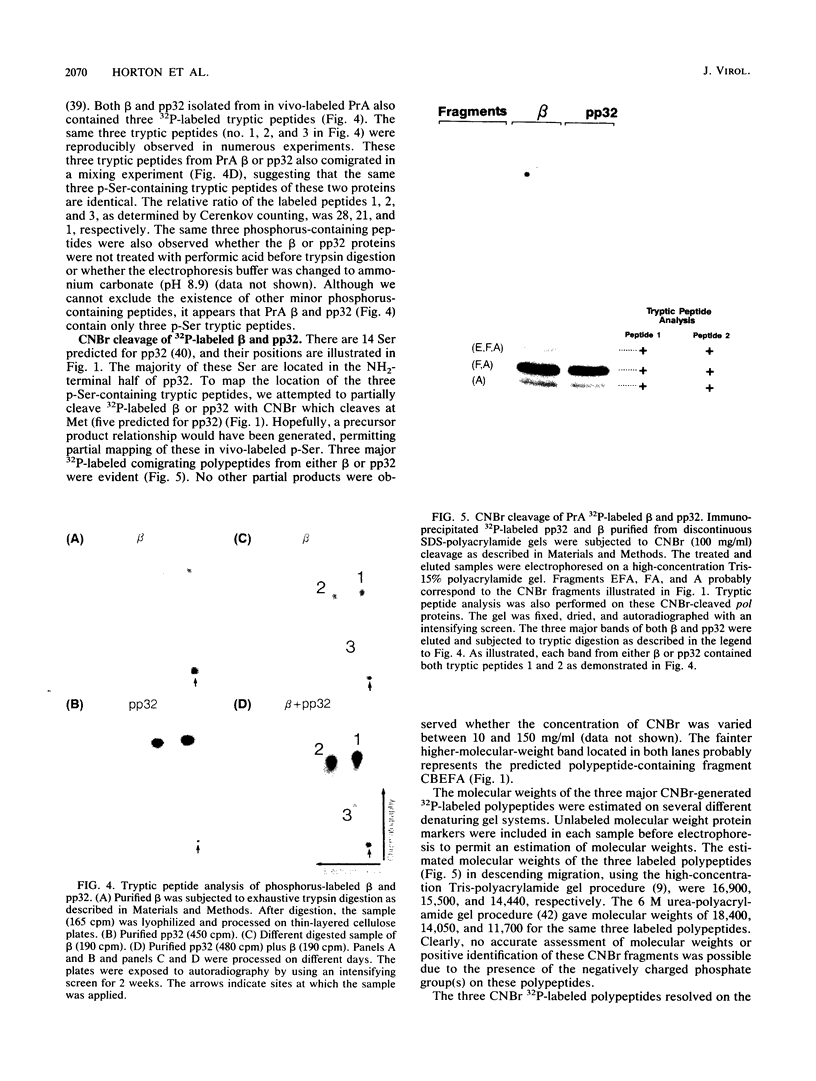
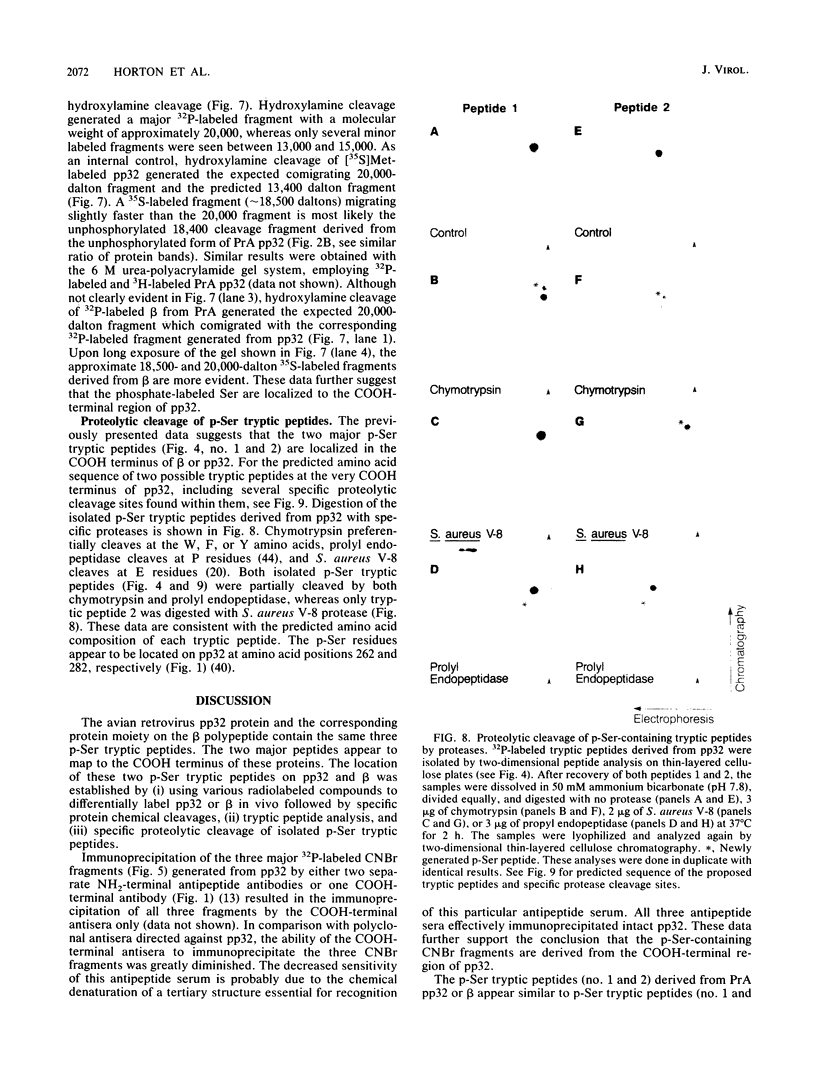
The avian retrovirus pp32 DNA endonuclease and the beta polypeptide of the reverse transcriptase contain the same three phosphoserine (p-Ser) tryptic peptides. At least 95% of the Pi label is nearly equally distributed between two major p-Ser tryptic peptides derived from either beta or pp32. These polymerase gene-derived proteins were metabolically labeled with various radioactive amino acids or Pi, and the purified protein was subjected to cyanogen bromide or hydroxylamine cleavage. The results indicated that the two major p-Ser tryptic peptides map to the COOH-termini of both proteins. The two major p-Ser tryptic peptides isolated from Pi-labeled pp32 were subjected to proteolysis by three separate specific proteases. Analysis of the data suggested that these p-Ser are located on pp32 at amino acid positions 262 and 282 from the amino terminus of pp32 (286 amino acids in length). At present, we cannot exclude the possibility that one or both p-Ser peptides map between amino acid positions 124 to 150. The role of this site-specific phosphorylation of pp32 and beta is also discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander F., Leis J., Soltis D. A., Crowl R. M., Danho W., Poonian M. S., Pan Y. C., Skalka A. M. Proteolytic processing of avian sarcoma and leukosis viruses pol-endo recombinant proteins reveals another pol gene domain. J Virol. 1987 Feb;61(2):534–542. doi: 10.1128/jvi.61.2.534-542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Callahan R., Chiu I. M., Wong J. F., Tronick S. R., Roe B. A., Aaronson S. A., Schlom J. A new class of endogenous human retroviral genomes. Science. 1985 Jun 7;228(4704):1208–1211. doi: 10.1126/science.2408338. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland T. D., Grandgenett D. P., Oroszlan S. Amino acid sequence analysis of reverse transcriptase subunits from avian myeloblastosis virus. J Virol. 1980 Oct;36(1):115–119. doi: 10.1128/jvi.36.1.115-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Varmus H. E. A mutant murine leukemia virus with a single missense codon in pol is defective in a function affecting integration. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6461–6465. doi: 10.1073/pnas.81.20.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyk G., Leis J., Longiaru M., Skalka A. M. Selective cleavage in the avian retroviral long terminal repeat sequence by the endonuclease associated with the alpha beta form of avian reverse transcriptase. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6745–6749. doi: 10.1073/pnas.80.22.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Mason W. S., Linial M. Synthesis and processing of polymerase proteins of wild-type and mutant avian retroviruses. J Virol. 1980 Oct;36(1):62–78. doi: 10.1128/jvi.36.1.62-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Grandgenett D. P. Endonuclease activity of purified RNA-directed DNA polymerase from avian myeloblastosis virus. J Biol Chem. 1979 Mar 10;254(5):1606–1613. [PubMed] [Google Scholar]
- Grandgenett D. P., Golomb M., Vora A. C. Activation of an Mg2+-dependent DNA endonuclease of avian myeloblastosis virus alpha beta DNA polymerase by in vitro proteolytic cleavage. J Virol. 1980 Jan;33(1):264–271. doi: 10.1128/jvi.33.1.264-271.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Schiff R. D. A 32,000-dalton nucleic acid-binding protein from avian retravirus cores possesses DNA endonuclease activity. Virology. 1978 Aug;89(1):119–132. doi: 10.1016/0042-6822(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C. Site-specific nicking at the avian retrovirus LTR circle junction by the viral pp32 DNA endonuclease. Nucleic Acids Res. 1985 Sep 11;13(17):6205–6221. doi: 10.1093/nar/13.17.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D., Quinn T., Hippenmeyer P. J., Oroszlan S. Structural characterization of the avian retrovirus reverse transcriptase and endonuclease domains. J Biol Chem. 1985 Jul 15;260(14):8243–8249. [PubMed] [Google Scholar]
- Hippenmeyer P. J., Grandgenett D. P. Requirement of the avian retrovirus pp32 DNA binding protein domain for replication. Virology. 1984 Sep;137(2):358–370. doi: 10.1016/0042-6822(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Hizi A., Gazit A., Guthmann D., Yaniv A. DNA-processing activities associated with the purified alpha, beta 2, and alpha beta molecular forms of avian sarcoma virus RNA-dependent DNA polymerase. J Virol. 1982 Mar;41(3):974–981. doi: 10.1128/jvi.41.3.974-981.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. C., Court D. L., Zweig M., Levin J. G. Murine leukemia virus pol gene products: analysis with antisera generated against reverse transcriptase and endonuclease fusion proteins expressed in Escherichia coli. J Virol. 1986 Oct;60(1):267–274. doi: 10.1128/jvi.60.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Skalka A. M. A C-terminal domain in the avian sarcoma-leukosis virus pol gene product is not essential for viral replication. J Virol. 1988 Feb;62(2):528–533. doi: 10.1128/jvi.62.2.528-533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick J. J., Harless J., Geisser B. S., Killam R., Hewitt R. R., Arlinghaus R. B. Endodeoxyribonuclease activity associated with Rauscher murine leukemia virus. J Virol. 1981 Jan;37(1):274–283. doi: 10.1128/jvi.37.1.274-283.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Miceli M. V., Jungmann R. A., Hung P. P. Protein kinase and its regulatory effect on reverse transcriptase activity of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2945–2949. doi: 10.1073/pnas.72.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoote M. M., Coligan J. E., Folks T. M., Fauci A. S., Martin M. A., Venkatesan S. Structural characterization of reverse transcriptase and endonuclease polypeptides of the acquired immunodeficiency syndrome retrovirus. J Virol. 1986 Nov;60(2):771–775. doi: 10.1128/jvi.60.2.771-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucher L. A., Loewenstein P. M., Green M. Phosphorylation in vitro of Escherichia coli-produced 235R and 266R tumor antigens encoded by human adenovirus type 12 early transformation region 1A. J Virol. 1985 Oct;56(1):183–193. doi: 10.1128/jvi.56.1.183-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K. C., Gilmore T. D., Panganiban A. T. The spleen necrosis virus int gene product expressed in Escherichia coli has DNA binding activity and mediates att and U5-specific DNA multimer formation in vitro. Virology. 1987 Mar;157(1):127–136. doi: 10.1016/0042-6822(87)90321-7. [DOI] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Baltimore D. Characterization of endonuclease activities in Moloney murine leukemia virus and its replication-defective mutants. J Virol. 1987 May;61(5):1756–1760. doi: 10.1128/jvi.61.5.1756-1760.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. The retrovirus pol gene encodes a product required for DNA integration: identification of a retrovirus int locus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7885–7889. doi: 10.1073/pnas.81.24.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Sefton B. M. Phosphorylation of the transforming protein of Rous sarcoma virus: direct demonstration of phosphorylation of serine 17 and identification of an additional site of tyrosine phosphorylation in p60v-src of Prague Rous sarcoma virus. J Virol. 1986 Jul;59(1):73–81. doi: 10.1128/jvi.59.1.73-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B. Localization of lipid-protein and protein-protein interactions within the murine retrovirus gag precursor by a novel peptide-mapping technique. J Biol Chem. 1983 Sep 25;258(18):11229–11235. [PubMed] [Google Scholar]
- Rettenmier C. W., Karess R. E., Anderson S. M., Hanafusa H. Tryptic peptide analysis of avian oncovirus gag and pol gene products. J Virol. 1979 Oct;32(1):102–113. doi: 10.1128/jvi.32.1.102-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel K. P., Papas T. S., Chirikjian J. G. DNA endonucleases associated with the avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2659–2663. doi: 10.1073/pnas.76.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., van Eenbergen J., Jenks B. G., Bloemers H. P. Hydroxylamine cleavage of proteins in polyacrylamide gels. Anal Biochem. 1983 Jul 1;132(1):54–67. doi: 10.1016/0003-2697(83)90425-6. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Partial phosphorylation in vivo of the avian retrovirus pp32 DNA endonuclease. J Virol. 1980 Dec;36(3):889–893. doi: 10.1128/jvi.36.3.889-893.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978 Oct;28(1):279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Construction and analysis of deletion mutations in the pol gene of Moloney murine leukemia virus: a new viral function required for productive infection. Cell. 1984 Jul;37(3):1043–1052. doi: 10.1016/0092-8674(84)90439-2. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tanese N., Roth M. J., Goff S. P. Analysis of retroviral pol gene products with antisera raised against fusion proteins produced in Escherichia coli. J Virol. 1986 Aug;59(2):328–340. doi: 10.1128/jvi.59.2.328-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Fischl M., Orlowski R. C., Walter R. Post-proline cleaving enzyme and post-proline dipeptidyl aminopeptidase. Comparison of two peptidases with high specificity for proline residues. J Biol Chem. 1978 May 25;253(10):3708–3716. [PubMed] [Google Scholar]