Abstract
Epilepsy is a devastating disease affecting more than 1% of the population. Yet, if one considers the neurobiological substrates of this disease, what is revealed is an array of phenomenon that exemplify the remarkable capacity for the brain to change its basic structure and function, that is, neural plasticity. Some of these alterations are transient and merely impressive for their extent, or for their robust nature across animal models and human epilepsy. Others are notable for their persistence, often enduring for months or years. As an example, the dentate gyrus, and specifically the principal cell of the dentate gyrus, the granule cell, is highlighted. This area of the brain and this particular cell type, for reasons that are currently unclear, hold an uncanny capacity to change after seizures. For those interested in plasticity, it is suggested that perhaps the best examples for study of plasticity lie in the field of epilepsy.
Keywords: Dentate gyrus, Granule cell, Hippocampus, Seizures, Neurotrophins, Neurogenesis, Neuropeptides
One of the most intriguing themes in neuroscience is the ability of the CNS to change, a phenomenon referred to as plasticity. Accordingly, an enormous literature now exists that has described examples of how the CNS can change, both transiently and chronically. This review will focus on the epileptic brain as perhaps the quintessential example of plasticity, although epilepsy is not always considered in this context. Indeed, if asked what was the best example of plasticity in the CNS, most neuroscientists would probably not think of epilepsy. A goal of this review is to suggest to those in the field of epilepsy research that their experiments may have relevance to basic neuroscience, and to suggest to those outside the field of epilepsy that some of the best examples of plasticity actually are phenomena associated with seizures. Insight from those in both groups will likely lead to a better understanding of both plasticity and epilepsy.
Plasticity in epilepsy has been most commonly studied in the brain area known as the hippocampus, and there are several reasons for this. First, the hippocampus is one of the areas of the brain that is quite susceptible to seizures, and many types of epilepsy involve the hippocampus. In temporal lobe epilepsy (TLE), a common type of epilepsy, the hippocampus is a key player, although it is not the only one. In addition, the laminar organization of the hippocampus makes changes perhaps more noticeable than might be in another region with a less obvious lamellar structure.
Although much of the work that involves plasticity in epilepsy has been conducted in rodents using animal models of epilepsy, there appears to be substantial similarity of results among species as phylogenetically distant as humans. Human studies generally are based on data from tissue specimens resected during surgery for pharmacologically unresponsive (“intractable”) epileptics. In these cases, anticonvulsants have not stopped seizures and the option of removing the area thought to trigger seizures is chosen. This often occurs in TLE, and the areas usually resected may include not only the hippocampus but also adjacent cortical regions and the amygdala.
Results of studies in the rodent hippocampus not only appear to apply to other species but also appear to be generalizable to other brain areas. Thus, many of the types of changes that have been documented in the hippocampus have been subsequently described in other areas, such as the neocortex. Thus, it is generally assumed that the changes in the rodent hippocampus after seizures serve as a model for plasticity in humans with epilepsy.
Background
The Basic Structure of the Dentate Gyrus
Within the hippocampus, both in rodents and man, lies a well-defined pyramidal cell layer and granule cell layer (Box 1). Areas CA1 and CA3 comprise the hippocampus proper, or the “hippocampal formation.” The granule cell layer and its associated lamina form a distinct area, the fascia dentata, also called the “dentate gyrus.” As shown in Box 1, the fundamental circuitry of this area, called the trisynaptic circuit, involves three glutamatergic synapses: 1) entorhinal cortical afferents (the “perforant path”) innervate the distal dendrites of granule cells, 2) granule cell axons (the “mossy fibers”) innervate the proximal apical dendrites of area CA3 pyramidal cells, 3) area CA3 innervates the apical dendrites of CA1 pyramidal cells. The circuitry of the hippocampus is actually much more complex because other glutamatergic synapses are present, other afferent systems exist, and these synapses are modulated in many ways by inhibitory neurons (often referred to as “interneurons”) that use GABA (γ-aminobutyric acid) as a neurotransmitter.
Box 1. Organization of the Rat Dentate Gyrus.
Left
Top
A schematic of the rodent hippocampus illustrates the major subfields: area CA1, area CA3, and the dentate gyrus.
Bottom
The trisynaptic pathway is illustrated. Afferents from the entorhinal cortex (the perforant path) innervate the outer two-thirds of the granule cell dendrites (synapse 1). Granule cell axons (the mossy fibers) project to the proximal apical dendrites of area CA3 pyramidal cells (synapse 2). CA3 pyramidal cell axon collaterals (the Schaffer collaterals) innervate the proximal apical dendrites of area CA1 pyramidal cells (synapse 3).
Right
A schematic of the rat dentate gyrus indicates the major cell types and the laminar organization. The molecular layer is subdivided into three distinct lamina, the outer, middle, and inner molecular layers. The granule cell layer is a packed layer of granule cells and some inhibitory neurons (“interneurons”). Interneurons are a heterogeneous population of neurons that contain GABA as well as many other peptides, and are distributed throughout the dentate gyrus. Glutamatergic “mossy” cells are located in the hilus and project primarily to the inner molecular layer. Although the major afferent input to the region is the perforant path, many other afferent systems, for example, CA3 pyramidal cell axons and various subcortical nuclei (e.g., dorsal raphe, locus coeruleus, septum), also innervate distinct areas of this region.
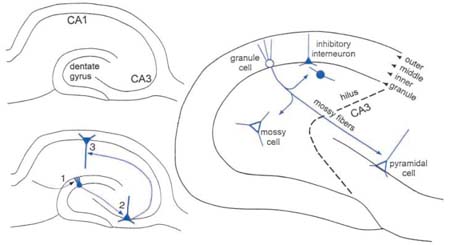
This review will focus on the dentate gyrus because it exemplifies some of the most dramatic types of structural and functional plasticity in the epileptic brain. As shown in Box 1, the basic structure of the dentate gyrus is highly organized. It is composed of a discrete granule cell layer, with each granule cell arranged similar to the next. The layer containing the dendrites of these cells is the molecular layer, which contains three sublayers: an inner molecular layer, middle molecular layer, and outer molecular layer (Box 1). Granule cell axons, the mossy fibers, descend into the hilus and then become restricted to the layer containing the proximal apical dendrites of CA3 pyramidal cells (Box 1). Various types of nongranule cells are scattered throughout the dentate gyrus, comprising a heterogeneous group of GABAergic inhibitory “interneurons” or a more homogenous group of glutamatergic neurons called “mossy cells” (Box 1). Many different peptides and potential neuromodulators are expressed in the hilar cells, as well as granule cells (see below).
Animal Models of Epilepsy
Many animal models of epilepsy exist (Table 1), but for the purposes of the present review, the focus will be on models of epilepsy that are thought to involve the hippocampus and dentate gyrus. Table 1 shows the types of models that are presently used to study hippocampal seizures in rats. It is assumed that seizures in these models enter the dentate gyrus and induce substantial depolarization and action potential discharge in many granule cells. Discharge then propagates to CA3 and CA1 pyramidal cells by the trisynaptic circuit. However, this has not necessarily been demonstrated for all seizures in a particular model. Indeed, some seizures that are thought to invade the hippocampus may not do so, whereas others may do so by pathways besides the trisynaptic circuit, and without activation of granule cells.
Table 1.
Animal models of epilepsy.
| Animal models used to address hippocampal seizures | ||
|---|---|---|
| Convulsants | Electrical Stimulation | Genetic |
| Excitatory agonists (NMDA, glutamate, kainic acid*, etc.) | Perforant path/angular bundle | Transgenic mice (stargazer) |
| Inhibitory antagonists (bicuculline, allylglycine, PTZ*, etc.) | Hippocampal kindling | Various knockouts/overexpressing mice |
| Cholinergics (pilocarpine*, anticholinesterases) | Ventral area CA3 (CHS; SSLSE) | E1 mouse |
| Other (tetanus toxin, fluorothyl) | Electroconvulsive shock | |
| Animal models used to address extrahippocampal involvement | ||
| Convulsants | Electrical Stimulation | Genetic |
| Focal treatment to sites in neocortex (penicillin, cobalt, iron, alumina gel, freeze lesions) | Focal stimuation of various brain regions (amygdala, cortex, subcortical regions) | Seizure-sensitive gerbil, Wag/Rij, Photo-sensitive baboon, SER |
| Focal injection into entorhinal cortex (aminooxyacetic acid, γ-acetylenic GABA) | Kindling of various sites (amygdala, anterior neocortex, other) | GAERS |
| Focal treatment to other areas (substantia nigra, deep prepiriform) | GEPR | |
| Various mutant mice (lethargic, tottering) | ||
| Various knockouts/overexpressing mice | ||
| In vitro models (hippocampal slices, organotypic cultures, cell cultures) | ||
| Convulsants | Electrical Stimulation | Genetic |
| GABAA receptor antagonists/modulators (bicuculline, picrotoxin, low [Cl-]o) | Stimulus induced bursting (STIB) | Gene targeting methods (transfection, gene gun, other) |
| K+ channel antagonists/modulators (4-aminopyridine, tetraethyl-ammonium, high [K+]o) | Perforant path stimulation | |
| NMDA receptor modulators zinc, glycine, (low [Mg2+]o) | Repeated stimulation* | |
models of status epilepticus; note that some of the unstarred drugs/stimuli can also induce status but their use in the literature does not usually involve status. Note that parentheses include only some of the examples that have been studied; many others exist. Abbreviations: CHS = continuous hippocampal stimulation; SSLSE = self-sustaining limbic status epilepticus. PTZ = pentylenetetrazol. ECS = electroconvulsive shock. E1 is an inbred strain of epileptic mice. GAERS = genetic epilespy rat of strasbourg. SER = spontaneous epileptic rat. Wag/Rij is an inbred strain of epileptic rat. GEPR = genetic model of absence epilepsy in rats from Strasbourg. Concentration terms (i.e., [Cl-]) refer to concentrations in the buffer used to incubate in vitro preparations.
Seizure Induced Gene Expression
Changes in Potential Neuromodulators
The fact that neuronal activity leads to changes in gene expression has been known for quite some time. However, it may be less appreciated just exactly how robust this phenomenon is after seizures. In the dentate gyrus, seizures induce a host of changes in granule cell gene expression, and changes in other cell types as well.
Table 2 shows some of the neurotrophins, many of which are normally expressed in granule cells, and change their expression dramatically after limbic seizures. One might think that each type of seizure would lead to different changes, depending on how the seizure was induced (by convulsant injection, electrical stimulation, etc.), how long the seizure lasted, and other factors specific to the model used. However, qualitatively similar changes can follow limbic seizures that were induced by different methods (Table 2). In these cases, the alterations in expression may be less dependent on the method used to cause limbic seizures than the phenomenon of seizure activity itself. However, it is interesting to note that many of the same changes are observed after experimental trauma or ischemia (Table 2), and seizure activity is likely to be absent in such experiments (but see below).
Table 2.
Changes in neurotrophins in the rat dentate granule cells after seizures, trauma, or ischemia.
| Epilepsy | |||||||
|---|---|---|---|---|---|---|---|
| Kainic Acid | Pilocarpine | Kindling | PTZ | ECS | Trauma | Ischemia | |
| NGF |
|
|
|
|
|
|
|
| BDNF |
|
|
|
|
|
|
|
| NT-3 | — |
|
|
 , — , — |
|
||
| NT-4 | — | ||||||
| trkA | — | — | |||||
| trkB |
|
|
|
|
|||
| trkC |
 , ,

|
|
|||||
| p75 |
|
— | |||||
References for Table 2 (information from more than one study is represented in each column): Ballarin and others (1991); Ernfors and others (1991); Isackson and others (1991); Dugich-Djordjevic (1992, 1995); Bayer and Milner (1993); Bengzon and others (1992, 1993); Gall (1993); Humpel and others (1993); Merlio and others (1993); Takeda and others (1993); Arai and others (1996); Bramham and others (1996); Elmer and others (1996); Mudo and others (1995, 1996); Schmidt-Kastner and others (1995, 1996); Kornblum and others (1997); Lee and others (1997); Inoue and others (1998); Kim and others (1998); Morimoto and others (1998); Castren and others (1998); Goutan and Ferrer (1998); Rudge and others (1998); Truettner and others (1999).
Note the similarity of changes in neurotrophins after seizures induced by different mechanisms, or after trauma or ischemia. Abbreviations:
 = increased expression relative to control;
= increased expression relative to control;
 = decreased expression; — = no change;
= decreased expression; — = no change;
 , — = some studies found no change and others demonstrated decreased expression;
, — = some studies found no change and others demonstrated decreased expression;
 ,
,

 = some studies found increased expression and another found an increase followed by a decrease to control levels. Note that some of the differences between studies may be due to different methods (immunocytochemistry vs. in situ hybridization) or examination at different latencies after seizures. Where no symbol is listed, data were unavailable. PTZ = pentylenetetrazol; ECS = electroconvulsive shock; trauma = specific models of trauma used for the studies in this table were sham lesions, implantation of a dialysis probe, hilar lesion (which induced seizures as well as damage), and entorhinal cortex lesion; ischemia = models of ischemia used in this table include transient forebrain ischemia/middle cerebral artery occlusion. Neurotrophins include NGF (nerve growth factor), BDNF (brain derived neurotrophic factor), NT-3 and NT-4 (neurotrophin-3, neurotrophin-4; neurotrophin 4 is sometimes referred to as neurotrophin 4/5). trk = tyrosine kinase, the high affinity receptor class for neurotrophins (NGF binds to trkA; BDNF to trkB; NT-3 to trk A,B,C but has preference for trkC; NT-4 to trkA and B but has preference for trk B). p75 is the low affinity receptor for all neurotrophins. For p75 studies, increases occur mainly in neurons that will die. Other studies apply to changes in expression of surviving dentate granule cells after seizures, trauma, or ischemia.
= some studies found increased expression and another found an increase followed by a decrease to control levels. Note that some of the differences between studies may be due to different methods (immunocytochemistry vs. in situ hybridization) or examination at different latencies after seizures. Where no symbol is listed, data were unavailable. PTZ = pentylenetetrazol; ECS = electroconvulsive shock; trauma = specific models of trauma used for the studies in this table were sham lesions, implantation of a dialysis probe, hilar lesion (which induced seizures as well as damage), and entorhinal cortex lesion; ischemia = models of ischemia used in this table include transient forebrain ischemia/middle cerebral artery occlusion. Neurotrophins include NGF (nerve growth factor), BDNF (brain derived neurotrophic factor), NT-3 and NT-4 (neurotrophin-3, neurotrophin-4; neurotrophin 4 is sometimes referred to as neurotrophin 4/5). trk = tyrosine kinase, the high affinity receptor class for neurotrophins (NGF binds to trkA; BDNF to trkB; NT-3 to trk A,B,C but has preference for trkC; NT-4 to trkA and B but has preference for trk B). p75 is the low affinity receptor for all neurotrophins. For p75 studies, increases occur mainly in neurons that will die. Other studies apply to changes in expression of surviving dentate granule cells after seizures, trauma, or ischemia.
As shown in Table 3, the granule cells (as well as many of the nongranule cells, mostly interneurons) express an array of peptides that are potential neuromodulators even under normal conditions. After seizures, most of them change. Receptors that normally transduce the signals of these peptides also change. For example, there are five receptor subtypes for neuropeptide Y (NPY), and the three that are expressed strongly in the dentate gyrus (Y1, Y2, Y5) all appear to change after seizures (Kofler and others 1997; Kopp and others 1999; Madsen and others 2000; Vezzani and others 2000). Thyroid releasing hormone (TRH) receptors are another example, as indicated by decreased binding in three different animal models of epilepsy (Kubek and others 1993; Jaworska-Feil and others 1999).
Table 3.
Changes in neuropeptides in the rat dentate gyrus after seizures.
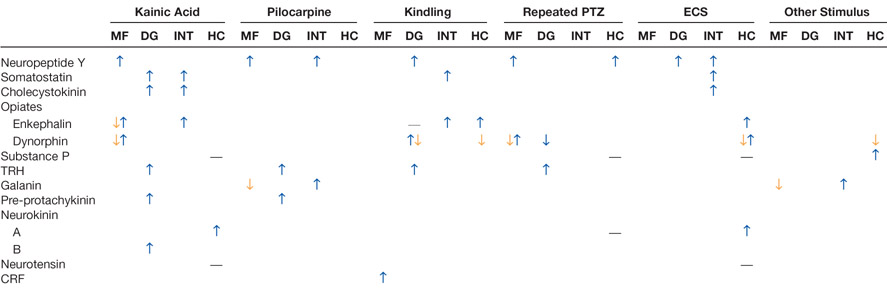 |
References for Table 3 (information from more than one study is included in each column): McGinty and others (1983, 1986); Kanamatsu and others (1986a, 1986b); Lason and others (1987); White and others (1987); Pitkanen and others (1989); Orzi and others (1990); Wanscher and others (1990); Hashimoto and Obata (1991); Xie and others (1991); Brene and others (1992); Lason and others (1992); Marksteiner and others (1992); Rosen and others (1992, 1994); Stenfors and others (1992a, 1992b); Bengzon and others (1993); Gruber and others (1993); Hong and others (1985, 1993); Kubek and others (1993); Passarelli and Orzi (1993); Kragh and others (1994); Mikkelsen and others (1994); Tonder and others (1994); Harrison and others (1995); Elmer and others (1996); Piwko and others (1996); Roder and others (1996); Sato and others (1996); Schwarzer and others (1996); Kofler and others (1997); Lurton and Cavalheiro (1997); Mazarati and others (1998); McCarthy and others (1998); Jaworska-Feil and others (1999); Pierce and others (1999); Scharfman and others (1999); Vezzani and others (1999b); Liu and others (2000); Wasterlain and others (2000).
Abbreviations: kainic acid = i.c.v. or systemic kainic acid; pilocarpine = pilocarpine or lithium/pilocarpine; PTZ = pentelenetetrazol; trauma = lesions, fluid percussive injury, or other models of trauma; ischemia = hypoxia or transient forebrain ischemia. Changes denoted reflect analysis of either the entire dentate gyrus (DG), granule cells (GC), mossy fibers (MF), hilar interneurons (INT), or hippocampus (HC).
 = increased relative to control;
= increased relative to control;
 = decrease; —- = no change. Where no symbol is listed, data were unavailable. Information listed includes multiple methods (immunocytochemical, in situ hybridization, other) and different latencies after seizures.
= decrease; —- = no change. Where no symbol is listed, data were unavailable. Information listed includes multiple methods (immunocytochemical, in situ hybridization, other) and different latencies after seizures.
 reflects that some studies described decreases at one latency after seizures and increases at a subsequent time. Data for enkephalin include studies that examined both met-enkephalin and those that did not specify. Dynorphin data include results for dynorphin A and B and were particularly variable over time and the dorsal-ventral axis of the hippocampus. TRH = thyrotropin-releasing hormone; CRF = corticotropin-releasing factor.
reflects that some studies described decreases at one latency after seizures and increases at a subsequent time. Data for enkephalin include studies that examined both met-enkephalin and those that did not specify. Dynorphin data include results for dynorphin A and B and were particularly variable over time and the dorsal-ventral axis of the hippocampus. TRH = thyrotropin-releasing hormone; CRF = corticotropin-releasing factor.
Importantly, the changes are not always in the positive direction, as exemplified by the simultaneous increase in NGF and BDNF but decrease in NT-3 (Table 2). For peptides, NPY increases its expression in mossy fibers after seizures but mossy fiber dynorphin, for example, decreases (Table 3). These patterns are quite robust across many animal models (Tables 2 and 3), but the temporal evolution of these changes is variable. How long they last is also variable, with some expression over within 24 h and other changes persisting for days or weeks.
Changes in Transmitter Systems
Although striking changes in potential neuromodulators have been well documented, a change in transmitters such as glutamate or GABA would seem almost heretical. Yet a number of laboratories have now described the expression of the inhibitory transmitter GABA in granule cells after seizures (Sandler and Smith 1991; Sloviter and others 1996). It has been well documented that granule cells are normally glutamatergic neurons and induce excitatory postsynaptic potentials (EPSPs) in the targets (Scharfman and others 1990; Jonas and others 1993). However, there is a normally low level of GABA expression, and after seizures, GABA expression increases (Sandler and Smith 1991; Schwarzer and Sperk 1995; Sloviter and others 1996). The synthetic enzyme for GABA, glutamic acid decarboxylase (GAD), also increases (Schwarzer and Sperk 1995; Makiura and others 1999). For many years, it was unclear whether the expression of GABA had functional relevance in light of the evidence that granule cells remained able to evoke EPSPs after seizures. A physiological change associated with increased GABA expression in granule cells has been difficult to prove, because any stimulation of granule cells usually stimulates interneurons as well. However, experiments reported in the last year suggest a potential physiological counterpart to GABA expression in granule cells because short latency inhibitory synaptic potentials (IPSPs) were evoked in pyramidal cells by granule cell stimulation after seizures, but not before seizures (Gutierrez 2000). Responses were pharmacologically similar to mossy fiber-evoked events. However, given that seizures induce so many changes in receptors and neuromodulators (Tables 2–4), as well as other proteins influencing neurotransmission, it is possible that the pharmacology of mossy fiber transmission may change after seizures, potentially complicating the interpretation of these intriguing new data. Yet similar results were found without seizures in another, more recent study (Walker and others 2001).
Table 4.
Seizure-induced changes in NMDA and AMPA receptor subunit expression in rat and human dentate gyrus granule cells.
| Pilocarpine | Kindling | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kainic Acid | Early | Early | Late | Early | Late | ptz | Elec. | ECS | SSLSE | TLE | |||||||
| 1 | 2 | 3 | 4 | 1 | 3 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |||
| NMDA | |||||||||||||||||
| NR1 | — | — | — |
|
|
|
— |
|
— | — | — | ||||||
| NR2A | — | — |
|
— |
|
|
|||||||||||
| NR2B |
|
— |
|
— |
|
|
|||||||||||
| NR2C | — | ||||||||||||||||
| NR2D | — | ||||||||||||||||
| AMPA | |||||||||||||||||
| GluR1 | — | — |
|
— | — |
 flop flop |
 flip flip |
|
|
||||||||
| GluR2 |
 flip flip |
 flip flip |
|
— |
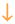 flip flip
 flop flop |
— |
|
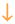 flip flip |
 flip flip |
 (2/3) (2/3) |
 (2/4) (2/4) |
||||||
| GluR3 |
|
|
|
|
|
 flip flip |
 (2/3) (2/3) |
||||||||||
| GluR4 |
 (2/4) (2/4) |
||||||||||||||||
References for Table 4 (each column represents a different study): 1) Condorelli and others (1994); 2) Pollard and others (1993); 3) Lason and others (1997); 4) Friedman and others (1994); 5) Mathern and others (1998b); 6) Lason and others (1998); 7) Kamphuis and others (1994); 8) Watkins and others (1998); 9) Naylor and others (1996); 10) Mathern and others (1998a); 11) Mathern and others (1998c, 1999); 12) Blumcke and others (1996); 13) Musshoff and others (2000).
Abbreviations:
 = increased relative to control;
= increased relative to control;
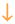 = decreased; — = no change; PTZ = pentylenetetrazol; elec. = electrical kindling of the Schaffer collaterals in the hippocampus; ECS = electroconvulsive shock; SSLSE = self-sustaining limbic status epilepticus; TLE = human temporal lobe epileptic specimens. Other methods used to examined changes were often different. Latencies after seizures also differed across studies, making exact comparisons difficult. (Flip) or (flop) refer to flip or flop splice variants of the particular GluR subunit in question. Data from three pilocarpine studies are shown, one that examined only early times (within a few days) after status and others that examined early or late (weeks or months after status) times after status. “(2/3)” or “(2/4)” refers to data using a label that did not discriminate well between subunits 2 and 3 or 2 and 4; note that the 2/4 data are primarily from the molecular layer, not the granule cell layer.
= decreased; — = no change; PTZ = pentylenetetrazol; elec. = electrical kindling of the Schaffer collaterals in the hippocampus; ECS = electroconvulsive shock; SSLSE = self-sustaining limbic status epilepticus; TLE = human temporal lobe epileptic specimens. Other methods used to examined changes were often different. Latencies after seizures also differed across studies, making exact comparisons difficult. (Flip) or (flop) refer to flip or flop splice variants of the particular GluR subunit in question. Data from three pilocarpine studies are shown, one that examined only early times (within a few days) after status and others that examined early or late (weeks or months after status) times after status. “(2/3)” or “(2/4)” refers to data using a label that did not discriminate well between subunits 2 and 3 or 2 and 4; note that the 2/4 data are primarily from the molecular layer, not the granule cell layer.
Even in the absence of evidence that granule cells change their fundamental excitatory/inhibitory nature, a number of laboratories have demonstrated major changes in glutamate/GABA receptors after seizures (Box 2, Tables 4 and 5), as well as transporters, ion channels (e.g., sodium, potassium, calcium, chloride), pumps, and numerous other important regulators of excitability. In addition to their influence on neuronal function, any change that is persistent is important to consider from a clinical perspective because anticonvulsants may act on these proteins.
Box 2. Glutamate and GABA Receptor Subunits.
| Glutamate Receptors | ||||
|---|---|---|---|---|
| Ionotropic | Metabotropic | |||
|
|
|
|||
| NMDA | AMPA | kainic acid | mGluR | |
| NR1 | GluR1 (A) | GluR5 | I | 1a,1b,1c,1d |
| NR2A | GluR2 (B) | GluR6 | 5a,5b | |
| NR2B | GluR3 (C) | GluR7 | II | 2 |
| NR2C | GluR4 (D) | KA1 | 3 | |
| NR2D | KA2 | III | 4a,4b | |
| NR3A | 6 | |||
| NR3B | 7a,7b | |||
| 8 | ||||
| GABA Receptors | ||||
|
| ||||
| Ionotropic | Metabotropic | |||
|
|
|
|||
| GABAA | GABAC (GABAAOr) | GABAB | ||
| α 1,2,3,4,5,6 | ρ(1) | 1 (a/b) | ||
| β 1,2,3 | 2 | |||
| γ 1,2,3 | ||||
| δ | ||||
| ε | ||||
Table 5.
Seizure-induced changes in GABA receptor subunit expression in rat and human dentate gyrus granule cells.
| Kainic Acid | Pilocarpine | Kindling | ECS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early | Late | Early | Late | Late | Early | Late | Early | Late | Early | TLE | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||
| GABAA | ||||||||||||
| K | ||||||||||||
| 1 |
|
|
|
|
|
— |
|
|
— |
|
— | |
| 2 |
|
 * * |
— | — |
|
— |
|
|||||
| 3 |
|
 ** ** |
|
— |
|
— | (
 ) ) |
|||||
| 4 |
|
|
|
|
|
— |
|
|||||
| 5 |
|
|
— | — |
|
— | ||||||
| 6 |
|
— | ||||||||||
| 2 | ||||||||||||
| 1 |
|
|
|
|
|
— |
|
|||||
| 2 |
|
|
— | — |
|
— |
 (2/3) (2/3) |
|
||||
| 3 |
|
|
|
|
|
|
|
— | — |
 (2/3) (2/3) |
|
|
| 3 | ||||||||||||
| 1 | — | — | — | — | ||||||||
| 2 |
|
|
— | — |
|
|
|
|
— |
|
|
|
| 3 | — | — | ||||||||||
| L |
|
|
|
|
||||||||
| M |
|
|
||||||||||
References for Table 5 (each column represents a different study): 1) Tsunashima and others (1997); Sperk and others (1998); 2) Brooks-Kayal and others (1998); 3) Fritschy and others (1999); 4) Kamphuis and others (1994); 5) Kokaia and others (1994); 6) Clark (1998); 7) Loup and others (2000); 8) Sperk and others (personal communication).
Abbreviations:
 = increased relative to control;
= increased relative to control;
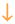 = decreased; — = no change, (
= decreased; — = no change, (
 ) = small increase that was not significant. Note methods used to examined changes were often different. Latencies after seizures also differed across studies, making exact comparisons difficult.
) = small increase that was not significant. Note methods used to examined changes were often different. Latencies after seizures also differed across studies, making exact comparisons difficult.
 * = increase in protein but not mRNA,
* = increase in protein but not mRNA,
 ** = increase in mRNA but not protein. Only data for the granule cells or granule cell layer are listed, except for human studies, which sometimes examined the dentate molecular layer but not the granule cell layer. “(2/3)” refers to results that did not discriminate between β2 or β3. For kainic acid and pilocarpine studies, early = within hours of status epilepticus, late = weeks after kainic acid or pilocarpine-induced status epilepticus. For kindling studies, early and late refer to 1 versus 28 days after the last kindled seizure (Kamphuis and others 1995) or 4 versus 12-48 h after the last of 40 kindled seizures (Kokaia and others 1994).
** = increase in mRNA but not protein. Only data for the granule cells or granule cell layer are listed, except for human studies, which sometimes examined the dentate molecular layer but not the granule cell layer. “(2/3)” refers to results that did not discriminate between β2 or β3. For kainic acid and pilocarpine studies, early = within hours of status epilepticus, late = weeks after kainic acid or pilocarpine-induced status epilepticus. For kindling studies, early and late refer to 1 versus 28 days after the last kindled seizure (Kamphuis and others 1995) or 4 versus 12-48 h after the last of 40 kindled seizures (Kokaia and others 1994).
Although some of these changes have been argued to be compensatory, in actuality some of the changes may perpetuate seizures. An example lies in the resistance of individuals in status epilepticus to anticonvulsants as the interval between status onset and drug administration lengthens. Early after status onset, the benzodiazepine diazepam, thought to work at the GABAA receptor, is the drug of choice. However, as the time from the onset of status increases, diazepam becomes increasingly ineffective, and this is thought to be due to rapid changes in the relevant GABA receptor subunits (Rice and others 1996; Kapur and Macdonald 1997; Brooks-Kayal and others 1998).
Changes in Gene Expression of Nongranule Cells
Granule cells are not the only type of cell in the dentate gyrus that changes after seizures. Indeed, one of the most difficult problems in epilepsy research is determining the ultimate implications of so many changes in such a diversity of cells, which is further complicated by the fact that some of these neurons are highly interconnected.
For the interneuron population, a number of changes have been described (Table 3). Some are similar to the changes in granule cells, for example, NPY is increased in some of the interneurons in the hilus after seizures. However, this change is not necessarily identical to the change in granule cells because increased expression of NPY in interneurons is primarily somatic, whereas increased expression of NPY in granule cells is primarily axonal (mossy fiber expression). An important point to consider in these studies is that some of the interneurons may be damaged after seizures, whereas granule cells are relatively resistant to seizures. In Tables 2 through 4, changes are listed for the surviving interneurons only; decreased expression due to loss of interneurons is self-explanatory and not included.
Mossy cells have been demonstrated to be one of the most vulnerable cell types to seizures. Therefore, there have not been many studies about this cell type after seizures. Only recently have the surviving mossy cells been examined after severe seizures (in human TLE: Blumcke and others 1999; in rat models of TLE: Scharfman and others 2001). Interestingly, mossy cells that survive become hyperexcitable (Scharfman and others 2001), which could have important implications for subsequent network activity. What are the changes in expression in surviving mossy cells? Currently very little is known. Of the two peptides that have been localized to mossy cells, calcitonin gene-regulatory peptide (CGRP; Bulloch and others 1996; Freund and others 1997) and the α8 subunit of the integrin receptor (Einheber and others 2000), it is only clear that the integrin receptor subunit increases after seizures (Einheber and others 2000). CGRP has not been examined in mossy cells after seizures, but it is known that CGRP increases in mossy cells after ischemia (Bulloch and others 1998).
Not to be forgotten are the glial cells, which undergo a metamorphosis after many types of seizures, presumably because of seizure-induced damage. Thus, glia in the vicinity of a damaged site undergo a well-described process of reactive gliosis. Until recently this was a phenomenon that few considered to have direct physiological effects, for example, most individuals considered tissue that was gliotic somewhat lifeless. However, new recordings from glia after injury (D'Ambrosio and others 1999), or in patients with epilepsy (Hinterkeuser and others 2000; Bordey and others 2001), suggest substantial potential to influence neuronal function. Even in astrocytes that are not necessarily part of a gliotic area, their physiology may change in a striking and complex manner, and this is likely to have functional consequences. The fact that many neuromodulators are synthesized in glia (e.g., growth factors, cytokines, kynurenines) and that seizures can increase synthesis (Du and others 1993; Vezzani and others 1999a) indicates that there may be an even greater influence of glia on neuronal function after seizures than under normal conditions.
Mechanisms Underlying Plasticity after Seizures
An important aspect of altered expression for all of the changes described above are their underlying mechanisms. In other words, how does seizure activity produce all of these changes? Given that synaptic depolarization activates several signal transduction mechanisms, which can end in an altered state of various transcription factors (e.g., AP-1, CREB), a prime candidate mechanism is the regulation of transcription factors. Indeed, one of the first indications that seizures change gene expression was the discovery that the immediate early gene c-fos increased greatly after seizures (Dragunow and Robertson 1987; Morgan and others 1987; White and Gall 1987). Since that time, innumerable other immediate early genes (FosB, c-jun, Krox, zif268; Gass and Herdegen 1995; Hughes and others 1999) have also been shown to increase after seizures, and not just in granule cells.
Structural Changes in the Dentate Gyrus after Seizures
Numerous examples of structural plasticity exist that have attracted much attention. In the dentate gyrus, a classic example is lesion-induced growth of axon collaterals into the molecular layer. In these studies, lesions to the entorhinal cortex lead to a cascade of events in the dentate gyrus that end in a substantially altered molecular layer. Although not necessarily considered at the time, it is interesting to consider whether some of these changes were associated with seizures, in light of evidence that entorhinal lesions can lead to seizures either acutely or after several days or weeks (Dashieff and McNamara 1982; Wu and others 2000). Importantly, some of these seizures did not have motor components (only EEG recordings detected them, that is, they were “electrographic” seizures) so they could have been missed quite easily.
In many animal models of epilepsy, a variety of structural changes occur in the dentate gyrus, some of which involve the growth of new axon collaterals or “sprouting.” Perhaps the most widely studied and remarkable example is the sprouting of mossy fibers after seizures (also termed “synaptic reorganization”), because the new collaterals can be extensive, and they target a very specific area, the inner molecular layer of the dentate gyrus. Mossy fiber sprouting also merits emphasis because similar changes appear to occur in human TLE (Sutula and others 1989; Houser and others 1990; Babb and others 1991). There also is evidence that other types of cells undergo sprouting after seizures, including GABA neurons (Davenport and others 1990; Morin and others 1999).
Mossy fiber sprouting has been identified in several animal models of epilepsy. It was first described after kainic acid administration and subsequently was shown after pilocarpine-induced seizures and kindling (Tauck and Nadler 1986; Cavazos and others 1991; Ben-Ari and Cossart 2000). Sprouting is demonstrable anatomically because the mossy fibers contain one of the highest levels of zinc in the hippocampus, so the Timm stain for heavy metals distinguishes the granule cell axons well. Figure 1 illustrates mossy fiber sprouting schematically and in the pilocarpine model, using Timm stain or NPY to demonstrate mossy fibers. GAP-43 also stains sprouted mossy fibers (Meberg and others 1993). Note that in contrast, there is only light Timm or NPY stain in the inner molecular layer normally (Scharfman and others 1999, 2000, 2001).
Fig. 1.
Mossy fiber sprouting. Left: Top. A schematic of the normal dentate gyrus granule cell projection, the mossy fibers, which mainly target CA3 pyramidal cells. Not shown are collaterals that innervate hilar dendrites of nonprincipal cells (interneurons and mossy cells of the dentate gyrus). Bottom. A schematic of the dentate gyrus granule cell axons after seizures that induce mossy fiber sprouting. Collaterals of the mossy fiber axons sprout into the inner molecular layer, where they are thought to innervate proximal dendrites of granule cells and inhibitory neuronal processes. Right: A, Mossy fiber sprouting from a pilocarpine-treated rat with recurrent seizures, illustrated by Timm stain for heavy metals. The sprouted fibers are stained darkly in the inner molecular layer (IML). Mossy fibers are also stained in the hilus (HIL). GCL = granule cell layer. Calibration = 100 μm. B, A section from a pilocarpine-treated rat that had status epilepticus and recurrent seizures, that was stained using an antibody to neuropeptide Y. Sprouted fibers are indicated by the arrows. DG = dentate gyrus. Calibration (in A) = 250 μm.
There continue to be several unsettled questions about this robust form of neural plasticity. First, it is not completely clear when sprouting occurs after seizures, although in models involving a period of status epilepticus (i.e., kainic acid or pilocarpine), it appears that sprouting begins sometime in the first 1 to 2 weeks, and is most robust after many weeks.
It is also not completely clear what the sprouted fibers actually do, although electron microscopy has shown that sprouted fibers make synapses and appear to target both granule cells and GABA neurons (Okazaki and others 1995; Kotti and others 1997; Zhang and Houser 1999; Wenzel and others 2000). Several physiological studies have provided evidence that the sprouted fibers have a functional role, but whether they have a primarily excitatory or inhibitory influence on the dentate gyrus is still somewhat unclear. Several studies have shown that stimulation of granule cells in hippocampal slices from kainic acid- or pilocarpine-treated rats activate recurrent excitatory circuits (Wuarin and Dudek 1996; Okazaki and others 1999). However, Sloviter (1992) showed that inhibition is actually greater in the dentate gyrus in vivo when sprouting occurs, which has been supported by evidence of sprouting on interneurons (Kotti and others 1997). The answer may be that each of these studies is fundamentally correct, because under some conditions the excitatory actions might dominate (e.g., if inhibition is compromised or mossy fiber transmission is enhanced by a hormonal fluctuation or neurotrophin; Scharfman and others 1999), but in other situations inhibition may have a greater influence. Indeed, this would seem logical given that human epileptics with sprouting have seizures only intermittently.
Another unsettled issue is the mechanism underlying mossy fiber sprouting. What is the trigger for sprouting, and what are the molecular cues that allow sprouted fibers to travel and then innervate the inner molecular layer? At the moment, it would be fair to say that this question is not answered. However, several studies have provided insight into this issue. For example, it was shown that there was a correlation between cell loss in the hilar region after seizures and the development of sprouting (Masukawa and others 1995). The loss of hilar neurons that project to the inner molecular layer (i.e., mossy cells) might provide an attractive opening site for sprouted axons. However, other studies have questioned the requirement for hilar cell loss (Stringer and others 1997), and in fact there have been reports of sprouting without detectable hilar cell loss, albeit not very robust sprouting. Thus, hilar damage may not be as important as originally believed. Other studies have addressed whether neurotrophins may play a role in sprouting, because seizures induce increases in neurotrophins in granule cells and neurotrophins foster process outgrowth in other systems. However, studies to date have not found evidence that neurotrophins fully address mechanisms of mossy fiber sprouting (Lowenstein and others 1993; Holtzman and Lowenstein 1995; Patel and McNamara 1995; Van Der Zee and others 1995).
Although mossy fiber sprouting may seem to be a phenomenon only in the hippocampus, several reports indicate that actually sprouting may occur in other areas of the brain after seizures. This indicates that the understanding of sprouting gained by studying mossy fiber sprouting might be able to address similar phenomenon elsewhere. Area CA1 has been one region where sprouting of axons after seizures has been reported (Perez and others 1996; Morin and others 1999; Smith and Dudek 2001). Sprouting has also been demonstrated in the neocortex (Salin and others 1995).
Structural changes in the dentate gyrus after seizures are not only confined to the example of mossy fiber sprouting. Another notable change is dispersion of the granule cell layer, which involves an expansion of the layer and a decrease in cell density within it (Houser 1990). Although the mechanisms are largely unknown, it is quite robust across various animal models and human epilepsy (Houser 1990) and quite striking in some mouse strains (Bouilleret and others 2000).
Another example is the formation of “basal” dendrites of granule cells (Spigelman and others 1998; Ribak and others 2000). After both pilocarpine-induced seizures and electrically induced seizures, some of the granule cells appear to develop dendrites from the opposite pole of the soma as those in the molecular layer (“apical”) dendrites. Basal dendrites have been examined ultrastructurally and appear to receive synaptic input similar to normal dendrite. Interestingly, basal dendrites have previously been described in primates (Seress and Mrzljak 1987) and in early development, adding evidence to the suggestion that seizures lead to a recapitulation of development (see below). Little is known about the physiological effects of basal dendrites, although it has been shown in slices from pilocarpine-treated animals that granule cells with basal dendrites have similar intrinsic properties as other granule cells and are not necessarily more excitable (Smith and others 2000).
Kindling
Kindling refers to the process of repeatedly stimulating the brain electrically with a stimulus that initially leads only to an afterdischarge. After triggering this stimulus repeatedly, for example, once a day for many days, the response to the stimulus increases, and eventually the stimulus leads to a stage 5 seizure. At this point, the animal is “fully kindled,” and the stimulus will continue to elicit a seizure if triggered subsequently.
Kindling is often studied as a model of epileptogenesis, that is, the development of the epileptic state. Arguments have been raised, however, that this is not an optimal model of epilepsy, because kindling does not result in a state of recurring spontaneous seizures, the common definition of epilepsy (however, it has been reported that this can occur under some conditions). Nevertheless, the state of the fully kindled animal is abnormally excitable, and kindling allows perhaps the best means to study the development of abnormal excitability in a well-controlled manner. In the context of plasticity, it is one of the best examples of neural plasticity that can be addressed in a controlled fashion, because the stimulation required is similar from animal to animal and the paradigm allows controlled influence on the development of the kindled state.
Neurogenesis
The dentate gyrus is one of the few areas of the brain that undergoes neurogenesis throughout life. These cells appear to come from the subgranular zone, which lies directly below the granule cell layer. After seizures, neurogenesis increases dramatically. This has now been shown for a variety of seizure types, including kindling, status epilepticus following convulsant injection (pilocarpine, kainic acid), electrical stimulation, and electroconvulsive shock (Bengzon and others 1997; Parent and others 1997, 1998; Gray and Sundstrom 1998; Scott and others 1998, 2000). Neurogenesis is quite robust and appears to continue for weeks following the initial stimulus. Perhaps just as important is the fact that many of the newly born cells appear to survive and develop into mature neurons that integrate into the circuitry around them (Scharfman and others 2000). Some of their developmental cues appear to occur normally, because many of them migrate to a position in the granule cell layer, with similar axons as normal granule cells (Markakis and Gage 1999; Scharfman and others 2000).
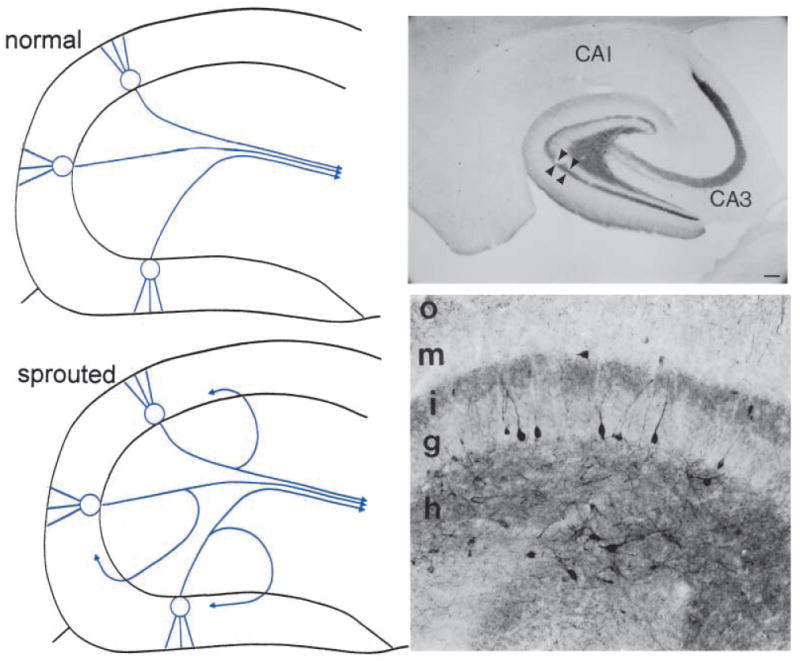
Other newly born cells appear to lose their way, ending up in the molecular layer or the hilus. In our studies of pilocarpine and kainic acid–treated rats, we found a subpopulation of newly born cells migrated all the way to the border of the hilus and area CA3 (Fig. 2; Scharfman and others 2000). There they appeared to cluster, as if an invisible barrier, presumably a combination of cellular and extracellular factors, prevented their further migration. The fact that no granule cells exist in this region normally allowed us a way to examine newly born cells that would be impossible in the granule cell layer, where they could be confused with normal adult granule cells.
Fig. 2.
Newly born granule cells in the hilus after seizures. A, The dentate gyrus of a pilocarpine-treated rat after status epilepticus and spontaneous recurrent seizures, stained using an antibody to the calcium binding protein calbindin D28K. There is a novel population of cells at the hilar/CA3 border that is not normally present. Many of these cells were double labeled with bromodeoxyuridine (BrdU), a marker of cells undergoing cell division, following BrdU injection after pilocarpine-induced status epilepticus, which indicates that these cells were born after seizures (Scharfman and others 2000). GCL = granule cell layer; HIL = hilus; PCL = pyramidal cell layer. Calibration = 100 μm. B, A higher magnification of the region at the hilar/CA3 border in part A shows the calbindin-immunoreactive cells at the CA3/hilar border (arrows). Calibration (in A) = 50 μm. C, An intracellularly labeled hilar neuron (arrow), recorded in a hippocampal slice from a pilocarpine-treated rat that had spontaneous seizures. This neuron had the morphology and electrophysiology of a granule cell but was located in the hilus and oriented differently from normal granule cells, some of which were stained for comparison (asterisks). Calibration same as B. The end of the pyramidal cell layer is on the right. D, Mossy fiber “giant” boutons (arrow), which distinguish granule cell axons from axons of other cell types, were located along the axons of the hilar/CA3 granule cells such as those shown in part C. This piece of the mossy fiber had two giant boutons. Calibration (in A) = 25 μm.
From Scharfman and others (2000), with permission from the Society for Neuroscience.
From these studies came several surprises. Electrophysiological recordings demonstrated that these cells have virtually identical membrane properties as adult granule cells located in the granule cell layer (Scharfman and others 2000). This is surprising in light of how distinct these properties are from other cell types in the region, such as the interneurons, pyramidal cells, and mossy cells (Scharfman 1992, 1999; Scharfman and others 2000). Therefore, even in an abnormal location and in the adult brain, new granule cells appear able to maintain the developmental program that underlies the maturation of a normal granule cell. Morphologically, these new granule cells also appear to develop normally, but there are several notable exceptions. Thus, although the size and shape of the soma and mossy fiber axon are difficult to distinguish from a normal granule cell, there are often extensive dendrites on both sides of the soma (Scharfman and others 2000). Thus, these neurons develop “basal” as well as “apical” dendrites. Yet this is not necessarily abnormal, because basal dendrites have been described in the granule cells located in the cell layer of the same animals (see above).
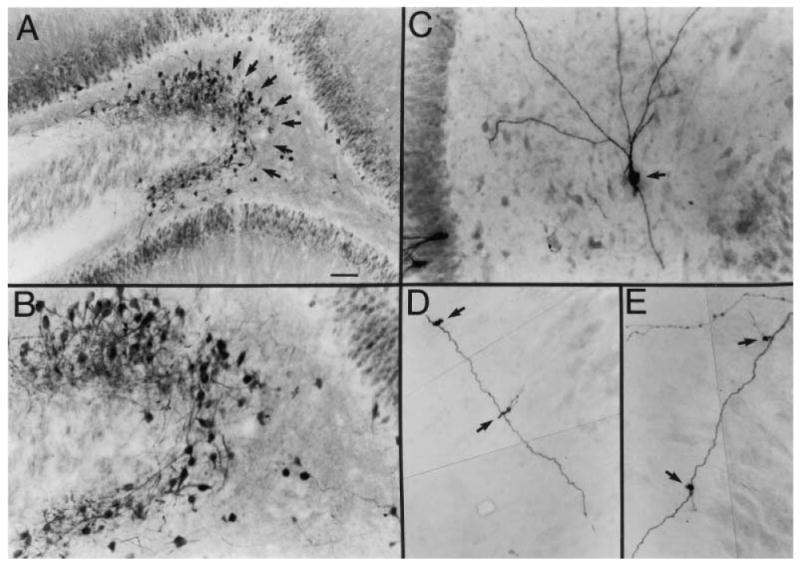
Another surprise was the physiological activity that the newly born granule cells demonstrated. Intracellular recordings showed that many of the cells had epileptiform discharges that occurred spontaneously at a periodicity of approximately 0.5 to 0.05 Hz (Fig. 3). This is completely abnormal for a granule cell, which, in the normal brain, requires unusual conditions to discharge spontaneously (such as exposure to increased extracellular potassium and GABA receptor antagonists, etc.). Furthermore, simultaneous recordings showed that CA3 pyramidal cells were synchronized with the newly born cells (Fig. 3). Synchrony was usually close, within several milliseconds. Therefore, it is possible that the newly born cells became coupled to pyramidal cells, either by synaptic or nonsynaptic mechanisms, either of which would be far from normal (Fig. 3). Thus, although the developmental program for membrane properties appeared to proceed unimpeded, the connectivity that developed was highly plastic.
Fig. 3.
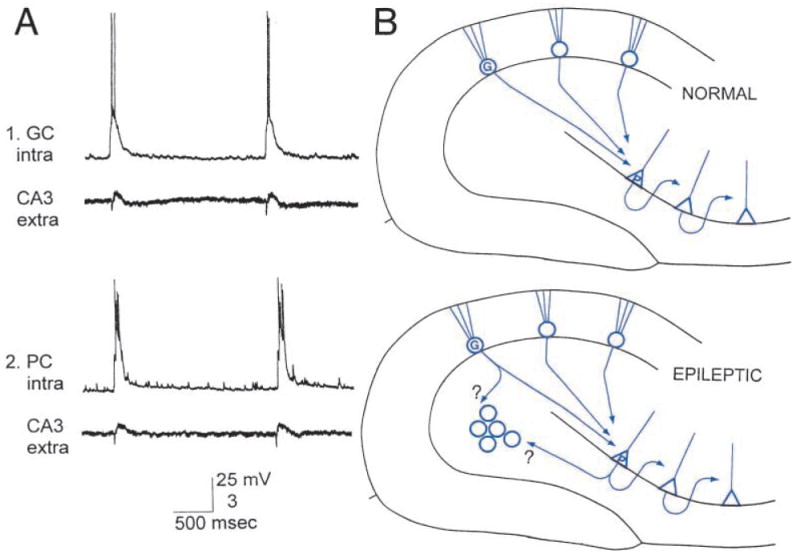
Functional implication of seizure-induced neurogenesis. A1, Simultaneous intracellular recordings from a granule cell (GC intra) at the hilar/CA3 border and CA3 pyramidal cell layer extracellularly (CA3 extra) in a hippocampal slice from a pilocarpine-treated rat with chronic seizures. For both 1 and 2, calibration for intracellular recordings, 25 mV; for extracellular recordings, 3 mV. A2, Same as A.1, but the intracellular recording is from a pyramidal cell in CA3, illustrating the synchrony of granule cells at the hilar/CA3 border with CA3 pyramidal cells. B: Top, Diagram of the normal mossy fiber pathway from granule cells to pyramidal cells. Area CA3 axons that innervate other CA3 neurons are also illustrated. Bottom, Diagram of the potential new excitatory circuits after seizure-induced neurogenesis and abnormal integration of newly born granule cells in the hilus with host circuitry. Granule cells located in the normal location may innervate the new granule cells, and the axon collaterals of CA3 pyramidal cells, which normally innervate hilar neurons, may also innervate the new cells.
Part A is from Scharfman and others (2000), with permission from the Society for Neuroscience.
Besides the dramatic plasticity evident in this form of adult neurogenesis, it may have etiological significance because similar types of abnormally developed clusters of new cells could occur elsewhere. Given the small size of these clusters of new cells, they could be missed by current neuroimaging methods, and therefore may be relevant to the epileptics with seizures of unclear origin.
Plasticity Induced by Seizures, Trauma, or Ischemia
Importantly, many of the plastic changes that follow seizures have also been reported after ischemia or injury/trauma, both in animal models and in humans (Table 2). Indeed, some of the first studies of stimulus-induced changes in gene expression in granule cells followed hilar lesions (Gall 1993). Although by no means are these situations (i.e., epilepsy, trauma, stroke) identical, this does raise the possibility that many of the changes that follow seizures are due to damage that occurs rather than the activity itself. One can also argue conversely that the changes following trauma must be considered with caution, because changes attributed to them may be, at least in part, a function of altered excitability, and possibly seizures, particularly seizures without obvious behavioral signs.
Clearly there are a variety of types of damage that occur after seizures, especially severe or prolonged seizures. In the dentate gyrus, cell loss is often observed in the hilar region and area CA3c (the part of area CA3 that enters the dentate gyrus). Similar to rodent models of epilepsy, human temporal lobe epileptics often exhibit hilar and pyramidal cell loss, with relative sparing of the granule cell layer (“hippocampal sclerosis”; note that in some cases, however, granule cell loss can also be extensive). In both animal models and human epileptics, there is often cell loss in other areas of the limbic system (entorhinal cortex, piriform cortex, etc.). The implication for plasticity is that the mechanisms involved may be indirect. That is, the seizure activity itself may not directly lead to changes, but actually lead to damage or cell death that is the real stimulus. Changes in glia, for example, could be related to the death or damage to juxtaposed neurons rather than the presumed passive depolarization of most glia during a seizure.
Human Epileptics
Human studies of epileptics have filled the literature with case studies of neural plasticity. Particularly if seizures occur in childhood, the areas of the brain that are responsible for language, speech, and so on, can substantially change position. Similar changes can occur after surgical removal of a part of the brain. Remarkably, some individuals can resume normal function after large parts of the brain are removed, even after hemispherectomy. It is assumed that the brain reorganizes functionally, but the manner in which this occurs, that is, the cellular and molecular mechanisms, has not been well explained.
A well publicized example is a young concert violinist whose seizures interfered with her ability to perform in concert. The seizures appeared to originate from the area believed to control her ability to play the violin. However, surgery in this area did not block her ability to perform, because her brain had evidently already adapted in such a way that the normal locations of, for example, the auditory area, were elsewhere. It is assumed that the brain's sensory function had already moved outside the normal location because of damage to the normal areas where these functions usually develop.
The actual area where seizures originate, the “focus,” is often the area that is targeted for removal. Although successful in many cases, sometimes the surgical removal of one area is not enough to stop seizures, because another area of the brain has developed the capacity to trigger seizures, a phenomenon known as “secondary epileptogenesis,” which is itself an extraordinary example of plasticity (Morrell and DeToledo-Morrell 1999; Sutula 2001; Teyler and others 2001). Thus, whereas some changes that occur in the brain after seizures can be adaptive (functional reorganization), others are certainly not (secondary epileptogenesis).
Perspectives and Horizons
Recapitulating Development
General issues have evolved as a result of the numerous demonstrations of seizure-induced or seizure-associated changes in the brain. A common theme, particularly strengthened after recent demonstrations of seizure-induced increases in dentate gyrus neurogenesis, is whether the series of changes that occurs after seizures represents a recapitulation of development. Even without consideration of neurogenesis, there are many examples of seizure-induced changes that support the concept that seizures induce a pattern of expression reminiscent of the developing brain. For example, the increase in growth factors and neurotrophins is robust. Increased expression of molecules associated with synaptogenesis also occurs. But even more persuasive are changes in expression that are extremely different from the adult, only observed in the immature state. One example is the increased expression of the α6 subunit of the GABAA receptor. Normally this subunit is expressed in granule cells only during development, but in the pilocarpine model, granule cells begin to express this subunit (Brooks-Kayal and others 1998).
Epilepsy at the End of a Continuum of Activity-Dependent Change
Another important issue is whether the changes that are observed after epilepsy are similar to those that have been described to follow increased neural activity. Are seizures merely at the end of a continuum that begins with the influence of a single EPSP or action potential in a single cell (Fig. 4)? If so, perhaps seizures merely induce a greater change than a more modest period of neural activity, rather than a different type of change altogether. This is of interest mechanistically because of the wealth of information on changes that follow brief periods of neural activity, such as short- and long-term potentiation. Can the responses of granule cells to a stimulus that produces LTP be generalized to responses of granule cells to a limbic seizure? Certainly there are some similarities; for example, immediate early gene expression and neurotrophins are increased by both a tetanus used to elicit LTP and seizures that involve the dentate gyrus (Bramham and others 1996). In area CA1, there does appear to be extensive similarities in some postulated mechanisms of epileptogenesis and LTP (Morris and others 2000). However, there is at least one major difference between seizures, particularly severe seizures, and lesser activity: there can be cell death and damage that sets into motion a number of changes that may never occur after limited neuronal activity (Fig. 4). In summary, one could argue, in the context of plasticity, that seizures induce qualitatively similar changes as lesser degrees of activity, differing perhaps only quantitatively, with the exception being seizure-associated damage (Fig. 4). Whether mechanisms underlying LTP and mechanisms underlying epileptogenesis are similar remains a difficult question to answer (Baudry 1986; McNamara 1993; Kullmann and others 2000).
Fig. 4.
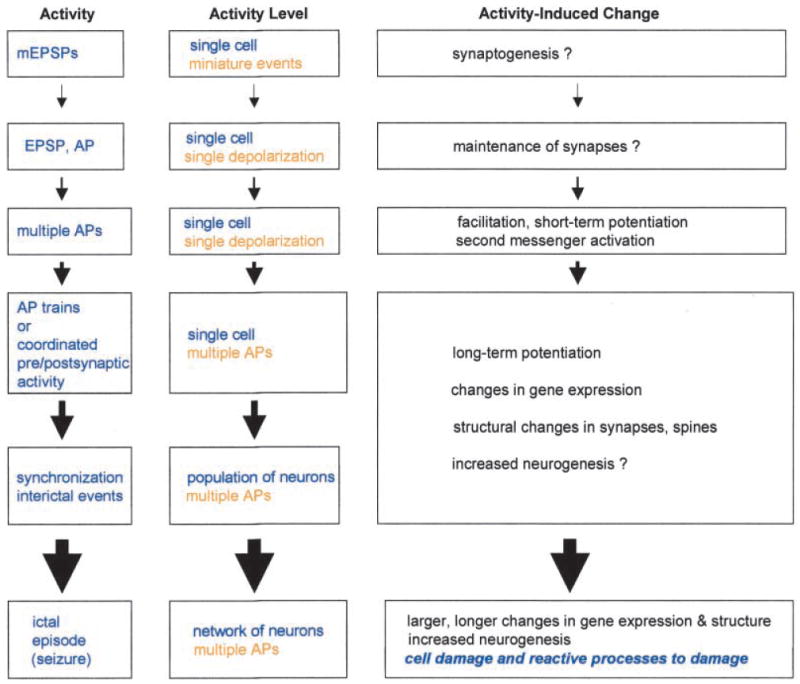
Activity-dependent changes in the dentate gyrus as a continuum. A diagram illustrates the concept of a continuum from plasticity induced by simple forms of neural activity, such as a single depolarization in a single cell, to the changes following seizures. Physiological levels of activity are listed from top to bottom, in increasing order. The actual physiological events are described according to the number of neurons involved and extent of depolarization in the center column, as the “Activity level.” Examples of the types of plastic changes associated with each level of activity are listed on the right as “Activity-induced changes,” some of which are not completely clear (“?”). Set apart from the rest is the one major difference between seizures and lesser forms of activity, at least as currently understood, which are the changes that follow cell death or damage due to seizures (see text). EPSP = excitatory postsynaptic potential; mEPSP = miniature EPSP; AP = action potential.
Interaction of Seizure-Associated Changes
Some of the most interesting insights into epileptogenesis may come from the understanding of how the various changes that occur after a seizure may interact. Figure 5 shows an example of the potential for changes to influence each other in the dentate gyrus. Specifically, Figure 5 considers the possible interaction between increased neurogenesis and seizure-induced increases in the neurotrophin BDNF and NPY.
Fig. 5.
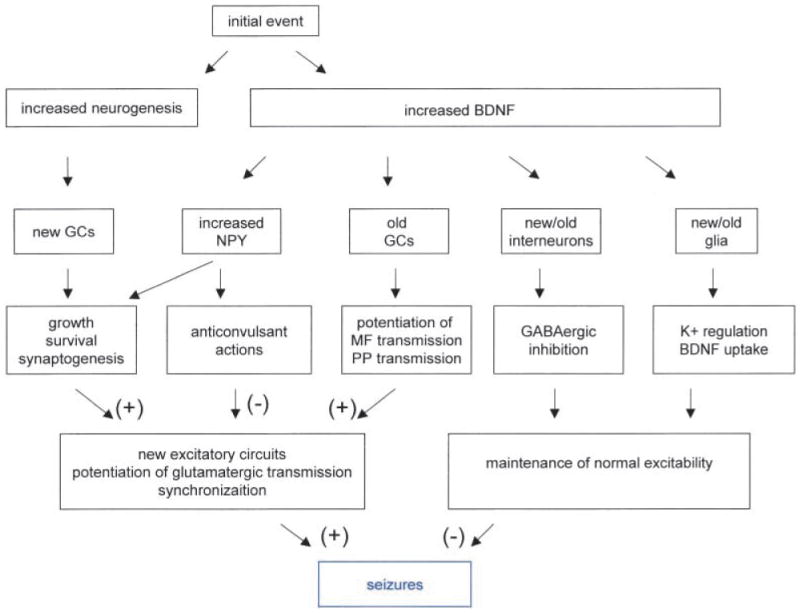
Schematic of interaction between BDNF and neurogenesis after an initial event that could contribute to consequent epileptogenesis. Potential interactions between three robust changes after seizures: increased neurogenesis of granule cells, increased BDNF expression in granule cells, and increased NPY in granule cells. Both BDNF and NPY may have actions on both newly born cells and “old” cells. Their effects on newly born cells would be likely given that both BDNF and NPY have effects on proliferation; BDNF may also influence the survival of newly born cells because of its neuroprotective actions, and foster synaptogenesis of new/old cell circuits. Effects on old cells would be likely because both BDNF and NPY have been shown to influence synaptic transmission. Whereas BDNF's effects appear to primarily increase excitability, most of the effects of NPY appear to be “anticonvulsant.” Thus, the interplay between newly born granule cells and increased expression of various modulators such as BDNF and NPY leads to a more complicated result than any one of these factors occurring alone.
Initially, either a seizure or injury could lead to increased neurogenesis and increased BDNF in granule cells. This might be the initial precipitating event that many have believed to be the beginning of epileptogenesis in TLE. Subsequently there has been description of a “silent” period, both in animal models and clinically, when little sign of seizures is apparent, after which chronic seizures occur. It may be no coincidence that the silent period in animal models is somewhat similar to the latency for newly born granule cells to mature.
It is possible that the newly born cells in the dentate gyrus interact with the increased production of BDNF in the surrounding, adult granule cells. BDNF may facilitate the growth of these new cells, be neuroprotective, as well as foster synaptogenesis. At the same time that BDNF is facilitating the growth, development, and synaptogenesis of newly born cells, it may also have profound influence on adult granule cells. For example, BDNF could protect the mature cells from seizure-induced damage. Indeed, one could suggest that this is one of the reasons why granule cells are so resistant to insults that kill adjacent neurons. In addition, BDNF would be likely to potentiate perforant path transmission and/or mossy fiber transmission, because BDNF does so in vivo and in vitro in normal tissue (Scharfman 1997; Messaoudi and others 1998). Taken together, these events could facilitate synchronized activity in the dentate gyrus and area CA3 (Fig. 5), although other effects of BDNF, that is, on interneurons (Marty and others 1997), glia, and NPY (Croll and others 1994; Fig. 5), could be anticonvulsant.
NPY is an important neuropeptide to consider in this context because it has been known for some time that seizures increase NPY expression in granule cells (Lurton and Cavalheiro 1997; Vezzani and others 1999b), BDNF induces NPY (Croll and others 1994), and NPY has generally anticonvulsant effects (Vezzani and others 1999b). In addition, and particularly relevant to neurogenesis after seizures, NPY has been shown recently to enhance proliferation of olfactory cells (Hansel and others 2001). Thus, perhaps NPY is yet another player that modulates the development and survival of newly born granule cells after seizures (Fig. 5).
Summary
Consideration of all the types of changes in the dentate gyrus following seizures will hopefully clarify the disease process, that is, epileptogenesis. It may also lead to better treatment strategies for epileptics that currently are not well controlled with available anticonvulsants. The ultimate goal is to determine which plastic changes are adaptive and which are maladaptive, and to determine how one might foster the former and hinder the latter. Of course, this approach may be too simplistic given that changes in a single protein can have multiple consequences, some good and some bad (Fig. 5). Clearly, to clarify how our understanding of plasticity can be used to better understand epilepsy, and vice versa, the collective expertise of neuroscientists, developmental/molecular biologists, and epilepsy researchers/clinicians will be necessary.
Acknowledgments
This work was supported by NS 37562, 38285, 16102 from NIH and the Human Frontiers Science Program.
References
- Arai SKH, Akabane A, Owada Y, Kamii H, Kawase M, Yoshimoto T. Induction of brain-derived neurotrophic factor (BDNF) and the receptor trkB mRNA following middle cerebral artery occlusion in rat. Neurosci Lett. 1996;211:57–60. doi: 10.1016/0304-3940(96)12720-8. [DOI] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–63. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Ballarin M, Ernfors P, Lindefors N, Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991;114:35–43. doi: 10.1016/0014-4886(91)90082-n. [DOI] [PubMed] [Google Scholar]
- Baudry M. Long-term potentiation and kindling: similar biochemical mechanisms? Adv Neurol. 1986;44:401–10. [PubMed] [Google Scholar]
- Bayer LE, Milner TA. Transient increases in neuropeptide Y-like immunoreactivity in dentate hilar neurons following fimbria/fornix transection. J Neurosci Res. 1993;34:434–41. doi: 10.1002/jnr.490340408. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–7. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvile A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–7. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Ernfors P, Kokaia M, Leanza G, Nilsson Og, et al. Regulation of neurotrophin and trkB and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 1993;53:433–46. doi: 10.1016/0306-4522(93)90207-v. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Soderstrom S, Kokaia Z, Kokaia M, Ernfors P, Persson H, et al. Widespread increase of nerve growth factor protein in the rat forebrain after kindling-induced seizures. Brain Res. 1992;587:338–42. doi: 10.1016/0006-8993(92)91016-8. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Beck H, Scheffler B, Hof PR, Morrison JH, Wolf HK, et al. Altered distribution of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionate receptor subunit GluR2(4) and the N-methyl-D-aspartate receptor subunit NMDAR1 in the hippocampus of patients with temporal lobe epilepsy. Acta Neuropathol (Berl) 1996;92:576–87. doi: 10.1007/s004010050564. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Zuschratter W, Schewe JC, Suter B, Lie AA, Riederer BM, et al. Cellular pathology of hilar neurons in Ammon's horn sclerosis. J Comp Neurol. 1999;414:437–53. doi: 10.1002/(sici)1096-9861(19991129)414:4<437::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J Neurophysiol. 2001;85:1719–31. doi: 10.1152/jn.2001.85.4.1719. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium binding proteins parvalbumin, calbindin, or calretinin. Neuroscience. 2000;97:47–58. doi: 10.1016/s0306-4522(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Sourhard T, Sarvey JM, Herkenham M, Brady LS. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: evidence for interhemispheric communication. J Comp Neurol. 1996;368:371–82. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brene S, Lindefors N, Ballarin M, Persson H. Kainic acid mediated increase of preprotachykinin A messenger RNA expression in the rat hippocampus and a region-selective attenuation by dexamethasone. Neuroscience. 1992;50:611–18. doi: 10.1016/0306-4522(92)90450-g. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nature Med. 1998;4:1166–72. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Bulloch K, Milner TA, Prasad A, Hsu M, Buzsaki G, McEwen BS. Induction of calcitonin gene-related peptide-like immunoreactivity in hippocampal neurons following ischemia: a putative regional modulator of the CNS injury/immune response. Exp Neurol. 1998;150:195–205. doi: 10.1006/exnr.1997.6765. [DOI] [PubMed] [Google Scholar]
- Bulloch K, Prasad A, Conrad CD, McEwen BS, Milner TA. Calcitonin gene-regulated peptide level in the rat dentate gyrus increases after damage. Neuroreport. 1996;7:1036–40. doi: 10.1097/00001756-199604100-00016. [DOI] [PubMed] [Google Scholar]
- Castren E, Berninger B, Leingartner A, Lindholm D. Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Prog Brain Res. 1998;117:57–64. doi: 10.1016/s0079-6123(08)64007-8. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;11:2795–803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli DF, Belluardo N, Mudo G, Dell'Albani P, Jiang X, Giuffrida-Stella AM. Changes in gene expression of AMPA-selective glutamate receptor subunits induced by status epilepticus in rat brain. Neurochem Int. 1994;25:367–76. doi: 10.1016/0197-0186(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clark M. Sensitivity of the rat hippocampal GABA (A) receptor alpha-4 subunit to electroshock seizures. Neurosci Lett. 1998;250:17–20. doi: 10.1016/s0304-3940(98)00422-4. [DOI] [PubMed] [Google Scholar]
- Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci. 1994;6:1343–53. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K+ homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–62. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashieff RM, McNamara JO. Electrolytic entorhinal lesions cause seizures. Brain Res. 1982;231:444–50. doi: 10.1016/0006-8993(82)90381-x. [DOI] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–90. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987;329:441–2. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Du F, Williamson J, Bertram E, Lothman E, Okuno E, Schwarcz R. Kynurenine pathway enzymes in a rat model of chronic epilepsy: immunohistochemical study of activated glial cells. Neuroscience. 1993;55:975–89. doi: 10.1016/0306-4522(93)90312-4. [DOI] [PubMed] [Google Scholar]
- Dugich-Djordjevic MM, Lapchak PA, Pasinetti GM, Najm I, Baudry M, Hefti F. Regionally-specific and rapid increases in brain-derived neurotrophic factor messenger RNA in the adult rat brain following seizures induced by systemic administration of kainic acid. Neuroscience. 1992;47:303–15. doi: 10.1016/0306-4522(92)90246-x. [DOI] [PubMed] [Google Scholar]
- Dugich-Djordjevic MM, Ohsawa F, Ikazaki T, Mori N, Day JR, Beck KD, et al. Differential regulation of catalytic and non-catalytic trkB messenger RNAs in the rat hippocampus following seizures induced by systemic administration of kainate. Neuroscience. 1995;66:861–77. doi: 10.1016/0306-4522(94)00631-e. [DOI] [PubMed] [Google Scholar]
- Einheber S, Pierce JP, Chow D, Znamensky V, Schnapp LM, Milner TA. Dentate hilar mossy cells and somatostatin-containing neurons are immunoreactive for the α8 integrin subunit: characterization in normal and kainic acid-treated rats. Neuroscience. 2000 doi: 10.1016/s0306-4522(01)00205-6. In press. [DOI] [PubMed] [Google Scholar]
- Elmer E, Kokaia M, Kokaia Z, Ferencz I, Lindvall O. Delayed kindling development after rapidly recurring seizures: relation to mossy fiber sprouting and neurotrophin, GAP-43 and dynorphin gene expression. Brain Res. 1996;712:19–34. doi: 10.1016/0006-8993(95)01424-1. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–76. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Pellegrini-Giampiertro DE, Sperber EF, Bennett MV, Moshe SL, Zukin RS. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in sutu hybridization study. J Neurosci. 1994;14:2697–707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Hajos N, Acsady L, Gorcs TJ, Katona I. Mossy cells of the rat dentate gyrus are immunoreactive for calcitonin-gene regulated peptide (CGRP) Eur J Neurosci. 1997;9:1815–30. doi: 10.1111/j.1460-9568.1997.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABA(A) receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–45. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Gall CM. Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp Neurol. 1993;124:150–66. doi: 10.1006/exnr.1993.1186. [DOI] [PubMed] [Google Scholar]
- Gass P, Herdegen T. Neuronal expression of AP-1 proteins in excitotoxic-neurodegenerative disorders and following nerve fiber lesions. Prog Neurobiol. 1995;47:257–69. [PubMed] [Google Scholar]
- Goutan EME, Ferrer I. BDNF, and full length and truncated TrkB expression in the hippocampus of the rat following kainic acid excitotoxic damage. Evidence of complex time-dependent and cell-specific responses. Mol Brain Res. 1998;59:154–64. doi: 10.1016/s0169-328x(98)00156-9. [DOI] [PubMed] [Google Scholar]
- Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–9. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Gruber B, Greber S, Sperk G. Kainic acid seizures cause enhanced expression of cholecystokinin-octapeptide in the cortex and hippocampus of the rat. Synapse. 1993;15:221–8. doi: 10.1002/syn.890150307. [DOI] [PubMed] [Google Scholar]
- Gutierrez R. Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J Neurophysiol. 2000;84:3088–90. doi: 10.1152/jn.2000.84.6.3088. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y as a neuroproliferative factor. Nature. 2001;410:940–4. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Harrison MB, Shumate MD, Lothman EW. Opioid peptide expression in models of chronic temporal lobe epilepsy. Neuroscience. 1995;65:785–95. doi: 10.1016/0306-4522(94)00529-e. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Obata K. Induction of somatostatin by kainic acid in pyramidal and granule cells of the rat hippocampus. Neurosci Res. 1991;12:514–27. doi: 10.1016/s0168-0102(09)80004-7. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–96. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Lowenstein DH. Selective inhibition of axon outgrowth by antibodies to NGF in a model of temporal lobe epilepsy. J Neurosci. 1995;15:7062–70. doi: 10.1523/JNEUROSCI.15-11-07062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JS, McGinty JF, Lee PH, Xie CW, Mitchell CL. Relationship between hippocampal opioid peptides and seizures. Prog Neurobiol. 1993;40:507–28. doi: 10.1016/0301-0082(93)90020-s. [DOI] [PubMed] [Google Scholar]
- Hong JS, Yoshikawa K, Kanamatsu T, McGinty JF, Mitchell CL, Sabol SL. Repeated electroconvulsive shocks alter the biosynthesis of enkephalin and concentration of dynorphin in the rat brain. Neuropeptides. 1985;5:557–60. doi: 10.1016/0143-4179(85)90078-2. [DOI] [PubMed] [Google Scholar]
- Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535:195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–82. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Alexi T, Walton M, Williams CE, Dragunow M, Clark RG, Gluckman PD. Activity and injury-dependent expression of inducible transcription factors, growth factors, and apoptosis-related genes within the central nervous system. Prog Neurobiol. 1999;57:421–50. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Humpel C, Ebendal T, Cao Y, Olson L. Pentylenetetrazol seizures increase pro-nerve growth factor-like immunoreactivity in the reticular thalamic nucleus and nerve growth factor mRNA in the dentate gyrus. J Neurosci Res. 1993;35:419–27. doi: 10.1002/jnr.490350409. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hirai H, Onteniente B, Suzuki F. Correlated long-term increase of brain-derived neurotrophic factor and TrkB proteins in enlarged granule cells of mouse hippocampus after kainic acid injection. Neuroscience. 1998;86:723–8. doi: 10.1016/s0306-4522(98)00112-2. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–48. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jaworska-Feil L, Turchan J, Przewlocka B, Budziszewska B, Leskiewicz M, Lason W. Effects of pilocarpine- and kainate-induced seizures on thyrotropin-releasing hormone biosynthesis and receptors in the rat brain. J Neural Trans. 1999;106:395–407. doi: 10.1007/s007020050167. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fiber synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993;472:615–63. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W, De Rijik TC, Lopes da Silva FH. Expression of GABAA receptor subunit mRNAs in hippocampal pyramidal and granular neurons in the kindling model of epileptogenesis: an in situ hybridization study. Mol Brain Res. 1995;31:33–47. doi: 10.1016/0169-328x(95)00022-k. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, De Rijk TC, Talamini LM, Lopes da Silva FH. Rat hippocampal kindling induces changes in the glutamate receptor mRNA expression patterns in dentate granule neurons. Eur J Neurosci. 1994;6:1119–27. doi: 10.1111/j.1460-9568.1994.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Kanamatsu T, McGinty JF, Mitchell CL, Hong JS. Dynorphin- and enkephalin-like immunoreactivity is altered in basal ganglia regions of rat brain after repeated electroconvulsive shock. J Neurosci. 1986a;6:644–9. doi: 10.1523/JNEUROSCI.06-03-00644.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamatsu T, Obie J, Grimes L, McGinty JF, Yoshikawa K, Sabol S, et al. Kainic acid alters the metabolism of met5-enkephalin and the level of dynorphin A in the rat hippocampus. J Neurosci. 1986b;6:3094–102. doi: 10.1523/JNEUROSCI.06-10-03094.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiapepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–40. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Smith MA, Post RM, Rosen JB. Attenuation of kindling-induced decreases in NT-3 mRNA by thyroid hormone depletion. Epilepsy Res. 1998;29:211–20. doi: 10.1016/s0920-1211(97)00087-9. [DOI] [PubMed] [Google Scholar]
- Kofler N, Kirchmair E, Schwarzer C, Sperk G. Altered expression of NPY-Y1 receptors in kainic acid induced epilepsy in rats. Neurosci Lett. 1997;230:129–32. doi: 10.1016/s0304-3940(97)00492-8. [DOI] [PubMed] [Google Scholar]
- Kokaia M, Pratt GD, Elmer E, Bengzon J, Fritschy JM, Kokaia Z, et al. Biphasic differential changes of GABAA receptor subunit mRNA levels in dentate gyrus granule cells following recurrent kindling-induced seizures. Mol Brain Res. 1994;23:323–32. doi: 10.1016/0169-328x(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Kopp J, Nanobashvili A, Kokaia Z, Lindvall O, Hokfelt T. Differential regulation of mRNAs for neuropeptide Y and its receptor subtypes in widespread areas of the rat limbic system during kindling epileptogenesis. Mol Brain Res. 1999;72:17–29. doi: 10.1016/s0169-328x(99)00191-6. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Sankar R, Shin DH, Wasterlain CG, Gall CM. Induction of brain derived neurotrophic factor mRNA by seizure neonatal and juvenile rat brain. Mol Brain Res. 1997;44:219–28. doi: 10.1016/s0169-328x(96)00224-0. [DOI] [PubMed] [Google Scholar]
- Kotti T, Riekkinen PJ, Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol. 1997;146:323–30. doi: 10.1006/exnr.1997.6553. [DOI] [PubMed] [Google Scholar]
- Kragh J, Tonder N, Finsen BR, Zimmer J, Bolwig TG. Repeated electroconvulsive shocks cause transient changes in rat hippocampal somatostatin and neuropeptide Y immunoreactivity and mRNA in situ hybridization signals. Exp Brain Res. 1994;98:305–13. doi: 10.1007/BF00228418. [DOI] [PubMed] [Google Scholar]
- Kubek MJ, Knoblach SM, Sharif NA, Burt DR, Buterbaugh GG, Fuson KS. Thyrotropin-releasing hormone gene expression and receptors are differentially modified in limbic foci by seizures. Ann Neurol. 1993;33:70–6. doi: 10.1002/ana.410330112. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F, Walker MC. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cell Mol Life Sci. 2000;57:1551–61. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lason W, Przewlocka B, Przewlocki R. Single and repeated electroconvulsive shock differentially affects the prodynorphin and proopiomelanocortin system in the rat. Brain Res. 1987;403:301–7. doi: 10.1016/0006-8993(87)90067-9. [DOI] [PubMed] [Google Scholar]
- Lason W, Przewlocka B, Przewlocki R. The prodynorphin system in the rat hippocampus is differentially influenced by kainic acid and pentetrazole. Neuroscience. 1992;51:357–62. doi: 10.1016/0306-4522(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Lason W, Turchan J, Przewlocka B, Labuz D, Machelska H, Przewlocki R. Effects of pentylenetetrazol kindling on glutamate receptor gene expression in the rat hippocampus. Brain Res. 1998;785:355–8. doi: 10.1016/s0006-8993(97)01426-1. [DOI] [PubMed] [Google Scholar]
- Lason W, Turchan J, Przewlocka B, Labuz D, Mika J, Przewlocki R. Seizure-related changes in the glutamate R2 and R5 receptor genes expression in the rat hippocampal formation. J Neural Trans. 1997;104:125–33. doi: 10.1007/BF01273175. [DOI] [PubMed] [Google Scholar]
- Liu H, Sankar R, Shin DH, Mazarati AM, Wasterlain CG. Patterns of status epilepticus-induced substance P expression during development. Neuroscience. 2000;101:297–304. doi: 10.1016/s0306-4522(00)00383-3. [DOI] [PubMed] [Google Scholar]
- Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–19. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Seren MS, Longo FM. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience. 1993;56:597–604. doi: 10.1016/0306-4522(93)90359-n. [DOI] [PubMed] [Google Scholar]
- Lurton D, Cavalheiro EA. Neuropeptide-Y immunoreactivity in the pilocarpine model of temporal lobe epilepsy. Exp Brain Res. 1997;116:186–90. doi: 10.1007/pl00005739. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Greisen MH, Nielsen SM, Bolwig TG, Mikkelsen JD. Electroconvulsive stimuli enhance both neuropeptide Y receptor Y1 and Y2 messenger RNA expression and levels of binding in the rat hippocampus. Neuroscience. 2000;98:33–9. doi: 10.1016/s0306-4522(00)00078-6. [DOI] [PubMed] [Google Scholar]
- Makiura Y, Suzuki F, Chevalier E, Onteniente B. Excitatory granule cells of the dentate gyrus exhibit a double inhibitory neurochemical content after intrahippocampal administration of kainate in adult mice. Exp Neurol. 1999;159:73–83. doi: 10.1006/exnr.1999.7138. [DOI] [PubMed] [Google Scholar]
- Markakis A, Gage FH. Adult generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–60. [PubMed] [Google Scholar]
- Marksteiner JW, Walder R, Bellmann R, Ortler M, Krause JE, Sperk G. Limbic seizures cause pronounced changes in the expression of neurokinin in the hippocampus of the rat. Neuroscience. 1992;49:383–95. doi: 10.1016/0306-4522(92)90104-a. [DOI] [PubMed] [Google Scholar]
- Marty S, Da Penha Berzaghi M, Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- Masukawa LM, O'Connor WM, Lynott J, Burdette LJ, Uruno K, McGonigle P, et al. Longitudinal variation in cell density and mossy fiber reorganization in the dentate gyrus from temporal lobe epileptic patients. Brain Res. 1995;678:65–75. doi: 10.1016/0006-8993(95)00167-o. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Leite JP, Kornblum HI, Mendoza D, Lozada A, et al. Hippocampal AMPA and NMDA mRNA levels and subunit immunoreactivity in human temporal lobe epilepsy patients and a rodent model of chronic mesial limbic epilepsy. Epilepsy Res. 1998a;32:154–71. doi: 10.1016/s0920-1211(98)00048-5. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Mendoza D, Leite JP, Chimelli L, Born DE, et al. Hippocampal N-methyl-D-aspartate receptor subunit mRNA levels in temporal lobe epilepsy patients. Ann Neurol. 1999;46:343–58. doi: 10.1002/1531-8249(199909)46:3<343::aid-ana10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Mendoza D, Lozada A, Kornblum HI. Hippocampal AMPA and NMDA mRNA levels correlate with aberrant fascia dentata mossy fiber sprouting in the pilocarpine model of spontaneous limbic epilepsy. J Neurosci Res. 1998b;54:734–53. doi: 10.1002/(SICI)1097-4547(19981215)54:6<734::AID-JNR2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Mendoza D, Lozada A, Leite JP, Chimelli L, et al. Increased hippocampal AMPA and NMDA receptor subunit immunoreactivity in temporal lobe epilepsy. J Neuropathol Exp Neurol. 1998c;57:615–34. doi: 10.1097/00005072-199806000-00008. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, et al. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–7. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JB, Walker M, Pierce J, Camp P, White JD. Biosynthesis and metabolism of native and oxidized neuropeptide Y in the hippocampal mossy fiber system. J Neurochem. 1998;70:1950–63. doi: 10.1046/j.1471-4159.1998.70051950.x. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Henriksen SJ, Goldstein A, Terenius L, Bloom FE. Dynorphin is contained within hippocampal mossy fibers: immunochemical alterations after kainic acid administration and colchicine-induced neuotoxicity. Proc Natl Acad Sci U S A. 1983;80:589–93. doi: 10.1073/pnas.80.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Kanamatsu T, Obie J, Dyer RS, Mitchell CL, Hong JS. Amygdaloid kindling increases enkephalin-like immunore-activity but decreases dynorphin-A-like immunoreactivity in rat hippocampus. Neurosci Lett. 1986;71:31–6. doi: 10.1016/0304-3940(86)90252-1. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Excitatory amino acid receptors and epilepsy. Curr Opin Neurol Neurosurg. 1993;6:583–7. [PubMed] [Google Scholar]
- Meberg PJ, Gall CM, Routtenberg A. Induction of F1/GAP-43 gene expression in hippocampal granule cells after seizures. Mol Brain Res. 1993;17:295–9. doi: 10.1016/0169-328x(93)90014-g. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of brain-derived neurotrophic factor induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–9. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Woldbye D, Kragh J, Larsen PJ, Bolwig TG. Electroconvulsie shocks increase the expression of neuropeptide Y (NPY) mRNA in the piriform cortex and the dentate gyrus. Mol Brain Res. 1994;23:317–22. doi: 10.1016/0169-328x(94)90241-0. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–7. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Sato K, Sato S, Yamada N, Hayabara T. Time-dependent changes in neurotrophic factor mRNA expression after kindling and long-term potentiation in rats. Brain Res Bull. 1998;45:599–605. doi: 10.1016/s0361-9230(97)00459-0. [DOI] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC. Alterations of perisomatic GABA synapses on hippocampal CA1 inhibitory interneurons and pyramidal cells in the kainate model of epilepsy. Neuroscience. 1999;93:457–67. doi: 10.1016/s0306-4522(99)00199-2. [DOI] [PubMed] [Google Scholar]
- Morrell F, DeToledo-Morrell L. From mirror focus to secondary epileptogenesis in man: a historical review. Adv Neurol. 1999;81:11–23. [PubMed] [Google Scholar]
- Morris TA, Jafari N, DeLorenzo RJ. Chronic delta FosB expression and increased AP-1 transcription factor binding are associated with the long term plasticity changes in epilepsy. Mol Brain Res. 2000;79:138–49. doi: 10.1016/s0169-328x(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Mudo G, Salin T, Condorelli DF, Jiang XH, Dell'Albani P, Timmusk T, et al. Seizures increase trkC mRNA expression in the dentate gyrus of rat hippocampus. Role of glutamate receptor activation. J Mol Neurosci. 1995;6:11–22. doi: 10.1007/BF02736755. [DOI] [PubMed] [Google Scholar]
- Mudo GJX, Timmuske T, Bindoni M, Belluardo N. Change in neurotrophins and their receptor mRNAs in the rat forebrain after status epilepticus induced by pilocarpine. Epilepsia. 1996;37:198–207. doi: 10.1111/j.1528-1157.1996.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Musshoff U, Schunke U, Kohling R, Speckmann EJ. Alternative splicing of the NMDAR1 glutamate receptor subunit in human temporal lobe epilepsy. Mol Brain Res. 2000;76:377–84. doi: 10.1016/s0169-328x(00)00030-9. [DOI] [PubMed] [Google Scholar]
- Naylor P, Stewart CA, Wright SR, Pearson RC, Ried IC. Repeated ECS induces GluR1 mRNA but not NMDAR1A-G mRNA in the rat hippocampus. Mol Brain Res. 1996;35:349–53. doi: 10.1016/0169-328x(95)00264-s. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515–34. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Molnar P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol. 1999;81:1645–60. doi: 10.1152/jn.1999.81.4.1645. [DOI] [PubMed] [Google Scholar]
- Orzi F, Zoli M, Passarelli F, Ferraguti F, Fieschi C, Agnati LF. Repeated electroconvulsive shock increase glial fibrillary acidic protein, ornithine decarboxylase, somatostatin, and cholecystokinin immunoreactivities in the hippocampal formation of the rat. Brain Res. 1990;533:223–31. doi: 10.1016/0006-8993(90)91343-f. [DOI] [PubMed] [Google Scholar]
- Parent JM, Janumpalli S, McNamara JO, Lowenstein DH. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neurosci Lett. 1998;247:9–12. doi: 10.1016/s0304-3940(98)00269-9. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–8. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli F, Orzi F. Effect of electroconvulsive shock treatment on the opioid mediated inhibition of serotonin release in rat hippocampal slices. Neuroreport. 1993;4:1108–10. [PubMed] [Google Scholar]
- Patel MN, McNamara JO. Selective enhancement of axonal branching of cultured dentate gyrus neurons by neurotrophic factors. Neuroscience. 1995;69:763–70. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Beaulieu C, Lacaille JC. Axonal sprouting of CA1 pyramidal cells in hyperexcitable hippocampal slices of kainate-treated rats. Eur J Neurosci. 1996;8:736–48. doi: 10.1111/j.1460-9568.1996.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–76. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Beal MF, Sirvio J, Swartz KJ, Mannisto PT, Riekkinen PJ. Somatostatin, neuropeptide Y, GABA and cholinergic enzymes in brain of pentylenetetrozol-kindled rats. Neuropeptides. 1989;14:197–207. doi: 10.1016/0143-4179(89)90045-0. [DOI] [PubMed] [Google Scholar]
- Piwko C, Thoss VS, Samanin R, Hoyer D, Vezzani A. Status of somatostatin receptor messenger RNAs and binding sites in rat brain during kindling epileptogenesis. Neuroscience. 1996;75:857–68. doi: 10.1016/0306-4522(96)00304-1. [DOI] [PubMed] [Google Scholar]
- Pollard H, Heron A, Moreau J, Ben-Ari Y, Khrestchatisky M. Alterations of the GluR-B AMPA receptor subunit flip/flop expression in kainate-induced epilepsy and ischemia. Neuroscience. 1993;57:545–54. doi: 10.1016/0306-4522(93)90004-y. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–53. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA, DeLorenzo RJ. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc Natl Acad Sci U S A. 1996;93:9665–9. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder C, Schwarzer C, Vezzani A, Gobbi M, Mennini T, Sperk G. Autoradiographic analysis of neuropeptide Y receptor binding sites in the rat hippocampus after kainic acid induced limbic seizures. Neuroscience. 1996;70:47–55. doi: 10.1016/0306-4522(95)00332-d. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Crain CJ, Weiss SR, Post RM. Alterations in mRNA of enkephalin, dynorphin and thyrotropin releasing hormone during amygdala kindling: an in situ hybridization study. Mol Brain Res. 1992;15:247–55. doi: 10.1016/0169-328x(92)90115-r. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Kim SY, Post RM. Differential regional and time course increases in thyrotropin-releasing hormone, neuropeptide Y, and enkephalin mRNAs following an amygdala kindled seizure. Mol Brain Res. 1994;27:71–80. doi: 10.1016/0169-328x(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Rudge JSMP, Pasnikowski EM, Ning C, Corcoran T, Acheson A, Anderson K, et al. Endogenous BDNF protein is increased in adult rat hippocampus after a kainic acid induced excitotoxic insult but exogenous BDNF is not neuroprotective. Exp Neurol. 1998;149:398–410. doi: 10.1006/exnr.1997.6737. [DOI] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 1995;15:8234–45. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991;303:177–92. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- Sato K, Kashihara K, Morimoto K, Hayabara T. Regional increases in brain-derived neurotrophic factor and nerve growth factor mRNAs during amygdaloid kindling, but not in acidic and basist growth factor mRNAs. Epilepsia. 1996;37:6–14. doi: 10.1111/j.1528-1157.1996.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells, and aspiny, “fast-spiking” cells. In: Ribak CE, Gall CM, Mody I, editors. The dentate gyrus and its role in seizures. New York: Elsevier; 1992. pp. 93–109. [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–95. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. In: Delgado-Escueta AV, Wilson W, Olsen RW, Porter RJ, editors. Basic mechanisms of the epilepsies: molecular and cellular approaches. 3rd. New York: Lippincott-Raven; 1999. pp. 805–20. [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Actions of BDNF in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19:5619–31. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–58. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons established by simultaneous intracellular recording in rat hippocampal slices. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Smith KL, Goodman JH, Sollas AL. Dentate hilar mossy cells after pilocarpine-induced status epilepticus, recurrent seizures and mossy fiber sprouting. Neuroscience. 2001 doi: 10.1016/s0306-4522(01)00132-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Humpel C, Wetmore C, Olson L. Cellular hybridization for BDNF, trkB, and NGF mRNAs and BDNF-immunoreactivity in rat forebrain after pilocarpine-induced status epilepticus. Exp Brain Res. 1996;107:331–47. doi: 10.1007/BF00230416. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Olson L. Decrease of neurotrophin-3 mRNA in adult rat hippocampus after pilocarpien seizures. Exp Neurol. 1995;136:199–204. doi: 10.1006/exnr.1995.1096. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–9. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wang S, Burnham WM, De Boni U, Wojtowicz JM. Kindling induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett. 1998;248:73–7. doi: 10.1016/s0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–6. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- Seress L, Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Res. 1987;405:169–74. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–6. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, et al. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Short- and long-term changes in CA1 network excitability after kainate treatment in rats. J Neurophysiol. 2001;85:1–9. doi: 10.1152/jn.2001.85.1.1. [DOI] [PubMed] [Google Scholar]
- Smith KL, Sollas AL, Goodman JH, Scharfman HE. Characteristics of dentate gyrus granule cells with basal dendrites after pilocarpine-induced status epilepticus and recurrent seizures. Epilepsia. 2000;41(7):77–8. [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Kandlhofer S. Expression of GABA(A) receptor subunits in the hippocampus of the rat after kainic acid-induced seizures. Epilepsy Res. 1998;32:129–39. doi: 10.1016/s0920-1211(98)00046-1. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Yan XX, Obenaus A, Lee EY, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–20. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Stenfors C, Srinivasan BR, Theodorsson E, Mathe AA. Electroconvulsive stimuli and brain peptides: effect of modification of seizure duration on neuropeptide Y, neurokinin A, substance P and neurotensin. Brain Res. 1992a;596:251–8. doi: 10.1016/0006-8993(92)91555-s. [DOI] [PubMed] [Google Scholar]
- Stenfors C, Theodoersson E, Mathe AA. Brain neuropeptides: changes by treatment with the convulsants pentylenetetrazole and bicuculline. Prog Neuropsychopharmacol Biol Psychiatry. 1992b;16:747–53. doi: 10.1016/0278-5846(92)90030-i. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Agarawal KS, Dure LS. Is cell death necessary for hippocampal mossy fiber sprouting? Epilepsy Res. 1997;27:67–76. doi: 10.1016/s0920-1211(97)01025-5. [DOI] [PubMed] [Google Scholar]
- Sutula TP. Secondary epileptogenesis, kindling, and intractable epilepsy: a reappraisal from the perspective of neural plasticity. Int Rev Neurobiol. 2001;45:355–86. doi: 10.1016/s0074-7742(01)45019-7. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–30. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Takeda A, Onodera H, Sugimoto A, Kogure K, Obinata M, Shibahara S. Coordinated expression of messenger RNAs for nerve growth factor, brain-derived neurotrophic factor and neurotropin-3 in the rat hippocampus following transient forebrain ischemia. Neuroscience. 1993;55:23–31. doi: 10.1016/0306-4522(93)90451-k. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1986;5:1016–22. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Morgan SL, Russell RN, Woodside BL. Synaptic plasticity and secondary epileptogenesis. Int Rev Neurobiol. 2001;45:253–67. doi: 10.1016/s0074-7742(01)45014-8. [DOI] [PubMed] [Google Scholar]
- Truettner J, Schmidt-Kastner R, Busto R, Alonso OF, Loor JY, Dietrich WD, et al. Expression of brain-derived neurotrophic factor, nerve growth factor, and heat shock protein HSP70 following fluid percussion brain injury in rats. J Neurotrauma. 1999;16:471–86. doi: 10.1089/neu.1999.16.471. [DOI] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Seighart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience. 1997;80:1019–32. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Van Der Zee CE, Rashid K, Le K, Moore KA, Stanisz J, Diamond J, et al. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995;15:5316–23. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, et al. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999a;19:5054–65. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Mule F, Ravizza T, Gobbi M, French-Mullen J. Plastic changes in neuropeptide Y receptor subtypes in experimental models of limbic seizures. Epilepsia. 2000;41(S6):115–21. doi: 10.1111/j.1528-1157.2000.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999b;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–15. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Wanscher B, Kragh J, Barry DI, Bolwig T, Zimmer J. Increased somatostatin and enkephalin-like immunoreactivity in hippocampus following hippocampal kindling. Neurosci Lett. 1990;118:3–36. doi: 10.1016/0304-3940(90)90242-2. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Liu H, Mazarati AM, Balwin RA, Shirasaka Y, Katsumori H, et al. Self-sustaining status epilepticus: a condition maintained by potentiation of glutamate receptors and by plastic changes in substance P and other peptide neuromodulators. Epilepsia. 2000;41(S6):S134–43. doi: 10.1111/j.1528-1157.2000.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Watkins CJ, Pei Q, Newberry NR. Differential effects of electroconvulsive shock on the glutamate receptor mRNAs for NR2A, NR2B, and mGluR5b. Mol Brain Res. 1998;61:108–13. doi: 10.1016/s0169-328x(98)00211-3. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Wooley CS, Robbins CA, Schwartzkroin PA. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. 2000;10:244–60. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- White JD, Gall CM. Differential regulation of neuropeptide and proto-oncogene mRNA content in the hippocampus following recurrent seizures. Brain Res. 1987;427:21–9. doi: 10.1016/0169-328x(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Bertram EH, Scharfman HE, Schwarcz RS. Neurodegeneration and chronic seizures after bilateral entorhinal cortex gamma-acetylenic GABA injections in rats. Epilepsia. 2000;41(7):19. [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci. 1996;16:4438–48. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CW, McGinty JF, Lee PH, Mitchell CL, Hong JS. A glutamate antagonist blocks perforant path stimulation-induced reduction of dynorphin peptide and prodynorphin mRNA levels in rat hippocampus. Brain Res. 1991;562:243–50. doi: 10.1016/0006-8993(91)90627-8. [DOI] [PubMed] [Google Scholar]
- Zhang N, Houser CR. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: a study of reorganized mossy fiber synapses. J Comp Neurol. 1999;405:472–90. [PubMed] [Google Scholar]


