Abstract
Purpose: The aim of this study was to evaluate the usefulness of CT guided adrenal biopsy in patients with equivocal MR chemical shift imaging findings.
Material and Methods: Fifty seven patients, 32 with history of malignancy and 25 without history of malignancy, 30 men and 27 women (33-82 years, mean age:58.8 years), with equivocal findings in chemical shift MR imaging, were subjected to CT guided percutaneous adrenal biopsy.
Results: From the 57 lesions that were sent for histopathological evaluation, 31 proved to be metastases (54.4%), 20 adenomas (35.1%), 3 cortical carcinomas (5.3%), 1 benign pheochromocytoma (1.8%) and 2 samples were non diagnostic(3.5%). In oncology patients metastases were found in 28/32 of the patients (87.5%) and adenomas in 3/32 (9.4%), while in patients without history of malignancy, metastases were found in 3/25 of the patients (12%) and adenomas in 17/25 (68%).
Conclusion: CT guided percutaneous adrenal biopsy is a safe and accurate method for a definite diagnosis of adrenal lesions. Since most adrenal lesions are benign, dedicated adrenal imaging is necessary for choosing which of them have to be further evaluated by biopsy. Chemical shift adrenal imaging alone seems to be a reliable method and can be used alternatively to CT enhancement washout technique for selecting which lesions are suspicious of malignancy and have to be investigated with biopsy, especially in cases where iodine contrast media is contraindicated.
Keywords: adrenal biopsy, MRI, chemical shift, CT, enhancement washout
Adrenal lesions are very commonly found during CT and MR examinations of the abdomen. Most of them represent benign adenomas, even in patients with a history of malignancy, with the exception of small cell lung cancer1–4. It has been reported that adrenal adenomas are discovered in 5% of abdominal CT examinations and in 2-9% of autopsy studies5,6. Before the application of advanced CT and MR techniques for the evaluation of these lesions, the only mean to specify the nature of an adrenal mass was US or CT guided percutaneous biopsy. However, advances in CT and MR imaging during the last 15 years, have changed the diagnostic approach of adrenal lesions reducing the number of adrenal biopsies7–13. Using a threshold of 0 or 10 HU in unenhanced CT imaging , the percentage enhancement wash-out technique and the in and opposed phase MR imaging, have changed the concept in characterizing adrenal masses. Nowadays, there are several imaging algorithms for the evaluation of adrenal lesions at different institutions. The goal of these algorithms is to distinguish only the suspicious lesions that have to be biopsied. This is essential in cancer patients, in whom an adrenal metastasis may be the only marker of metastatic disease and alters the stage of the disease and the treatment plan1,14.
The objective of this study was to evaluate the usefulness of CT guided adrenal biopsy in the evaluation of adrenal lesions with equivocal imaging characteristics in chemical shift MR imaging , that were discovered either during staging or following up of a known malignancy or coincidently during imaging of the abdomen for other reasons. With the term "equivocal", we mean lesions without evidence of lipids that may include true non-adenomas and lipid-poor adenomas.
Materials and Methods
From January 2000 to April 2005, 388 patients (M: 208, F: 180, 23-91 years old, mean age: 54.6), with recently discovered adrenal mass in CT or MR imaging, were enrolled in the study. All lesions were further evaluated with MRI and chemical shift imaging. Patients with advanced metastatic disease, including adrenal glands, patients with lesions with typical CT findings of benignity (myelolipoma or cyst) and 2 patients with huge masses, typical of malignancy(finally proven adrenal carcinomas) were excluded from the study. Three hundred twenty nine of them (84.8%) were not subjected to biopsy because MR imaging findings were suggestive of benignity (signal loss less than 20% during opposed phased sequences), while in 2 patient (0.5%), laboratory tests before biopsy were suggestive of pheochromocytoma, and adrenal biopsy wasn't performed because of the risk of hemodynamic instability during biopsy15.
Finally, 57 patients (14.7%, M:30, F:27, 33-82 years old, mean age: 58.8) , with equivocal findings in chemical shift MR imaging, were subjected to CT guided percutaneous adrenal biopsy. Before biopsy, coagulative mechanism and serum and urine catecholamine levels were controlled. 32 of them were monitored for a known malignancy (mean age: 63.1 years), while the rest 25 were subjected to abdominal imaging for other reasons (mean age: 53.2 years). As equivocal lesions were defined lesions with signal loss less than 20% during opposed phased sequences. In oncology patients, inhomogenous lesions with areas devoid of lipids (2 cases), even when the rest of the lesion had imaging findings typical of adenoma in chemical shift MR imaging, were considered suspicious of collision tumors (metastasis to adrenal adenoma), and were subjected to biopsy. In these cases, the nonlipid region in the lipid containing mass was targeted during biopsy. In non oncology patients, such areas were considered as central degeneration of an adenoma, and follow up was proposed.
Patients with findings typical of benignity in chemical shift MR imaging (329 patinets-84.8%) were not subjected to biopsy and were advised to re-evaluate the lesion after a specified time interval, either with CT or with MRI (Figure 1).
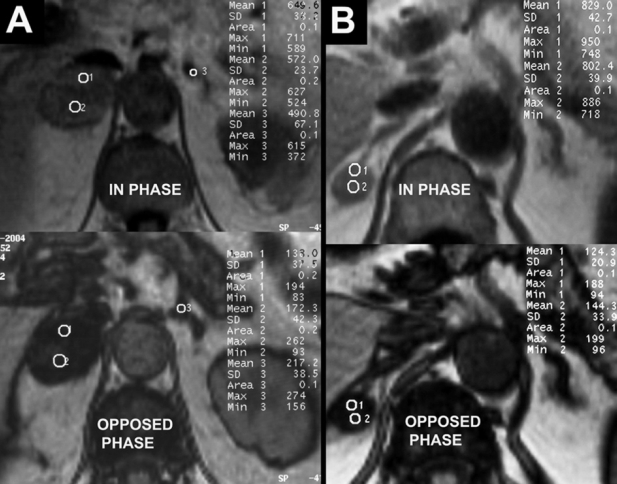
Figure 1. A) Bilateral adrenal lesions in a 64 year old female subjected in abdominal CT for cholecystidis. B) Right adrenal lesion in a 65 year old woman with history of large intestine cancer. MR evaluation of the lesions in both cases with in phase and opposed phase images is characteristic of bilateral adenomas, with mean signal loss at opposed phase sequences more than 40%. In 1A, the central lipid poor strap represents central degeneration of the adenoma. In oncology patients, such lesions were subjected to biopsy because of the risk of collision tumor. Follow up of the lesions in both cases, showed stability of the findings.
CT scans were obtained with a Picker PQ 5000 CT scanner device with 3 mm slice thickness, pitch 1-1.5, reconstruction interval 3 mm, scan time 1sec, 120 kVp, 200 mA, with a FOV ranging from 140-160 mm, depending on the patients size. Images were obtained before and after contrast agent administration. A bolus injection of 100 ml of non-ionic contrast medium (Ultravist 300, Shering, Germany) was given.
MR scans were obtained with a Siemens 1 T scanner (Siemens Expert Plus). Before administration of contrast agent, Haste T2WI and Flash T1WI images were obtained, with a slice thickness of 8 mm, FOV 340-400 mm, depending on patients size and a matrix of 256X192. In phase (TR=150ms, TE=7 ms) and opposed phase (TR=150 ms, TE=3.6 ms) images were also obtained before contrast administration with a dual GRE sequence, with a slice thickness of 7 mm, FOV 340-400 mm, depending on patient's size and a matrix of 256X144. After the administration of contrast agent (15-20 ml, Magnevist, Shering, Germany), Flash T1WI images in axial and coronal plane were obtained, using the same parameters as before the administration of contrast agent.
CT guided adrenal biopsies were performed using 18-22 gauge aspiration needles (29 patients) or 16-20 gauge cutting needles with the coaxial technique (28 patients). All biopsies were performed by one specialised radiologist in adrenal biopsies, with 18 years of experience (I.T). Posterior paraspinal approach was used in 48 patients and lateral decubitus position in 9 patients, with the side to be biopsied dependent, in order to reduce the risk of pneumothorax. Triangulation methods weren not used. Local anaesthesia with lidocaine was used in all patients and 2-4 biopsy samples were obtained for every patient. Samples were sent to the pathologist the same day. All patients were informed about the purpose of the study and gave their written consent before CT guided biopsy.
The size of the lesion was expressed at its maximum axial dimension.
Biopsy results were classified in 4 groups: 1) metastases, 2) adenoma, including samples with benign cortical like tissue, 3) other, including cortical carcinoma and pheochromocytoma and 4) non diagnostic.
Statistical analysis was performed by the Statistical Package for Social Sciences (SPSS) v.12.0. ANOVA, T-test, and x2 test were used, and differences were considered significant when p<0.05.
Results
Adrenal biopsies under CT guidance were performed in 57 patients (mean age: 58.8 years), 30 men (mean age: 58.17 years) and 27 women (mean age: 59.5 years). In 28 patients the mass was in the right adrenal gland (49.1%) , in 26 in the left adrenal gland (45.6%) whereas 3 patients had bilateral lesions (5.3%). Two of the patients (3.5 %) developed a small hematoma immediately after biopsy, which resolved spontaneously and 1 patient (1.8%) developed self-limited asymptomatic pneumothorax. No other major complications were observed.
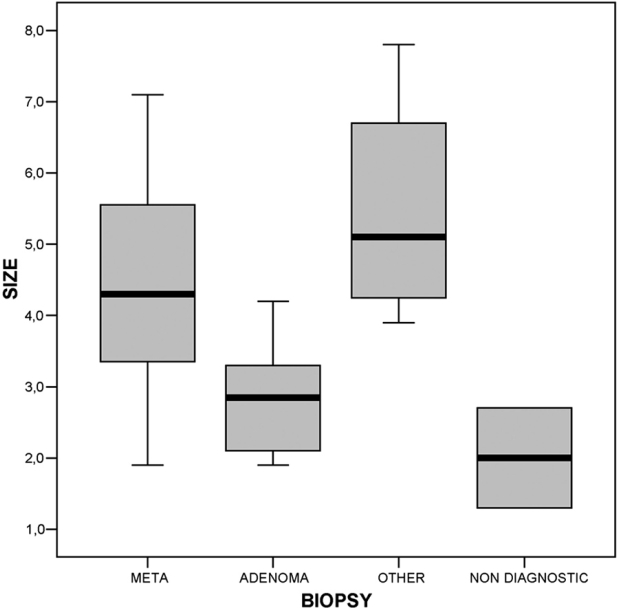
The mean size of the lesions was 3.9 cm (1.3 -7.8 cm). In patients with history of malignancy the mean size of the lesions was 4.3 cm, while in patients without history of malignancy the mean size was 3.4 cm, differing significantly (t=2.287, df=55, p<0.05). Metastases ranged from 1.9 cm to 7.1 cm with mean size 4.5cm, while adenomas ranged from 1.9 cm to 4.2 cm with mean size 2.9cm, differing significantly (t=5.11, df=49, p<0.01) (Figure 2).
Figure 2. Boxplot of the lesions size. There is a statistically significant difference between lesions size (F=12,4, df=3, p<0,01).
Of the 57 lesions biopsied, 31 lesions proved to be adrenal metastases (54.4%), 20 lesions adenomas (35.1%), 3 lesions adrenal carcinomas (5.3%), 1 lesion benign pheochromocytoma(1.8%) and 2 tissue sambles were non diagnostic (3.5%) (Figures 3,4,5). Metastases were solitary in 21 cases (67.7%) and among other metastases in 10 cases (32.3%).
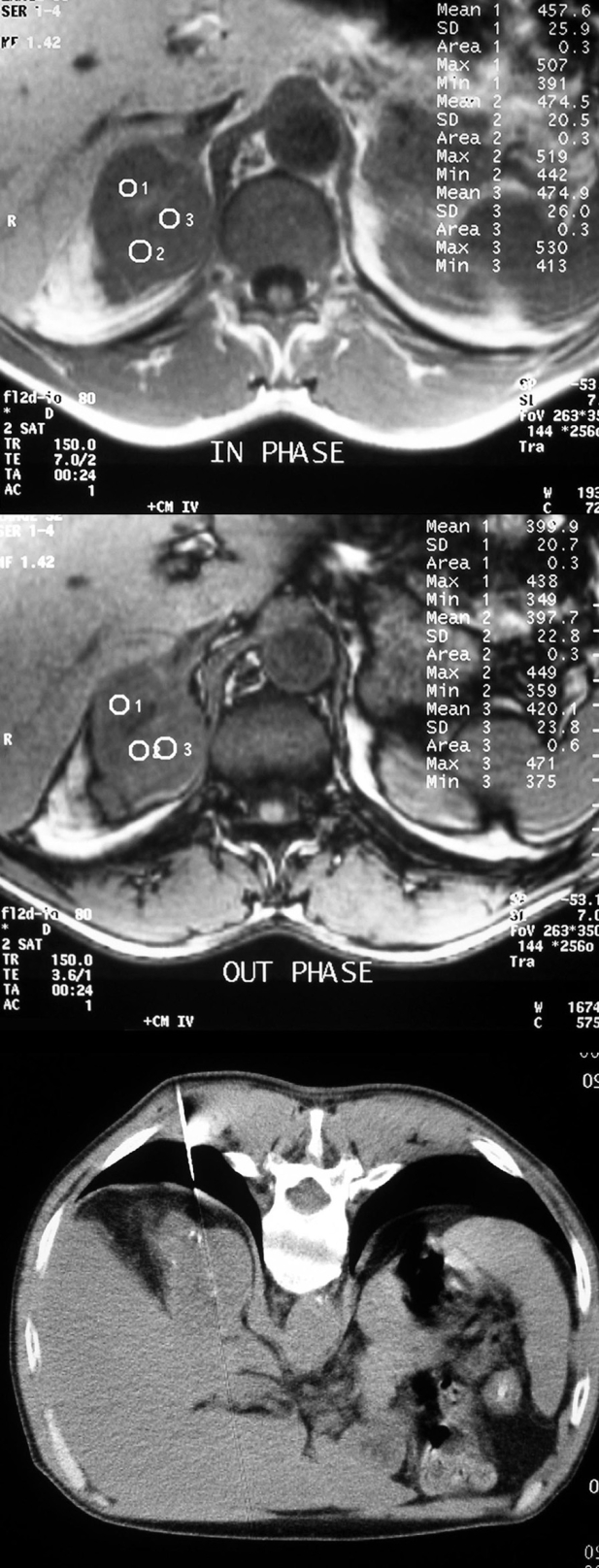
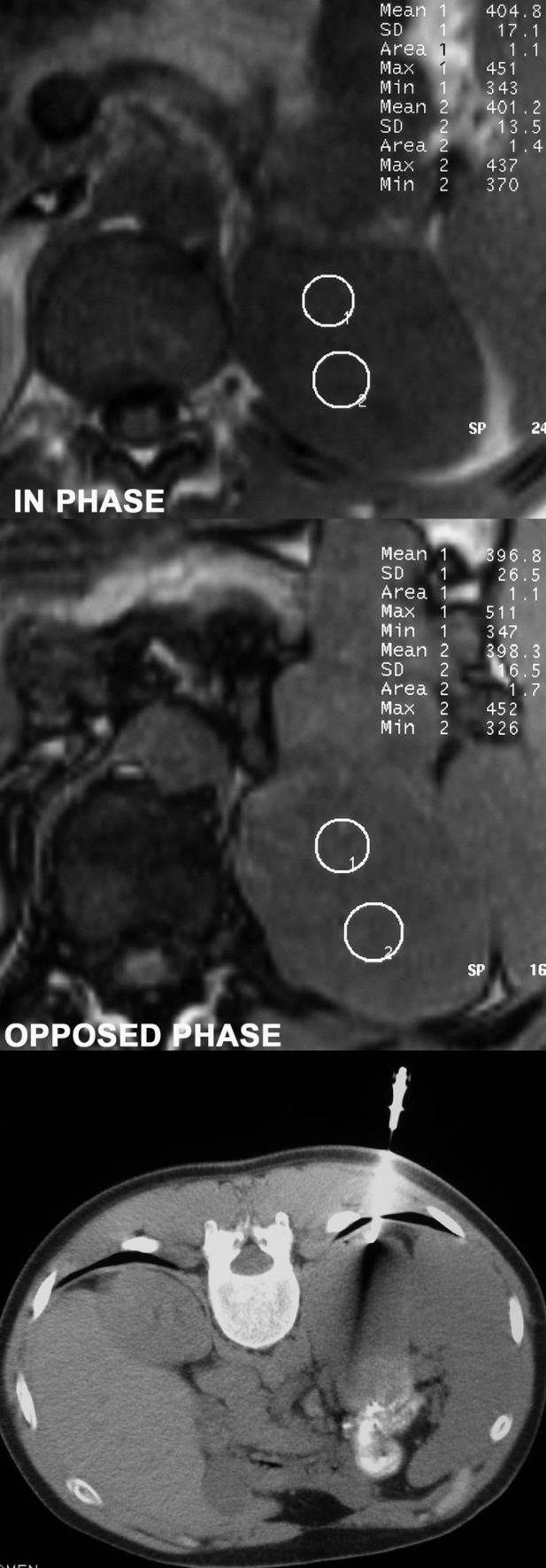
Figure 3. Adrenal lesion of a man discovered during staging of lung cancer. Right decubitus position was difficult in this non co-operative patient and posterior paraspinal approach was used. Chemical shift MR findings were highly suspicious of a non adenomatous lesion. Biopsy result was metastatic adenocarcinoma.
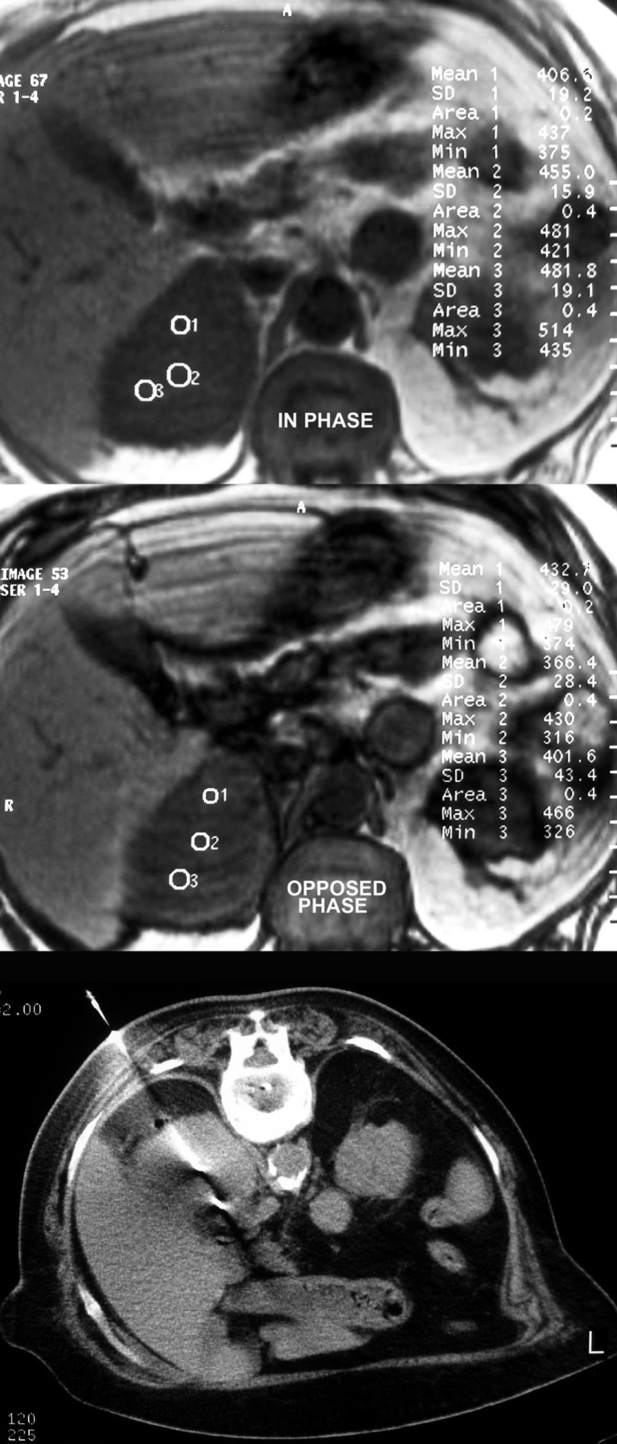
Figure 4. Right adrenal lesion of a woman with history of low grade NHL. In visual assessment, lesion seems not to contain lipids. Quantitative chemical shift MR findings are suggestive of malignancy, since the mean signal loss is lower than 20%. Biopsy confirmed the infiltration of the adrenal gland by the lymphoma.
Figure 5. Left adrenal lesion incidentally discovered in a man subjected in abdominal CT for lumbago. Chemical shift MR findings were highly suspicious of a non adenomatous lesion. Biopsy result was nonfunctioning benign pheochromocytoma.
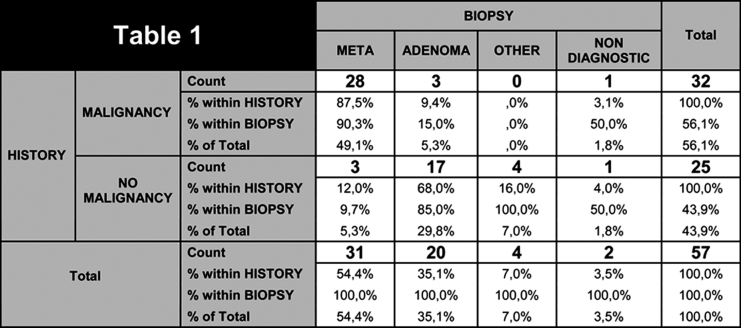
In the 3 cases of cortical carcinoma and the case of pheochromocytoma, diagnosis was confirmed after surgical removal. In patients with history of malignancy, biopsy revealed metastasis in 28/32 (87.5%), adenoma in 3/32(9.4%) and in 1 case (3.1%) tissue sample was non diagnostic. In patients without history of malignancy, 3/25 (12%) of the lesions proved to be unsuspected metastases from unknown origin, 17/25 (68%) adenoma, 3/25 (12%) adrenal carcinoma, 1/25(4%) pheochromocytoma and in 1 case (4%) tissue sample was non diagnostic. Table statistical analysis showed correlation between biopsy result and patient history (x2= 33.609, df=3, p<0.01) (Table 1).
Table 1. Biopsy results in oncology and non oncology patients.
The primary neoplasm in patients with adrenal metastases was lung carcinoma in 18/31(58.1) of the cases, breast carcinoma in 4/31(12.9%), lymphoma in 2/31(6.5), renal carcinoma in 2/31(6.5), melanoma in 1/31(3.2%), pancreatic carcinoma in 1/31(3.2%), esophageal carcinoma in 1/31(3.2%), large intestinal carcinoma in 1/31(3.2%) and osteosarcoma in 1/ 31(3.2%). In the cases with unknown primary lesion, the primary lesion finally proved to be 1 lung carcinoma, 1 breast carcinoma and 1 melanoma.
In the 2 lesions with non diagnostic biopsy results, core biopsy tissue sample contained blood clots, adipose tissue and connective tissue. Follow up of these 2 patients, revealed stability of the lesion of the patient without history of malignancy while in the other patient with history of lung cancer, the lesion increased from 1.3 cm to 3.1 cm within 6 months, and a second biopsy confirmed the diagnosis of metastasis.
Six to eighteen months follow up in patients without malignancy and lesions with chemical shift MR findings typical of benignity that did not require biopsy, either with CT or with MRI, did not reveal suspicious enlargement of any of the lesions. The 2 patients with laboratory evidences of pheochromocytoma were operated, and diagnosis was confirmed after biopsy.
Discussion
Adrenal glands, weighing about 5 gr. each, are one of the commonest organs involved in metastatic disease, especially in lung cancer. Moreover, adrenal adenomas are a very common incidental finding during abdominal imaging, comprising the majority of adrenal lesions, even in patients with a history of malignancy, with the exception of small cell lung cancer1–4,16,17. Consequently, the definite diagnosis of an adrenal lesion is of major importance, especially in oncology patients, where it may be the only marker of metastatic disease with significant impact in management of these patients18.
Adrenal biopsy is nowadays considered as a safe procedure for establishing the final diagnosis of an adrenal mass, but the risk of a complication, the psychological impact to the patient and the high probability of an adrenal lesion being benign, have upgraded the significance of dedicated adrenal imaging with CT and MR techniques, in order to reduce as much as possible the needless adrenal biopsies7–13. Furthermore, recent advances in PET and PET/CT are continuously altering the role of imaging in characterizing adrenal lesions, due to the increased glycolytic activity and 18F-FDG uptake by the metastatic adrenal lesions19–21.
Although the percentage of adenomas in our study was lower than previously published studies4,22–25, it would have been much lower if we had used more strict chemical shift MR criteria (higher threshold of signal loss in opposed phase sequences) and combination with CT enhancement washout findings in order to exclude lesions with adenoma like imaging findings, especially in patients without a history of malignancy. The risk of missing a malignant adrenal lesion enforced us to be more aggressive in these patients.
In our study, 6/25 (24%) of the incidental adrenal lesions with equivocal chemical shift MR findings in patients with no history of malignancy, finally proved to be malignant (3 metastases and 3 cortical carcinomas). The risk of malignancy in incidental adrenal masses in our study was higher than previously reported rates22.
In patients with a history of malignancy, 28/32 (87.5%) of the lesions that were submitted to biopsy, proved to be metastases. Previous published studies however, reported that the most frequent adrenal lesions, even in oncology patients, are benign adenomas. We believe that dedicated MR imaging prevented needless biopsies in this group of patients. Positive predictive value of an adrenal biopsy which demonstrates malignancy in a patient with known malignancy is believed to be 100% 4 .We consider that all of the biopsies that demonstrated metastases in our oncology patients were true positive. Negative predictive value of negative biopsies in oncology patients, is also mentioned to be 100%18. Follow up of our patients with negative biopsy (3 adenomas and 1 non diagnostic), revealed that only the patient with the non diagnostic sample had adrenal metastasis that biopsy failed to discover, while the 3 cases of adenoma remained stable. Thus, negative predictive of biopsy in our group of oncology patients was 75% (3/4).
Non diagnostic biopsies were only 2/57(3.5%), much lower than previously reported rates4,15,25. We consider that the relatively small size of the lesions (1.3 and 2.7 cm) was the cause of non diagnostic tissue samples.
Despite of the significant difference between the mean size of the metastatic adrenal lesions (4.49 cm) and adenomas (2.8cm), (t=5.114, df=49, p<0.01), there is a wide overlap that prevents the definition of the nature of the lesion according to its size (Figure 2). However, an adrenal lesion with a maximum diameter bigger than 4 cm, especially in cancer patients, is highly suspicious of a metastasis. Similar results have been already published7,12,22,26.
In the case of pheochromocytoma, the patient had normal urine catecholamine levels before biopsy, but fortunately, biopsy didn't cause blood-pressure alteration. It is mentioned that 14% of patients with pheochromocytoma may be asymptomatic and have normal biochemical studies27.
The major limitation of our study is that CT enhancement washout technique was exceptionally used since it is not included in our institution default imaging algorithm when encountering with adrenal lesions. We believe that our results would have been stronger if we have compared CT washout findings with chemical shift MR findings and some of the biopsies in adrenal adenomas might have been avoided.
In conclusion, CT guided percutaneous adrenal biopsy is a safe and specific technique and is probably the method of choice for characterizing adrenal lesions with doubtful MR imaging findings. Chemical shift adrenal imaging alone seems to be a reliable method for selecting which lesions are suspicious of malignancy and have to be investigated with biopsy.
This seems to be especially important in cases where iodine contrast media is contraindicated (allergy, renal failure), where an alternative to CT enhancement washout technique has to be established in order to characterize adrenal lesions.
References
- 1.Remer EM, Obuchowski N, Ellis JD, Rice TW, Adelstein DJ, Baker ME. Adrenal mass evaluation in patients with lung carcinoma: a cost-effectiveness analysis. AJR. 2000;174:1033–1039. doi: 10.2214/ajr.174.4.1741033. [DOI] [PubMed] [Google Scholar]
- 2.Korobkin M, Francis IR, Kloos RT, Dunnick NR. The incidental adrenal mass. Radiol Clin North Am. 1996;34:1037–1054. [PubMed] [Google Scholar]
- 3.Oliver TW, Jr, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984;153:217–218. doi: 10.1148/radiology.153.1.6473783. [DOI] [PubMed] [Google Scholar]
- 4.Silverman SG, Mueller PR, Pinkney LP, Koenker RM, Seltzer SE. Predictive value of image-guided adrenal biopsy: analysis of results of 101 biopsies. Radiology. 1993;187:715–718. doi: 10.1148/radiology.187.3.8497619. [DOI] [PubMed] [Google Scholar]
- 5.Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110:1014–1021. [PubMed] [Google Scholar]
- 6.Wu HH, Cramer HM, Kho J, Elsheikh TM. Fine needle aspiration cytology of benign adrenal cortical nodules. A comparison of cytologic findings with those of primary and metastatic adrenal malignancies. Acta Cytol. 1998;42:1352–1358. doi: 10.1159/000332167. [DOI] [PubMed] [Google Scholar]
- 7.Lee MJ, Hahn PF, Papanicolaou N, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology. 1991;179:415–418. doi: 10.1148/radiology.179.2.2014283. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell DG, Crovello M, Matteucci T, Petersen RO, Miettinen MM. Benign adrenocortical masses: diagnosis with chemical shift MR imaging. Radiology. 1992;185:345–351. doi: 10.1148/radiology.185.2.1410337. [DOI] [PubMed] [Google Scholar]
- 9.Korobkin M, Lombardi TJ, Aisen AM, et al. Characterization of adrenal masses with chemical shift and gadolinium-enhanced MR imaging. Radiology. 1995;197:411–418. doi: 10.1148/radiology.197.2.7480685. [DOI] [PubMed] [Google Scholar]
- 10.Korobkin M, Brodeur FJ, Yutzy GG, et al. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR. 1996;166:531–536. doi: 10.2214/ajr.166.3.8623622. [DOI] [PubMed] [Google Scholar]
- 11.Tsushima Y, Ishizaka H, Matsumoto M. Adrenal masses: differentiation with chemical shift, fast low-angle shot MR imaging. Radiology. 1993;186:705–709. doi: 10.1148/radiology.186.3.8430178. [DOI] [PubMed] [Google Scholar]
- 12.van Erkel AR, van Gils APG, Lequin M, Kruitwagen C, Bloem JL, Falke THM. CT and MR distinction of adenomas and nonadenomas of the adrenal glands. J Comput Assist Tomogr. 1994;18:432–438. doi: 10.1097/00004728-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Mayo-Smith WW, Lee MJ, McNicholas MM, Hahn PF, Boland GW, Saini S. Characterization of adrenal masses (<5 cm) by use of chemical shift MR imaging: observer performance versus quantitative measures. AJR. 1995;165:91–95. doi: 10.2214/ajr.165.1.7785642. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz LH, Panicek DM, Doyle MV, et al. Comparison of two algorithms and their associated charges when evaluating adrenal masses in patients with malignancies. AJR. 1997;168:1575–1578. doi: 10.2214/ajr.168.6.9168729. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen SD, Nghiem HV, Korobkin M, Caoili EM, Higgins EJ. Changing role of imaging-guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR. 2004;182:1033–1037. doi: 10.2214/ajr.182.4.1821033. [DOI] [PubMed] [Google Scholar]
- 16.Candel AG, Gattuso P, Reyes CV, Prinz RA, Castelli MJ. Fine-needle aspiration biopsy of adrenal masses in patients with extraadrenal malignancy. Surgery. 1993;114:1132–1137. [PubMed] [Google Scholar]
- 17.Falke THM. Imaging of adrenal metastases. Curr Opin Radiol. 1991;3:681–686. [PubMed] [Google Scholar]
- 18.Harisinghani MG, Maher MM, Hahn PF, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol. 2002;57:898–901. doi: 10.1053/crad.2002.1054. [DOI] [PubMed] [Google Scholar]
- 19.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the Evaluation of Adrenal Masses. J Nucl Med. 2006;47:32–37. [PubMed] [Google Scholar]
- 20.Kumar R, Xiu Y, Yu JQ, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–2062. [PubMed] [Google Scholar]
- 21.Minn H, Salonen A, Friberg J, et al. Imaging of adrenal incidentalomas with PET using (11)C-metomidate and (18)F-FDG. J Nucl Med. 2004;45:972–979. [PubMed] [Google Scholar]
- 22.Lumachi F, Borsato S, Brandes AA, et al. Fine-needle aspiration cytology of adrenal masses in noncancer patients: clinicoradiologic and histologic correlations in functioning and non-functioning tumors. Cancer. 2001;93:323–329. doi: 10.1002/cncr.9047. [DOI] [PubMed] [Google Scholar]
- 23.Welch TJ, Sheedy PF, Stephens DH, Johnson CM, Swensen SJ. Percutaneous adrenal biopsy: review of a 10-year experience. Radiology. 1994;193:341–344. doi: 10.1148/radiology.193.2.7972740. [DOI] [PubMed] [Google Scholar]
- 24.Mody MK, Kazerooni EA, Korobkin M. Percutaneous CT-guided biopsy of adrenal masses: immediate and delayed complications. J Comput Assist Tomogr. 1995;19:434–439. doi: 10.1097/00004728-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Bernardino ME, Walther MM, Phillips VM, et al. CT-guided adrenal biopsy: accuracy, safety, and indications. Am J Roentgenol. 1985;144:67–69. doi: 10.2214/ajr.144.1.67. [DOI] [PubMed] [Google Scholar]
- 26.Khafagi FA, Gross MD, Shapiro B, Glazer GM, Francis I, Thompson NW. Clinical significance of the large adrenal masses. Br J Surg. 1991;78:828–833. doi: 10.1002/bjs.1800780720. [DOI] [PubMed] [Google Scholar]
- 27.Casola G, Nicolet V, VanSonnenberg E, et al. Unsuspected pheochromocytoma: risk of blood-pressure alterations during percutaneous adrenal biopsy. Radiology. 1986;159:733–735. doi: 10.1148/radiology.159.3.3517958. [DOI] [PubMed] [Google Scholar]