Abstract
To guarantee specific tRNA and amino acid pairing, several aminoacyl-tRNA synthetases correct aminoacylation errors by deacylating or “editing” misaminoacylated tRNA. A previously developed variant of Escherichia coli tyrosyl-tRNA synthetase (iodoTyrRS) esterifies or “charges” tRNATyr with a nonnatural amino acid, 3-iodo-l-tyrosine, and with l-tyrosine less efficiently. In the present study, the editing domain of phenylalanyl-tRNA synthetase (PheRS) was transplanted into iodoTyrRS to edit tyrosyl-tRNATyr and thereby improve the overall specificity for 3-iodo-l-tyrosine. The β-subunit fragments of the PheRSs from Pyrococcus horikoshii and two bacteria were tested for editing activity. The isolated B3/4 editing domain of the archaeal PheRS, which was exogenously added to the tyrosylation reaction with iodoTyrRS, efficiently reduced the production of tyrosyl-tRNATyr. In addition, the transplantation of this domain into iodoTyrRS at the N terminus prevented tyrosyl-tRNATyr production most strongly among the tested fragments. We next transplanted this archaeal B3/4 editing domain into iodoTyrRS at several internal positions. Transplantation into the connective polypeptide in the Rossmann-fold domain generated a variant that efficiently charges tRNATyr with 3-iodo-l-tyrosine, but hardly produces tyrosyl-tRNATyr. This variant, iodoTyrRS-ed, was used, together with an amber suppressor derived from tRNATyr, in a wheat germ cell-free translation system and incorporated 3-iodo-l-tyrosine, but not l-tyrosine, in response to the amber codon. Thus, the editing-domain transplantation achieved unambiguous pairing between the tRNA and the nonnatural amino acid in an expanded genetic code.
Keywords: aminoacyl-tRNA synthetase, nonnatural amino acid, expanded genetic code, iodotyrosine, protein engineering
Aminoacyl-tRNA synthetases (aaRSs) esterify an amino acid to the 3′ end of tRNA (1). The 20 aaRSs are divided into two classes, each comprising 10 aaRSs, on the basis of characteristic sequence motifs (2). Class I aaRSs have the Rossmann-fold aminoacylation domain, whereas class II aaRSs have the aminoacylation site built around antiparallel β-sheets. To guarantee correct decoding of the genetic code, the aaRS must esterify or “charge” only the cognate amino acid to the tRNA. However, because some amino acids have similar sizes or chemical properties, several aaRSs fail to distinguish among these amino acids at the aminoacylation site, and thus mischarge noncognate amino acids to tRNA (3).
To correct such errors, these aaRSs hydrolyze the ester bond formed between the tRNA and the noncognate amino acid, or “edit” misaminoacylated tRNA by using an extra domain for editing (4–6). The class I aaRSs with editing functions such as the isoleucyl-, valyl-, and leucyl-tRNA synthetases (IleRS, ValRS, and LeuRS, respectively), belong to subclass Ia, and a connective-polypeptide 1 (CP1) domain, which is inserted in the middle of the Rossmann-fold domain, catalyzes the editing reaction (7–10). In contrast, the editing domains of the class II aaRSs, such as the phenyl-, alanyl-, threonyl-, and prolyl-tRNA synthetases (PheRS, AlaRS, ThrRS, and ProRS, respectively), have diverse structures and are inserted at different sites in the enzymes (11–15).
The repertoire of genetically encoded amino acids has recently been expanded to include nonnatural amino acids in Escherichia coli, yeast, and mammalian cells (16–18). Nonnatural amino acids are specifically coupled with their “carrier” tRNAs by engineered aaRS molecules. A variety of such aaRSs are derived from the tyrosyl-tRNA synthetases (TyrRS) from E. coli (17–19) and Methanocaldococcus jannaschii (20). Several useful variants of tryptophanyl-tRNA synthetase (21), lysyl-tRNA synthetase (22), PheRS (23), and LeuRS (22, 24) have also been produced. These engineered aaRSs must discriminate against the formerly cognate amino acid and efficiently recognize its nonnatural analogue. We previously developed a variant of E. coli TyrRS for genetic encoding of 3-iodo-l-tyrosine, which is designated hereafter as iodoTyrRS (19). The only difference between 3-iodo-l-tyrosine and l-tyrosine is the bulky iodine atom at the phenolic ring. Our x-ray crystallographic study has revealed that the reshaped pocket of iodoTyrRS has sufficient space to accommodate the iodine atom [supporting information (SI) Fig. S1A] (25). Therefore, iodoTyrRS efficiently and specifically produces iodotyrosyl-tRNATyr in the presence of both 3-iodo-l-tyrosine and tyrosine. Nevertheless, iodoTyrRS still produces tyrosyl-tRNATyr in the absence of 3-iodo-l-tyrosine (19).
In this context, in the recently reported engineering of LeuRS, the reshaping of the aminoacylation site was not sufficient to achieve the specificity for dansylalanine; therefore, the editing domain was also modified to hydrolyze leucyl-tRNA (24). This case suggested that the editing mechanism could be useful for improving the specificity for nonnatural amino acids. However, two-thirds of the aaRS species, including TyrRS, have no editing domain (26). Thus, we planned to use the editing domain of PheRS, which can hydrolyze tyrosyl-tRNAPhe (11). The PheRS editing domain does not appear to hydrolyze iodotyrosyl-tRNA, because of the limited size of the editing pocket for the bulky iodine atom (Fig. S1B) (27, 28). Therefore, the transplantation of the PheRS editing domain would enable iodoTyrRS to edit tyrosyl-tRNATyr, thus producing no tyrosyl-tRNATyr, even in the absence of 3-iodo-l-tyrosine.
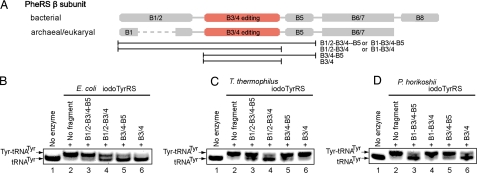
PheRS belongs to class IIc and is a tetrameric enzyme consisting of two αβ heterodimers. The B3/4 domain of the β-subunit (Fig. 1A) catalyzes the editing (11, 29). The domain architecture and the amino acid sequence of PheRS are totally different from those of TyrRS, which belongs to class Ic and is homodimeric. The TyrRS subunit has the Rossmann-fold domain, with the CP domain inserted in the middle (30). The CP domain is inserted at a similar position to that in the CP1 editing domain in the monomeric class Ia IleRS, ValRS, and LeuRS, but it has no editing activity and its fold is unrelated to that of the CP1 editing domain. This CP1 editing-domain fold is totally different from that of the PheRS B3/4 domain (11). Furthermore, the tRNA-binding manner of the class Ic TyrRS is completely distinct from those of class Ia and class IIc (30). Therefore, there are no hints on how the PheRS B3/4 domain should be transplanted into iodoTyrRS.
Fig. 1.
Isolation of editing domains. (A) The domains of the bacterial and archaeal/eukaryotic PheRS β-subunits and the analyzed fragments. (B–D) Acidic PAGE analysis of tyrosyl-tRNATyr formation in the presence of iodoTyrRS and the E. coli (B), T. thermophilus (C), and P. horikoshii (D) fragments. The bands corresponding to tyrosyl-tRNATyr and uncharged tRNATyr are marked. The reactions for lanes 2–6 contained iodoTyrRS, whereas those for lanes 3–6 additionally contained the PheRS fragment indicated above each lane. Neither iodoTyrRS nor any fragment was included in the reaction for lanes 1.
In the present study, we planned to achieve “exclusive” specificity for 3-iodo-l-tyrosine. First, we examined whether the B3/4 editing domain fragments from three different organisms retain the activity to hydrolyze tyrosyl-tRNA. The best editing domain fragment was then transplanted into several different positions of iodoTyrRS, to find an appropriate position. Thus, the editing domain transplantation drastically improved the overall specificity of iodoTyrRS for 3-iodo-l-tyrosine: the new variant never produces tyrosyl-tRNA. The engineered iodoTyrRS exclusively incorporated iodoTyrRS into the specified site of a protein in the wheat germ cell-free system.
Results
Editing Activity of the Isolated B3/4 Editing Domain from PheRS.
First, we searched for a minimal fragment that retains the editing activity of the PheRS β-subunit. The bacterial PheRSs from E. coli and Thermus thermophilus and the archaeal/eukaryal PheRS from Pyrococcus horikoshii were selected as the donors. T. thermophilus PheRS has eight structural domains, B1–B8, in the β-subunit, where B2, B4, and B7 are inserted into B1, B3, and B6, respectively (29) (Fig. 1A). On the other hand, the archaeal/eukaryal PheRS β-subunit lacks B2 and B8, and its editing domain, B3/4, differs from those of the bacterial B3/4 domains with respect to the sequence and catalytic residues (28). The B3/4 domain is clustered with the B1/2 and B5 domains, whereas the B6/7 and B8 domains are structurally independent (28, 29). Therefore, the B1/2–B3/4–B5, B1/2–B3/4, B3/4–B5, and B3/4 fragments were constructed from the E. coli and T. thermophilus PheRSs, and the B1–B3/4–B5, B1–B3/4, B3/4–B5, and B3/4 fragments were constructed from the P. horikoshii PheRS (Fig. 1A). Each of the overproduced and purified fragments was added to the aminoacylation reaction with E. coli tRNATyr, iodoTyrRS, and l-tyrosine, and the production of tyrosyl-tRNATyr was analyzed by acidic PAGE (31).
In the absence of the PheRS fragment, the tRNATyr molecules were fully charged with l-tyrosine (Fig. 1 B–D, lanes 2). The yield of tyrosyl-tRNATyr was reduced by the addition of the E. coli B1/2–B3/4, B3/4–B5, and B3/4 (Fig. 1B, lanes 4–6), T. thermophilus B1/2–B3/4 (Fig. 1C, lane 4), and P. horikoshii B1–B3/4–B5 and B3/4 fragments (Fig. 1D, lanes 3 and 6). Thus, the isolated B3/4 editing domains from the E. coli and P. horikoshii PheRSs reduced the yield of tyrosyl-tRNATyr, whereas the B3/B4 domain from T. thermophilus required the extension to the N terminus for the effect. Furthermore, these fragments worked on the noncognate tRNATyr. The observed decrease in the yield of tyrosyl-tRNATyr is consistent with the hydrolysis activity of each isolated fragment.
Editing Activity of the Editing Domains Fused to the N Terminus of IodoTyrRS.
E. coli B3/4, P. horikoshii B3/4, and T. thermophilus B1/2–B3/4 were each fused to the N terminus of iodoTyrRS to generate N-Eed-IYRS, N-Ped-IYRS, and N-Ted-IYRS, respectively (Fig. 2A). N-Eed-IYRS and N-Ted-IYRS produced tyrosyl-tRNATyr less efficiently than iodoTyrRS, and N-Ped-IYRS hardly produced tyrosyl-tRNATyr (Fig. 2B, filled symbols). The replacement of the conserved Ala residue by Trp in the editing site reportedly abolishes the editing activities of T. thermophilus and P. horikoshii PheRSs, by plugging the pocket (11, 28). The Ala-to-Trp mutation was introduced into the fusion variants to create N-Eedmu-IYRS, N-Tedmu-IYRS, and N-Pedmu-IYRS. These editing-deficient mutants produced tyrosyl-tRNATyr as efficiently as iodoTyrRS (Fig. 2B, open symbols). Consequently, the N-terminally fused editing domains hardly affect the aminoacylation active site of iodoTyrRS. In addition, the finding that a specific mutation restored the yield of tyrosyl-tRNATyr suggested that the decreased yield was caused by the ability of the fused domains to hydrolyze tyrosyl-tRNATyr.
Fig. 2.
Transplantation of editing domains into iodoTyrRS. (A) The domains of iodoTyrRS and its variants. Numbers indicate the first residue of each domain. A filled symbol represents each enzyme, and the same symbol is used to indicate the data for the enzyme in B–G. (B and D) Time courses of tyrosyl-tRNATyr formation in the presence of iodoTyrRS, the indicated fusion/insertion variants, the indicated editing-deficient fusion variants, and no enzyme. (C and E) Formation of tyrosyl-tRNATyr (lanes Y) and iodotyrosyl-tRNATyr (lanes IY) in the presence of the indicated enzymes. The bands corresponding to aminoacyl-tRNATyr and uncharged tRNATyr are marked. (F) Time courses of tyrosyl-tRNATyr hydrolysis in the presence of N-Ped-IYRS, CP-Ped-IYRS, and no enzyme. (G) The translation of GST(Am) in the wheat germ cell-free system. The indicated enzymes were added to the reactions, together with the suppressor tRNA, in the absence (−IY) and presence (IY) of 3-iodo-l-tyrosine. All of the reactions contained l-tyrosine. The produced full-length GST was detected by Western blotting.
The orientation of the B3/4 domain relative to the B1 and B5 domains in P. horikoshii PheRS reportedly differs from that in T. thermophilus PheRS (28) and probably from that in E. coli PheRS as well. The location of the C terminus in the archaeal B3/4 domain is quite different from those in the bacterial B3/4 domains, as the last strand in the β-sheet is oppositely oriented. Accordingly, the orientation of the fused B3/4 domain relative to the aminoacylation domain of iodoTyrRS should differ between the archaeal B3/4 domain and the bacterial B3/4 domains. The higher editing activity of N-Ped-IYRS may reflect the better accessibility of the editing active site to the aminoacylated end of the tRNA.
Aminoacyl-tRNATyr production by N-Eed-IYRS, N-Ted-IYRS, and N-Ped-IYRS was compared, using 3-iodo-l-tyrosine and l-tyrosine, by acidic PAGE. These variants produced only iodotyrosyl-tRNATyr, but not tyrosyl-tRNATyr (Fig. 2C, lanes 4–9), whereas the parent iodoTyrRS produced both aminoacyl-tRNAs at similar levels (Fig. 2C, lanes 2 and 3). The synthesized iodotyrosyl-tRNATyr is hardly recognized by the fused editing domains, which is consistent with the steric considerations based on the editing active site structures. Thus, the fusion of the editing domains successfully improved the overall amino acid specificity of iodoTyrRS in favor of 3-iodo-l-tyrosine.
Transplantation of the P. horikoshii Editing Domain into IodoTyrRS at Internal Sites.
We tested the feasibility of the transplantation of an editing domain into internal positions of iodoTyrRS. The P. horikoshii B3/4 domain at the N terminus was more efficient in editing tyrosyl-tRNA than the other bacterial domains. In addition, the N and C termini of the archaeal domain are much closer to each other than those of the bacterial domains, suggesting that the archaeal domain is more suitable for insertion into internal sites of iodoTyrRS.
The loop regions in the CP and anticodon (AC)-binding domains (residues 163–169 and 294–298, respectively) (Fig. S2) were selected as internal insertion sites, and various amino acid sequences with different lengths were tested for connecting the editing domain and the iodoTyrRS. Thus, we created and tested >30 insertion variants and obtained two variants that could be prepared by overproduction in E. coli cells and retained the activity toward 3-iodo-l-tyrosine. These variants, CP-Ped-IYRS and AC-Ped-IYRS, accommodate the editing domain in the loops of the CP and AC-binding domains, respectively (Fig. 2A).
For analyzing CP-Ped-IYRS and AC-Ped-IYRS, the mutation that abolishes the editing activity was introduced to create CP-Pedmu-IYRS and AC-Pedmu-IYRS, respectively. These variants were compared with N-Ped-IYRS and N-Pedmu-IYRS in in vitro assays. CP-Ped-IYRS and N-Ped-IYRS hardly produced tyrosyl-tRNATyr, whereas their editing-deficient mutants produced tyrosyl-tRNATyr as efficiently as iodoTyrRS (Fig. 2D). Therefore, the insertion in the CP domain does not impair the catalytic activity at the aminoacylation active site of iodoTyrRS, but results in the efficient editing of tyrosyl-tRNATyr. In contrast, AC-Ped-IYRS produced tyrosyl-tRNATyr as efficiently as iodoTyrRS (Fig. 2D). Therefore, although the insertion of the editing domain in the AC-binding domain does not impair the aminoacylation activity of iodoTyrRS, this transplanted editing domain failed to edit tyrosyl-tRNATyr.
The overall amino acid specificity of CP-Ped-IYRS was examined by acidic PAGE, in comparison with those of iodoTyrRS and AC-Ped-IYRS. CP-Ped-IYRS only produced iodotyrosyl-tRNATyr, and not tyrosyl-tRNATyr, whereas iodoTyrRS and AC-Ped-IYRS produced both (Fig. 2E). Thus, the overall specificity of CP-Ped-IYRS was significantly improved in favor of 3-iodo-l-tyrosine.
The hydrolytic activity of the editing domain was compared between N-Ped-IYRS and CP-Ped-IYRS. These variants were examined for the activity to accelerate the deacylation of tyrosyl-tRNATyr. As a result, both variants significantly accelerated the deacylation, thus showing the hydrolytic activity of the editing domain isolated from the archaeal PheRS (Fig. 2F). CP-Ped-IYRS accelerated the reaction more efficiently than N-Ped-IYRS, indicating that CP-Ped-IYRS is more active in the editing reaction.
Application of N-Ped-IYRS and CP-Ped-IYRS in a Eukaryotic Cell-Free Translation for the Site-Specific Incorporation of 3-Iodo-l-Tyrosine into Proteins.
The iodoTyrRS has been used in cell-free translation using a wheat germ extract, together with an amber suppressor tRNA derived from E. coli tRNATyr, to incorporate 3-iodo-l-tyrosine into proteins in response to an amber codon introduced within the coding region (19). Because the bacterial TyrRS-tRNATyr pair does not cross-react with the eukaryotic aaRS-tRNA pairs, the nonnatural amino acids recognized by bacterial TyrRS mutants are expected to be incorporated only at amber codons (18, 19). In fact, the E. coli pair of iodoTyrRS and the suppressor tRNA reportedly incorporates 3-iodo-l-tyrosine only at amber codons, but not at other codons, such as tyrosine codons, in the wheat germ cell-free translation (19).
We translated the GST gene with an amber codon at position 25. The suppressor tRNA alone did not produce the full-length product of GST(Am) (Fig. 2G, lane 1). The pair of iodoTyrRS and the suppressor tRNA produced GST in the presence of 3-iodo-l-tyrosine (Fig. 2G, lane 3) and generated a smaller amount of GST in the absence of 3-iodo-l-tyrosine (Fig. 2G, lane 2). In the absence of competing 3-iodo-l-tyrosine, the l-tyrosine was probably incorporated at the amber position.
N-Ped-IYRS and CP-Ped-IYRS were then used in place of iodoTyrRS. Both variants produced the full-length GST in the presence of 3-iodo-l-tyrosine (Fig. 2G, lanes 5 and 9). On the other hand, N-Ped-IYRS produced an appreciable amount of GST in the absence of this amino acid (Fig. 2G, lane 4). In contrast, GST production by CP-Ped-IYRS was not detectable when 3-iodo-l-tyrosine was absent (Fig. 2G, lane 8). The complete repression of GST production by CP-Ped-IYRS is ascribed to the ability of CP-Ped-IYRS to edit tyrosyl-tRNA, because CP-Pedmu-IYRS produced an appreciable amount of GST in the absence of 3-iodo-l-tyrosine (Fig. 2G, lane 10).
Although neither CP-Ped-IYRS nor N-Ped-IYRS produced tyrosyl-tRNA in the aminoacylation assay (Fig. 2F), translation of the amber codon in the absence of 3-iodo-l-tyrosine was completely repressed by the editing activity of CP-Ped-IYRS, but was tolerated by that of N-Ped-IYRS (Fig. 2G). In this context, the presence of elongation factor Tu (EF-Tu), which binds to aminoacyl-tRNA, reportedly facilitates the production of misacylated tRNA, probably because EF-Tu protects the misacylated tRNA from hydrolysis by the editing domains (32). The weaker editing activity of N-Ped-IYRS than that of CP-Ped-IYRS (Fig. 2F) was probably insufficient to overcome the protection of tyrosyl-tRNA by EF-Tu.
Discussion
In the present study, we showed that the isolated B3/4 editing domain of the P. horikoshii PheRS β-subunit retains the hydrolytic activity for tyrosyl-tRNA. The archaeal editing domain was transplanted into the CP domain of iodoTyrRS. The CP-Ped-IYRS thus generated efficiently edits tyrosyl-tRNATyr and hardly incorporates l-tyrosine into proteins at the amber position in a eukaryotic translation system, thus achieving the unambiguous translation of the amber codon into 3-iodo-l-tyrosine.
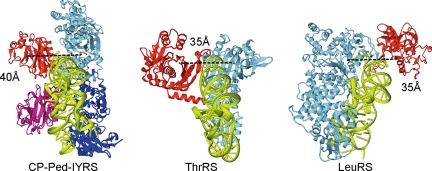
The structural model of CP-Ped-IYRS in the tRNATyr-bound form is shown in Fig. 3. The editing domain of CP-Ped-IYRS is linked with the Rossmann-fold domain by two linker peptides, and the two editing domains in the dimer are located close to each other. These factors restrict the movement of the editing domain and moderately fix the editing active site at a distance of 40 Å from the aminoacylation active site. The distance between the aminoacylation and editing active sites is reportedly 30–40 Å for the editing aaRS species (7, 8, 14, 27, 33); two examples are shown in Fig. 3. The positions of the editing active site, the aminoacylation active site, and the 3′ end of tRNA relative to one another are similar between CP-Ped-IYRS and ThrRS, a class II aaRS. This resemblance to the naturally occurring editing aaRS probably explains the efficient editing by CP-Ped-IYRS. The tRNA binding mode of class Ic including TyrRS, which is different from those of classes Ia and Ib, but is similar to that of class II (30), underlies the putative similarity in the editing mechanism between ThrRS and CP-Ped-IYRS.
Fig. 3.
Comparison among the tertiary structures of editing aaRSs. (Left) Structural model of a CP-Ped-IYRS dimer complexed with a tRNATyr. The model was built as described in SI Text. (Center and Right) The crystal structures of the E. coli ThrRS·tRNAThr complex (Center) (14) and the P. horikoshii LeuRS·tRNALeu complex (Right) (10) are shown for comparison. The two monomers of CP-Ped-IYRS are shown in blue and cyan with their editing domains in magenta and red, respectively. The editing domain is in red, with the remaining part of the enzyme in cyan, for ThrRS and LeuRS. tRNA is in lime green in each structure. The distances between the aminoacylation and editing active sites are shown.
The editing domain at the N terminus of N-Ped-IYRS can move relatively freely, and the distance between the editing and aminoacylation active sites may change from 40 to 60 Å (Fig. S3A). At a distance as far as 60 Å from the aminoacylation active site, the editing active site may not efficiently accommodate the aminoacylated end of the tRNA. As a result, this movement of the editing domain may reduce the overall editing efficiency. As for AC-Ped-IYRS, the editing active site is moderately fixed at a distance of 50 Å from the aminoacylation active site (Fig. S3B). Because this variant lacked editing activity, this distance is probably too long for efficient catalysis by the editing domain, although it is also possible that the transplanted domain may not assume the correct tertiary structure.
3-Iodo-l-tyrosine, at a normal concentration, competes and prevails against l-tyrosine for iodoTyrRS, which enables the specific incorporation of this nonnatural amino acid into proteins in eukaryotic translation systems (18, 19). In contrast, CP-Ped-IYRS is able not only to charge 3-iodo-l-tyrosine efficiently to tRNA but also to exclude l-tyrosine by itself, even in the absence of the competition between the two amino acids. Therefore, this insertion variant achieves exclusive specificity for 3-iodo-l-tyrosine and is here renamed as iodoTyrRS-ed. The transplantation and engineering of editing domains are expected to enable most aaRSs to hydrolyze their cognate aminoacyl-tRNAs, thus dramatically contributing to the creation of novel, useful variants for genetic code expansion.
Materials and Methods
Preparation of Fragments from the β-Subunit of PheRSs.
The B1/2–B3/4–B5, B1/2–B3/4, B3/4–B5, and B3/4 fragments from the E. coli PheRS β-subunit consist of residues 1–475, 1–403, 188–475, and 188–403, respectively, and those from T. thermophilus consist of residues 1–473, 1–401, 190–473, and 190–401, respectively (29). The B1–B3/4–B5, B1–B3/4, B3/4–B5, and B3/4 fragments from P. horikoshii consist of residues 1–353, 1–280, 83–353, and 83–280, respectively (28). A methionine residue was added at the N terminus of the B3/4–B5 and B3/4 fragments from these organisms. The genes encoding these fragments were cloned in the multiple cloning site of the vector pET-26b (Novagen) to express these fragments with a His tag at the C terminus. The expression plasmids thus generated were introduced into E. coli BL21 Star (DE3) cells (Invitrogen), which were grown at 37°C before the induction of fragment expression. After the induction, the temperature was lowered to 20°C. The expressed fragments were then purified by chromatography on a Ni Sepharose 6 Fast Flow column (GE Healthcare). The purified samples were stored at −20°C in 20 mM Tris·HCl buffer (pH 8.0), containing 37.5 mM NaCl and 50% glycerol. The preparation of iodoTyrRS was implemented through the same procedure.
Preparation of N-Fusion and Insertion Variants of IodoTyrRS.
The B3/4 domain (residues 188–403) of the E. coli PheRS β-subunit was N-terminally capped with a Met residue, and then connected to the N terminus of iodoTyrRS, which resulted in N-Eed-IYRS. The B1/2–B3/4 domain (residues 1–405) of the T. thermophilus PheRS β-subunit was added to the N terminus of iodoTyrRS, together with a flexible Gly-Ser-Ala-Pro-Ser-Gly linker, to generate N-Ted-IYRS. The B3/4 domain (residues 83–276) of the P. horikoshii PheRS β-subunit was added at the N terminus of iodoTyrRS, together with a Gly-Ser-Ala-Ser-Gly-Pro-Ala-Ser-Ala-Gly linker, to create N-Ped-IYRS, and the N terminus was then capped with a Met residue. The P. horikoshii B3/4 domain (residues 83–276), together with a linker (Gly-Ser-Ala-Ser-Gly-Pro-Ala-Ser-Ala) after this domain, was inserted between residues 166 and 167 of iodoTyrRS to generate CP-Ped-IYRS. The same P. horikoshii B3/4 domain was inserted between residues 295 and 296 of iodoTyrRS to generate CP-Ped-IYRS, together with the linkers Ala-Pro-Arg-Ala-Gln-Tyr-Val-Leu-Ala (identical to residues 296–304 of iodoTyrRS) and Gly-Ser-Ala-Ser-Gly-Pro-Ala-Ser-Ala-Gly, preceding and following this domain, respectively. To generate the editing-deficient fusion/insertion variants, Ala-356 (according to the numbering in the E. coli and T. thermophilus PheRS β-subunits) and Ala-141 (according to the numbering in the P. horikoshii subunit) were replaced by Trp to plug the editing pocket and prevent tyrosine binding (11, 28). To prepare these fusion/insertion variants, their genes were cloned in vector the pET-26b, and the products, with a His tag at the C terminus, were obtained as described for the preparation of the PheRS fragments.
Aminoacylation and Deacylation Assays.
E. coli tRNATyr was prepared by run-off transcription using T7 RNA polymerase (34). The aminoacylation reaction for the PheRS fragments was performed at 37°C in 100 mM Tris·HCl buffer (pH 7.5), containing 15 mM MgCl2, 4 mM ATP, 5 μM E. coli tRNATyr, 200 μM l-tyrosine (Sigma), 1.6 μM iodoTyrRS, and 2.5 μM PheRS fragment. The aminoacylation reaction for the fusion/insertion variants was performed at 24°C for 30 min in 100 mM Tris·HCl buffer (pH 7.5), containing 15 mM MgCl2, 4 mM ATP, 5 μM E. coli tRNATyr, 200 μM l-tyrosine or 3-iodo-l-tyrosine (Sigma), and 1 μM iodoTyrRS or a fusion/insertion variant. The aminoacylated tRNAs were analyzed by acidic PAGE on a 7% gel containing 7 M urea (31). When the radio isotope-labeled l-tyrosine was used for analysis, the aminoacylation was performed at 37°C in Tris·HCl buffer (pH 7.5), containing 15 mM MgCl2, 4 mM ATP, 5 μM E. coli tRNATyr, 1 μM iodoTyrRS or a fusion/insertion variant, and 30 μM l-[14C]tyrosine (GE Healthcare). Hydrolysis of tyrosyl-tRNATyr was performed at 37°C in 100 mM Tris·HCl buffer (pH 7.5), containing 15 mM MgCl2, 2.2 μM [14C]tyrosyl-tRNATyr, and 50 nM N-Ped-IYRS or CP-Ped-IYRS. These reactions were stopped by the addition of trichloroacetic acid. The radioactivity of the acid-insoluble fractions was measured with a liquid scintillation counter (LSC-5100; Aloka).
Cell-Free Translation Using a Wheat Germ Extract.
The RTS 100 Wheat Germ CECF Kit (Roche) was used for cell-free translation of the reporter gene, GST(Am) [see SI Text for the construction of the plasmid containing the GST(Am) gene]. The amber suppressor tRNATyr derived from E. coli tRNATyr was prepared by T7 transcription as described above. The reaction mixture (25 μl) contained 3-iodo-l-tyrosine (1 mM), the amber suppressor tRNA (8 μM), either iodoTyrRS, N-Ped-IYRS, or CP-Ped-IYRS (2 μM each), and the plasmid harboring the GST(Am) gene (40 ng/μl). The translation was performed at 24°C for 6 h. The products were detected by Western blotting using an anti-GST antibody (GE Healthcare).
Supplementary Material
Acknowledgments.
We thank Drs. D. Kiga (Tokyo Institute of Technology), T. Ito (University of Tokyo), and Ms. F. Iraha (RIKEN) for valuable advice. This work was supported by grants-in-aid for Science Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Targeted Proteins Research Program, the RIKEN Structural Genomics/Proteomics Initiative, and the National Project on Protein Structural and Functional Analyses of the Ministry of Education, Culture, Sports, Science and Technology of Japan. T.K. was supported by the Special Postdoctoral Research Program of RIKEN. H.M.S. was supported by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803531105/DCSupplemental.
References
- 1.Fersht AR, Kaethner MM. Mechanism of aminoacylation of tRNA. Proof of the aminoacyl adenylate pathway for the isoleucyl- and tyrosyl-tRNA synthetases from Escherichia coli K12. Biochemistry. 1976;15:818–823. doi: 10.1021/bi00649a014. [DOI] [PubMed] [Google Scholar]
- 2.Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- 3.Norris AT, Berg P. Mechanism of aminoacyl RNA synthesis: Studies with isolated aminoacyl adenylate complexes of isoleucyl RNA synthetase. Proc Natl Acad Sci USA. 1964;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fersht AR. Enzyme Structure and Mechanism. New York: Freeman; 1985. [Google Scholar]
- 5.Lin L, Hale SP, Schimmel P. Aminoacylation error correction. Nature. 1996;384:33–34. doi: 10.1038/384033b0. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt E, Schimmel P. Mutational isolation of a sieve for editing in a transfer RNA synthetase. Science. 1994;264:265–267. doi: 10.1126/science.8146659. [DOI] [PubMed] [Google Scholar]
- 7.Nureki O, et al. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 8.Fukai S, et al. Structural basis for double-sieve discrimination of l-valine from l-isoleucine and l-threonine by the complex of tRNAVal and valyl-tRNA synthetase. Cell. 2000;103:793–803. doi: 10.1016/s0092-8674(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 9.Cusack S, Yaremchuk A, Tukalo M. The 2-Å crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000;19:2351–2361. doi: 10.1093/emboj/19.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukunaga R, Yokoyama S. Aminoacylation complex structures of leucyl-tRNA synthetase and tRNALeu reveal two modes of discriminator-base recognition. Nat Struct Mol Biol. 2005;12:915–922. doi: 10.1038/nsmb985. [DOI] [PubMed] [Google Scholar]
- 11.Roy H, Ling J, Irnov M, Ibba M. Post-transfer editing in vitro and in vivo by the β-subunit of phenylalanyl-tRNA synthetase. EMBO J. 2004;23:4639–4648. doi: 10.1038/sj.emboj.7600474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsui WC, Fersht AR. Probing the principles of amino acid selection using the alanyl-tRNA synthetase from Escherichia coli. Nucleic Acids Res. 1981;9:4627–4637. doi: 10.1093/nar/9.18.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beebe K, Ribas De Pouplana L, Schimmel P. Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 2003;22:668–675. doi: 10.1093/emboj/cdg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dock-Bregeon A, et al. Transfer RNA-mediated editing in threonyl-tRNA synthetase: The class II solution to the double discrimination problem. Cell. 2000;103:877–884. doi: 10.1016/s0092-8674(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 15.Beuning PJ, Musier-Forsyth K. Hydrolytic editing by a class II aminoacyl-tRNA synthetase. Proc Natl Acad Sci USA. 2000;97:8916–8920. doi: 10.1073/pnas.97.16.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science. 2001;292:498–500. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 17.Chin JW, et al. An expanded eukaryotic genetic code. Science. 2003;301:964–967. doi: 10.1126/science.1084772. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto K, et al. Site-specific incorporation of an unnatural amino acid into proteins in mammalian cells. Nucleic Acids Res. 2002;30:4692–4699. doi: 10.1093/nar/gkf589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiga D, et al. An engineered Escherichia coli tyrosyl-tRNA synthetase for site-specific incorporation of an unnatural amino acid into proteins in eukaryotic translation and its application in a wheat germ cell-free system. Proc Natl Acad Sci USA. 2002;99:9715–9720. doi: 10.1073/pnas.142220099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Schultz PG. Expanding the genetic code. Angew Chem Int Ed Engl. 2004;44:34–66. doi: 10.1002/anie.200460627. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, et al. Selective incorporation of 5-hydroxytryptophan into proteins in mammalian cells. Proc Natl Acad Sci USA. 2004;101:8882–8887. doi: 10.1073/pnas.0307029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson JC, et al. An expanded genetic code with a functional quadruplet codon. Proc Natl Acad Sci USA. 2004;101:7566–7571. doi: 10.1073/pnas.0401517101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta D, Wang P, Carrico IS, Mayo SL, Tirrell DA. A designed phenylalanyl-tRNA synthetase variant allows efficient in vivo incorporation of aryl ketone functionality into proteins. J Am Chem Soc. 2002;124:5652–5653. doi: 10.1021/ja0177096. [DOI] [PubMed] [Google Scholar]
- 24.Summerer D, et al. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006;103:9785–9789. doi: 10.1073/pnas.0603965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi T, et al. Structural basis of nonnatural amino acid recognition by an engineered aminoacyl-tRNA synthetase for genetic code expansion. Proc Natl Acad Sci USA. 2005;102:1366–1371. doi: 10.1073/pnas.0407039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fersht AR, Shindler JS, Tsui WC. Probing the limits of protein-amino acid side-chain recognition with the aminoacyl-tRNA synthetases. Discrimination against phenylalanine by tyrosyl-tRNA synthetases. Biochemistry. 1980;19:5520–5524. doi: 10.1021/bi00565a009. [DOI] [PubMed] [Google Scholar]
- 27.Kotik-Kogan O, Moor N, Tworowski D, Safro M. Structural basis for discrimination of l-phenylalanine from l-tyrosine by phenylalanyl-tRNA synthetase. Structure (London) 2005;13:1799–1807. doi: 10.1016/j.str.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki HM, et al. Structural and mutational studies of the amino acid editing domain from archaeal/eukaryal phenylalanyl-tRNA synthetase. Proc Natl Acad Sci USA. 2006;103:14744–14749. doi: 10.1073/pnas.0603182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosyak L, Reshetnikova L, Goldgur Y, Delarue M, Safro MG. Structure of phenylalanyl-tRNA synthetase from Thermus thermophilus. Nat Struct Biol. 1995;2:537–547. doi: 10.1038/nsb0795-537. [DOI] [PubMed] [Google Scholar]
- 30.Yaremchuk A, Kriklivyi I, Tukalo M, Cusack S. Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 2002;21:3829–3840. doi: 10.1093/emboj/cdf373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varshney U, Lee CP, RajBhandary UL. Direct analysis of aminoacylation levels of tRNAs in vivo: Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J Biol Chem. 1991;266:24712–24718. [PubMed] [Google Scholar]
- 32.Ling J, Yadavalli SS, Ibba M. Phenylalanyl-tRNA synthetase editing defects result in efficient mistranslation of phenylalanine codons as tyrosine. RNA. 2007;13:1881–1886. doi: 10.1261/rna.684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crepin T, Yaremchuk A, Tukalo M, Cusack S. Structures of two bacterial prolyl-tRNA synthetases with and without a cis editing domain. Structure (London) 2006;14:1511–1525. doi: 10.1016/j.str.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.