Abstract
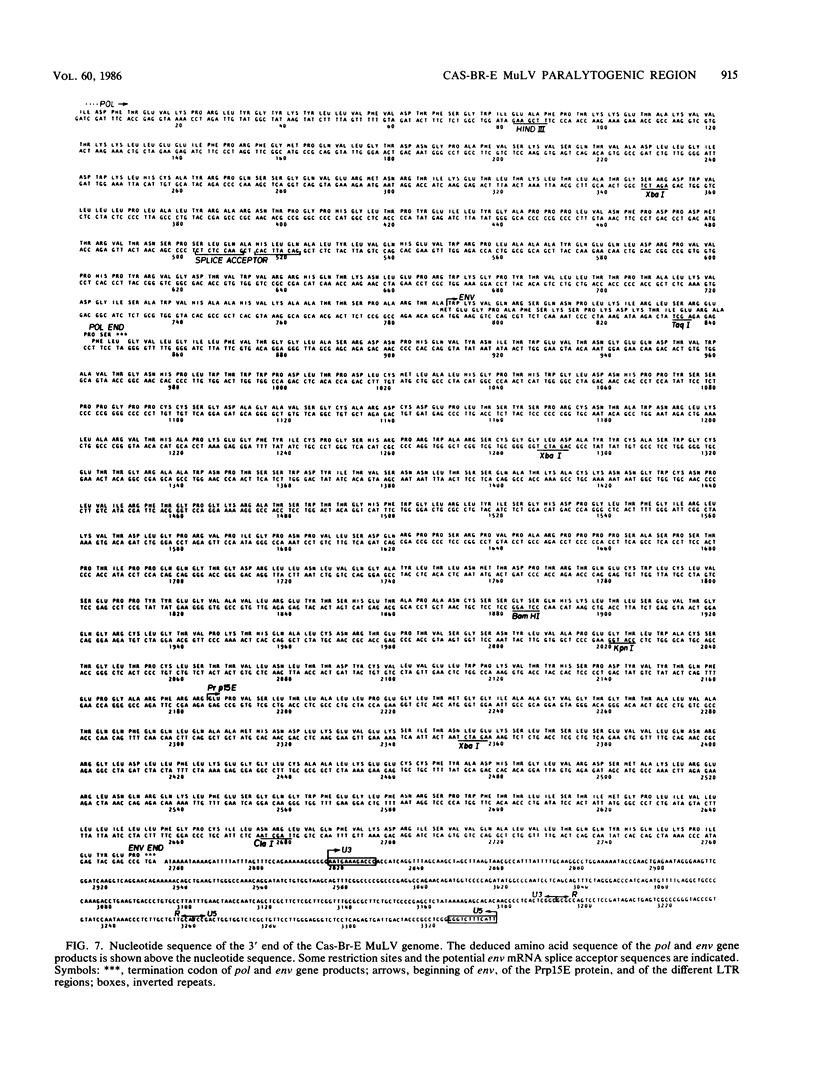
The ecotropic Cas-Br-E murine leukemia virus (MuLV) and its molecularly cloned derivative pBR-NE-8 MuLV are capable of inducing hind-limb paralysis and leukemia after inoculation into susceptible mice. T1 oligonucleotide fingerprinting, molecular hybridization, and restriction enzyme analysis previously showed that the env gene of Cas-Br-E MuLV diverged the most from that of other ecotropic MuLVs. To analyze proviruses in leukemic tissues, we derived DNA probes specific to Cas-Br-E sequences: two from the env region and one from the U3 long terminal repeat. With these probes, we found that this virus induced clonal (or oligoclonal) tumors and we documented the presence of typical mink cell focus-forming-type proviruses in leukemic tissues and the possible presence of other recombinant MuLV proviruses. Since the region harboring the determinant of paralysis was mapped within the pol-env region of the virus (L. DesGroseillers, M. Barrette, and P. Jolicoeur, J. Virol. 52:356-363, 1984), we performed the complete nucleotide sequence of this region covering the 3' end of the genome. We compared the deduced amino acid sequences of the pol carboxy-terminal domain and of the env gene products with those of other nonparalytogenic, ecotropic, and mink cell focus-forming MuLVs. This amino acid comparison revealed that this part of the pol gene product and the p15E diverged very little from homologous proteins of other MuLVs. However, the Cas-Br-E gp70 sequence was found to be quite divergent from that of other MuLVs, and the amino acid changes were distributed all along the protein. Therefore, gp70 remains the best candidate for harboring the determinant of paralysis.
Full text
PDF


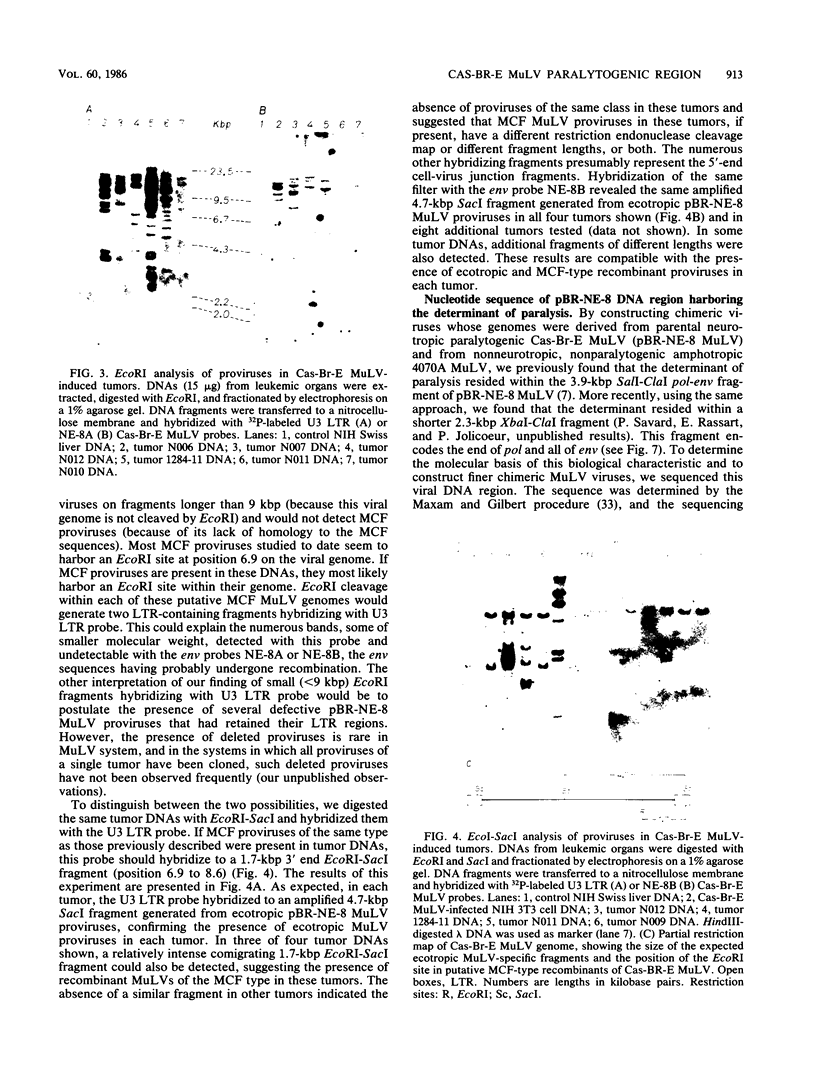





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Robbins K. C., Aaronson S. A. Wild mouse RNA tumor viruses. A nongenetically transmitted virus group closely related to exogenous leukemia viruses of laboratory mouse strains. J Exp Med. 1979 Jan 1;149(1):254–266. doi: 10.1084/jem.149.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Complete amino acid sequence and glycosylation sites of glycoprotein gp71A of Friend murine leukemia virus. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5788–5792. doi: 10.1073/pnas.79.19.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Barrette M., Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984 Nov;52(2):356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983 Dec;48(3):685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Robitaille Y., Jolicoeur P. Retrovirus-induced spongiform encephalopathy: the 3'-end long terminal repeat-containing viral sequences influence the incidence of the disease and the specificity of the neurological syndrome. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8818–8822. doi: 10.1073/pnas.82.24.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Famulari N. G., Buchhagen D. L., Klenk H. D., Fleissner E. Presence of murine leukemia virus envelope proteins gp70 and p15(E) in a common polyprotein of infected cells. J Virol. 1976 Nov;20(2):501–508. doi: 10.1128/jvi.20.2.501-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G. Murine leukemia viruses with recombinant env genes: a discussion of their role in leukemogenesis. Curr Top Microbiol Immunol. 1983;103:75–108. doi: 10.1007/978-3-642-68943-7_4. [DOI] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Schwartz D., Gilbert W. Isolation and mapping of cDNA hybridization probes specific for ecotropic and nonecotropic murine leukemia proviruses. Virology. 1983 Feb;125(1):139–154. doi: 10.1016/0042-6822(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Davidson W. F., Ruscetti S. K., Chused T. M., Morse H. C., 3rd Wild mouse ecotropic murine leukemia virus infection of inbred mice: dual-tropic virus expression precedes the onset of paralysis and lymphoma. J Virol. 1981 Aug;39(2):597–602. doi: 10.1128/jvi.39.2.597-602.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Hopkins N. Nucleotide sequence of the gp70 gene of murine retrovirus MCF 247. J Virol. 1983 Sep;47(3):413–420. doi: 10.1128/jvi.47.3.413-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., DesGroseillers L. Neurotropic Cas-BR-E murine leukemia virus harbors several determinants of leukemogenicity mapping in different regions of the genome. J Virol. 1985 Nov;56(2):639–643. doi: 10.1128/jvi.56.2.639-643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshin W. L., Arcement L. J., Naso R. B., Arlinghaus R. B. Common precursor for Rauscher leukemia virus gp69/71, p15(E), and p12(E). J Virol. 1977 Sep;23(3):787–798. doi: 10.1128/jvi.23.3.787-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Holland C. A., Lung M. L., Chattopadhyay S. K., Lowy D. R., Hopkins N. H. Nucleotide sequence of the 3' end of MCF 247 murine leukemia virus. J Virol. 1983 Jan;45(1):291–298. doi: 10.1128/jvi.45.1.291-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Hunsmann G., Friedrich R. Nucleotide sequence of the envelope gene of Friend murine leukemia virus. J Virol. 1983 Jan;45(1):1–9. doi: 10.1128/jvi.45.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Shimizu C. S., Rasheed S., Pal B. K., Gardner M. B. Characterization of genome structure of amphotropic and ecotropic wild mouse retroviruses. J Virol. 1982 Feb;41(2):605–614. doi: 10.1128/jvi.41.2.605-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder D., Stirm S., Schneider J., Hunsmann G., Smythers G., Oroszlan S. Glycoproteins of friend murine leukemia virus: separation and NH2-terminal amino acid sequences of gp69 and gp71. J Virol. 1982 Apr;42(1):352–355. doi: 10.1128/jvi.42.1.352-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Karshin W. L., Jamjoom G. A., Arlinghaus R. B. A fucose-deficient glycoprotein precursor to Rauscher leukemia virus gp69/71. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2326–2330. doi: 10.1073/pnas.73.7.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Jolicoeur P. Restriction analysis and molecular cloning of endogenous murine leukemia virus-specific DNA sequences of the mouse genome. Virology. 1982 Nov;123(1):175–186. doi: 10.1016/0042-6822(82)90304-x. [DOI] [PubMed] [Google Scholar]
- Rassart E., Shang M., Boie Y., Jolicoeur P. Studies on emerging radiation leukemia virus variants in C57BL/Ka mice. J Virol. 1986 Apr;58(1):96–106. doi: 10.1128/jvi.58.1.96-106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Tung J. S., Hopkins N., Robbins P. W. Relationship of GIX antigen expression to the glycosylation of murine leukemia virus glycoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6420–6424. doi: 10.1073/pnas.77.11.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Schultz A., Rein A., Henderson L., Oroszlan S. Biological, chemical, and immunological studies of Rauscher ecotropic and mink cell focus-forming viruses from JLS-V9 cells. J Virol. 1983 Mar;45(3):995–1003. doi: 10.1128/jvi.45.3.995-1003.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Villemur R., Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of viral DNA from leukemogenic Gross passage A murine leukemia virus and nucleotide sequence of its long terminal repeat. J Virol. 1983 Feb;45(2):539–546. doi: 10.1128/jvi.45.2.539-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Knupp C., Wong P. K. A 1.6-kilobase-pair fragment in the genome of the ts1 mutant of Moloney murine leukemia virus TB that is associated with temperature sensitivity, nonprocessing of Pr80env, and paralytogenesis. J Virol. 1985 May;54(2):364–373. doi: 10.1128/jvi.54.2.364-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven W. J., Vermorken A. J., Onnekink C., Bloemers H. P., Bloemendal H. Structural studies on Rauscher murine leukemia virus: isolation and characterization of viral envelopes. J Virol. 1978 Sep;27(3):595–603. doi: 10.1128/jvi.27.3.595-603.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven W. J., van Zaane D., Onnekink C., Bloemers H. P. Impaired processing of precursor polypeptides of temperature-sensitive mutants of Rauscher murine leukemia virus. J Virol. 1978 Feb;25(2):553–561. doi: 10.1128/jvi.25.2.553-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]