Abstract
The mechanisms by which cancer evolves and persists in natural systems have been difficult to ascertain. In the Xiphophorus melanoma model, a functional oncogene (Xiphophorus melanoma receptor kinase Xmrk) has been maintained for several million years despite being deleterious and in an extremely unstable genomic region. Melanomas in Xiphophorus spp. fishes (platyfishes and swordtails) have been investigated since the 1920s, and, yet, positive selection that could explain the maintenance of Xmrk has not been found. Here, we show that Xiphophorus cortezi females from two populations prefer males with the spotted caudal (Sc) melanin pattern, which is associated with the presence of the Xmrk oncogene and serves as the site of melanoma formation within this species. Moreover, X. cortezi females prefer males with an enhanced Sc to males with a reduced Sc pattern. RT-PCR analysis confirms tissue-specific Xmrk expression within the Sc pattern in X. cortezi. Because of the association of Xmrk with the Sc pigment pattern and the fact that melanoma formation augments this visual signal, sexual selection appears to be maintaining this oncogene because of a mating preference for Sc, as well as the exaggeration of this male trait. At the individual level, decreases in viability and fecundity because of Xmrk and subsequent melanoma formation may be mitigated via increases in mate acquisition. At the population level, maintenance of this oncogene appears to be under frequency dependent selection, as we detected female preference for males without Sc in a third population that had higher frequencies of Sc in females.
Keywords: cancer, evolution, sexual selection, Xiphophorus, Xmrk
The evolutionary origin of cancer may be an inevitable outcome of multicellularity, cell replacement, and genetic changes (1, 2). Yet this does not explain how cancer can (and does) persist over evolutionary time. Although seemingly counterintuitive, several cancer cell lineages recently have been shown at the genetic level to be under positive selection (for review, see ref. 3). For example, strong directional selection has been detected in the coding regions flanking the mammalian testis-determining gene (Sry) in humans and primates (4), despite Sry expression in human prostate cancer (5). However, the maintenance of such oncogenes is generally considered the byproduct of genomic conflict (6) and antagonistic coevolution (e.g., maternal-fetal interactions) (7, 8). One recent study has even implicated a possible tradeoff involving sexual selection: A shorter CAG repeat region within the androgen receptor gene can increase fertility but may also increase an individual's risk of developing prostate cancer (9). Genes with oncogenetic potential could benefit an individual if their expression is necessary for and/or enhances a phenotype under sexual selection. Pigment patterns function as sexually selected signals across extremely diverse taxa (10), with the most desirable mate often having the most pronounced visual trait (for review, see ref. 11). Sexual selection for enhanced pigment patterns would result in the increased propagation of pigment cells used in creating the trait. Because increasing the size of a pigment pattern can also be characteristic of melanoma formation, we examined the possibility that sexual selection could contribute to the maintenance of a cancer-causing gene.
The study of hybrid crosses within Xiphophorus spp. and the resulting melanomas led to the initial realization that cancers can have a heritable basis (12). More recently, nonhybrid melanoma formation has also been confirmed in three species of Xiphophorus (13, 14), including X. cortezi. In both hybrid and nonhybrid melanomas, overexpression of the Xmrk oncogene occurs within species-specific macromelanophore patterns (13, 15). X. cortezi is polymorphic for the macromelanophore pigment pattern spotted caudal (Sc) and the associated Xmrk oncogene (13, 16). Xmrk is an essential component for the expression of the Sc phenotype in X. cortezi (13, 15) (Fig. 1) and can be located on the X and/or Y chromosomes (19). Therefore, malignant melanoma formations originating from the Sc macromelanophore pattern have been documented in both sexes of X. cortezi (13, 16) (Fig. 2B). All individuals with the Sc phenotype have at least one copy of Xmrk. However, because of the incomplete penetrance of Sc, individuals that lack phenotypic expression of Sc can also have one or two copies of Xmrk (16). Screening six populations of X. cortezi for the Xmrk genotype has confirmed previous results (13, 15) of the consistent association between the Sc phenotype and the Xmrk genotype (i.e., the Xmrk genotype was found in all 98 Sc individuals screened) (A.A.F. and S. Tanda, unpublished work).
Fig. 1.
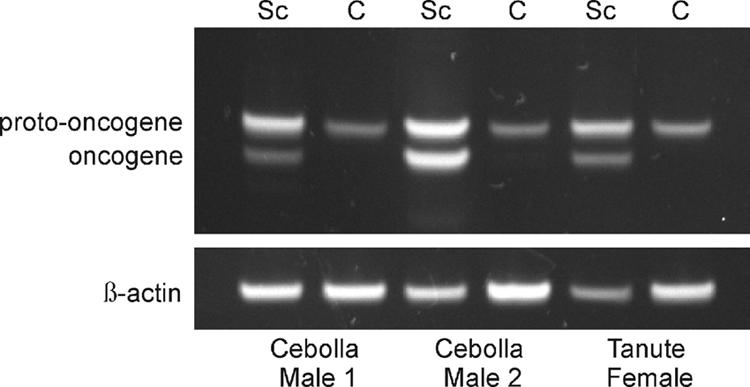
Xmrk and protooncogene expression in X. cortezi. Semiquantitative RT-PCR analyses using the same template primers for the oncogene (Xmrk, bottom bands of upper image) and protooncogene (top bands of upper image). In each of the three wild-caught individuals, all of which did not have visible melanoma formation, the Sc tissue was associated with both Xmrk and protooncogene expression, whereas in the nonpigmented control tissue (C), only the protooncogene was expressed. The housekeeping gene, β-actin, is included as a loading control (17). A 15-bp insertion associated with the protooncogene in X. cortezi accounts for the fractionation of the two bands by gel electrophoresis (A.A.F. and S. Tanda, unpublished data). This small insertion was found in the predicted signal peptide sequence at the beginning of the protooncogene. Polymorphisms within this region of the oncogene and protooncogene are common in individuals derived from wild populations of several Xiphophorus spp. (18).
Fig. 2.
Sc phenotype in X. cortezi. (A) X. cortezi male collected from Tanute, with an average expression of Sc on the caudal fin (nonmalignant). (Scale bar, 5 mm.) (B) X. cortezi male from Conchita with malignant melanoma extending from Sc into the caudal peduncle. Histopathology confirms malignant melanoma in this individual, classified as a melanophorous-macromelanophorous polymorphic melanoma (20 and A.A.F. and P. Bowser, unpublished data). Note on this male that substantial portions of the sword and caudal fin have sloughed-off, which ultimately impairs swimming ability. Specimens were photographed on the day they were collected in December 2005. (Scale bar, 5 mm.)
Using standard dichotomous choice tests, we examined female mate preference for the macromelanophore pattern Sc in three populations (Cebolla, Tanute, Conchita) of X. cortezi. In the first experiment, we examined whether there was a preference for larger Sc-patterned males that could explain the correlation between Sc and the Xmrk oncogene, as the overexpression of Xmrk can enhance this pigment pattern (13, 15). We used time associating with a stimulus as a measure of mating preference. Association time has been shown to be indicative of mate preference in fishes, such as in the closely related swordtail, Xiphophorus nigrensis, where association time was correlated with male mating success in the field (21, 22) and Gambusia holbrooki, where association time was correlated with male mating success in the laboratory (23). In addition, a recent study conducted on X. nigrensis found that association time is a more consistent and repeatable estimate of female preference than other female behaviors (24). In the second experiment, we examined whether the frequency of the Sc phenotype in a population could influence the strength of preference for Sc given the increased costs associated with having two copies of the Xmrk oncogene (16, 19).
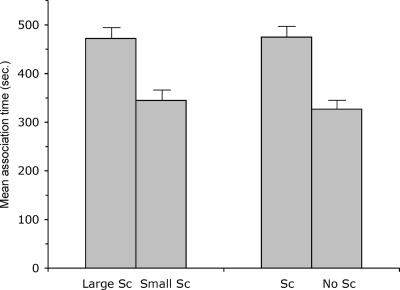
We found that Cebolla females spent significantly more time associating with the large Sc treatment than the small Sc treatment (Fig. 3) (mean large Sc time = 471.6 ± 27.9 s, mean small Sc time = 345.4 ± 26.2 s, Paired-Samples T test: t18 = 2.49, P < 0.02). This disparity is important because the Sc pigment pattern increases in size with melanoma formation (13). Theoretical studies suggest that even weak female mating preferences can produce strong selection on male traits (25). Therefore, the results of this first experiment provide compelling evidence for the continued evolutionary maintenance of a known oncogene through its role in the augmentation of a visual signal used in the selection of mates.
Fig. 3.
Female preferences for enhanced Sc phenotypes. The time that females from the Cebolla population spent associating with the large Sc treatment compared with the small Sc treatment, as well as with an average size Sc compared with no Sc. Bars represent the mean ± SEM.
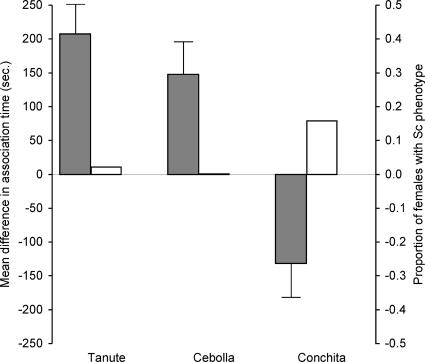
When females were given a choice between Sc and non-Sc males, we detected a significant preference for Sc males in two populations of X. cortezi (Tanute: mean Sc time = 496.8 ± 29.8 s, mean non-Sc time = 289.5 ± 24.3 s, t18 = 4.26, P < 0.001; Cebolla: mean Sc time = 474.9 ± 30.1 s, mean non-Sc time = 327.4 ± 23.6 s, Paired-Samples T test: t20 = 2.85, P < 0.01). These two populations are found in separate drainages (Table 1) that are genetically divergent (26); therefore, female preferences for the Sc phenotype appear widespread in X. cortezi. Because of the association between Sc and Xmrk, female preference for Sc-patterned males would also act to maintain the Xmrk oncogene. However, females from the third population (Conchita), which had the highest percentage of Sc females (Table 1), discriminated against Sc males, preferring to associate with non-Sc males (Fig. 4) (Conchita: mean Sc time = 343.2 ± 27.4 s, mean non-Sc time = 475.0 ± 28.2 s, Paired-Samples T test: t26 = −2.41, P < 0.02). Therefore, when the frequency of females with Sc is high in a population, there appears to be direct selection against female preference for males with Sc because individuals with Sc always have Xmrk, and offspring carrying two copies of Xmrk have both reduced viability (16, 19) and potential for a shorter reproductive lifespan (see following paragraph).
Table 1.
Percentage of Sc phenotype by sex across three study sites
| Population | Drainage | Sex | Sc individuals | Total individuals | Sc percentage |
|---|---|---|---|---|---|
| Tanute | Tampaón | Male | 19 | 63 | 30.2 |
| Female | 1 | 47 | 2.1 | ||
| Cebolla | Moctezuma | Male | 31 | 75 | 41.3 |
| Female | 0 | 30 | 0.0 | ||
| Conchita | Moctezuma | Male | 23 | 81 | 28.4 |
| Female | 9 | 57 | 15.8 |
All adult individuals collected were scored upon capture in December 2005 (Tanute, Conchita) and January 2007 (Cebolla). Juveniles were excluded from survey because of variance in the developmental timing of Sc expression.
Fig. 4.
Female preferences for Sc across populations. Primary y axis (left, gray bars) represents the mean amount of time females spent with painted Sc males minus the time spent with the non-Sc males across all four trials. Bars represent the mean ± SEM. Positive values indicate preference for Sc males, whereas negative values indicate discrimination against Sc males. Secondary y axis (right, white bars) represents the proportion of females with Sc phenotype for each of the three populations (all Cebolla females lacked Sc).
Traditionally, nonhybrid melanoma formation was thought to be an artifact of housing Xiphophorus spp. in the laboratory (13, 16) because individuals with melanomas were not found in the field or in museum collections. However, in a single day of collecting X. cortezi for this study, we found five males and one female of the 99 individuals surveyed with melanomas originating from Sc in the Conchita population. The rate of disease progression in wild populations is not known. In a laboratory setting, the formation and progression of melanomas in Xiphophorus spp. can take months before lethality (14), during which time males court and appear able to mate with females, thereby passing on the Xmrk oncogene. However, unlike Xiphophorus variatus, which is only known to develop melanomas during senescence (i.e., >18 months old) (13), the incidence of melanoma formation in nonhybrid X. cortezi is highest in sexually active males that are 9–12 months old (13). Kallman (16) documented the presence of severe melanomas in nonhybrid X. cortezi by 7 months of age. Xiphophorus spp. are thought to be able to live 2 years in natural populations (13, 27), and one study based on otolith counts of wild-caught X. nigrensis estimated that a 39-mm male (i.e., average male size for individuals in this study) would reach sexual maturity at ≈4.5 months of age (28). Therefore, melanoma formation in X. cortezi males occurring within this first year would represent a substantial decrease in an individual's reproductive lifespan. Whether increased male attractiveness associated with the Sc phenotype compensates for the potential reproductive loss because of early mortality is unclear. The greater incidence of melanoma formation in X. cortezi males, compared with females (13), is consistent with our results, demonstrating a mating advantage for male X. cortezi with larger Sc pigment patterns.
It has been hypothesized that female mating preferences for traits that make males more visible could have initially evolved as the result of a preexisting sensory bias (29). Once present, these preferences can be maintained through direct selection because of reduced mate search costs (30, 31). Thus, female preference for Sc patterns (and larger Sc patterns) in males may reflect a general bias for more visible males (i.e., with more pigmented area). In fact, X. cortezi females from the Conchita population have been documented to prefer males with more melanin irrespective of location (i.e., individual bars or a single larger bar with the same area) (32). However, X. cortezi females from the Conchita population discriminate against Sc-patterned males, despite the increased visibility of these males because of their pigmentation. We suggest that that the bias for more pigment in X. cortezi males has become more refined, possibly because of the increase in mating costs associated with choosing males with Sc (and Xmrk), outweighing the benefits to females of reduced mate search cost. The greater frequency of Sc in females at Conchita would increase the probability of producing offspring with two copies of Xmrk. These offspring are more likely to have melanomas that shorten their reproductive lifespan (13, 16). In addition, there is evidence that offspring with two copies of Xmrk may be nonviable (19). A negative relationship between male ornamentation and an indicator of male viability has also been detected in guppies (33), and such relationships are expected to influence the extent and direction of the selection on female mating preferences.
Explaining how a functional Xmrk has been evolutionarily maintained within divergent Xiphophorus spp. has remained a considerable challenge. Remarkably, Xmrk has become more efficient since its origin, incorporating two gain-of-function mutations within its coding region that lead to ligand-independent dimerization (12). Because Xmrk is always correlated with specific macromelanophore patterns (13, 15), these patterns have been suggested to play a key role in maintaining Xmrk (12, 34). Despite this, we are aware of only one other study that has detected selection for a macromelanophore pattern (34), and this study was conducted on Xiphophorus helleri, which lacks the Xmrk genotype (15). It is intriguing that both patterns, dabbed (X. helleri) (35) and spotted (X. coretzi) (16) caudal, are determined by autosomal genes and are not sex-linked. To date, however, the only mechanism that has been proposed to explain the preservation of Xmrk, which is located on the sex chromosomes (19), is the genetic hitch-hiking model (36). Although the genetic hitch-hiking model can increase the frequency of neutral or even slightly deleterious mutations, it necessitates close proximity on a chromosome (36). Thus, at least in the case of X. cortezi, which has retained a functional Xmrk oncogene despite the autosomal determination of Sc, this mechanism does not seem applicable.
Rapid morphological evolution, common for traits under sexual selection (37, 38), can increase an individual's susceptibility to cancer (1). Here, we present a system in which the onset of melanoma formation is advantageous for the acquisition of mates. This research provides evidence of sexual selection acting to favor the evolutionary maintenance of an oncogene. X. cortezi female preference for males with enhanced Sc phenotypes explains the continued genetic correlation between Xmrk and S, and may have an epistatic basis (39) with a mating advantage associated with a larger Sc compensating for the deleterious Xmrk. The fact that all Xiphophorus spp. that have maintained a functional Xmrk have a coupled macromelanphore pigment pattern (15) provides additional support that these genetic entities work synergistically in their capacity to enhance visual signals used in sexual selection.
Methods
Specimen Collection and Housing.
All fish used in these experiments were collected from the following natural populations in San Luis Potosí, Mexico: Arroyo Tanute (N 21.39.123, W.99.02.127); Arroyo Cebolla (N 21.23.472, W 98.59.885); and Arroyo Conchita (N 21.33.500, W 98.59.320). Representative samples were collected as adults during two field seasons: December 2005 (Conchita and Tanute) and January 2007 (Cebolla). Upon return to the United States, the fish were individually housed at Ohio University in 19-L tanks and visually isolated from one another for a minimum of 4 weeks before conducting female preference tests. All fish were maintained under standard laboratory conditions throughout the experiments, which included a 12L:12D cycle, daily feeding (Tetramin flakes), and a constant temperature of 22°C.
Male Treatments.
Pairs of males were matched for standard length within ±2 mm. Because X. cortezi females prefer males with symmetrical barring (40, 41) and have polymorphic preferences for males with vertical bars (42), males were also paired according to their bar state (i.e., not differing by more than a total of three vertical body bars), bar symmetry, and overall similarity in melanin pigment patterning. Paired males were always from the same population. All males used as stimulus males lacked the Sc pattern. Each experiment used seven pairs of stimulus males, with the exception of the experiment testing for preferences of large Sc vs. small Sc (Cebolla population), which used eight pairs. We randomized which pair of males was used with each female. We tested 19 females from Tanute, 21 females from Cebolla, and twenty-seven females from Conchita. All females were wild-caught. In the investigation of female preferences for the presence of Sc phenotype, one male in each pair was randomly chosen to receive a painted Sc treatment of average size using the antiseptic dye, Dr. Naylor's Blu-Kote dye (H. W. Naylor Co., Inc.) (43), whereas the other male received a mock water painting. The size of the Sc phenotype applied to males was consistent and representative of the average Sc expression for the population being tested, based on measurement of wild-caught males from that site (see supporting information (SI) Text and Figs. S1 and S2). Nineteen females from the Cebolla population were tested for their preferences of the enhanced Sc phenotype. Males in this experiment randomly received either a small Sc phenotype (≈20th percentile of expression) or a large Sc phenotype (≈80th percentile), giving the female a choice between large Sc and small Sc. This experiment was conducted with females from the Cebolla population only, and females were tested 5 weeks after we conducted the presence-absence Sc experiment. In both experiments, each female was tested with the same pair of males twice; however, the treatment that each male received was switched each test day. Switching males between treatments allowed us to control for any unforeseen behavioral or phenotypical differences between the pairs of stimulus males. Within 3–4 h, the antiseptic dye fades, which makes it possible to switch males between treatments on test days. We randomized which pair of males was tested with each female. All observation trials, including the painting of treatments in these trials, were conducted by A.A.F. to reduce variation in the painting of males.
Female Preference Tests.
We used a standard dichotomous-choice test design (for tank description, see ref. 32). A twin light fixture with Vitalite fluorescent bulbs (Durotest) was hung 30 cm above the tank, ensuring that females were not dark-adapted during the 8-min acclimation. After acclimation, we recorded the time the female spent associating in the zones adjacent to male stimuli for 8 min. After the first trial, the positions of males were switched to control for positional bias. Following reacclimation, the second 8-min trial was conducted. Two days later, the female and male pair was tested again with the only difference being that we switched the male who received the painted Sc treatment. In the investigation of enhanced Sc phenotypes, the treatment each stimulus male received was also switched across test days (e.g., large Sc treatment on first test day and small Sc treatment on second test day). Previous research in our laboratory has demonstrated that the use of Dr. Naylor's Blu-Kote dye does not adversely affect male behavior and that these painted phenotypes elicit female responses similar to natural phenotypes (43). We used the total time a female associated with each treatment as an indicator of female mate preference. A female was considered to have a side bias when she did not enter both choice zones during the two trials on that test day.
RT-PCR.
Two wild-caught males from Cebolla (January 2007) and a wild-caught female from Tanute (December 2005) were killed to determine the specificity of protooncogene and oncogene expression. Two tissue samples (≈20 mg each) were taken from each individual for purification of total RNA: one sample from the Sc pattern and the other from a nonpigmented region on the side of fish (control). Total RNA from these animal tissues was extracted by using the Qiagen RNeasy Mini Kit. The manufacturer's instructions were followed, including a homogenization with QIAshredder and an on-column DNase I digestion step (RNase-Free DNase Set, Qiagen) to extract total RNA. Total RNA was eluted in 50 μl of RNase-Free H2O. The Qiagen QuantiTect Reverse Transcription Kit was used to synthesize the first strand cDNA from 26 ng of total RNA. cDNA equivalent to 2.6 ng of total RNA was subjected to amplification (LA Taq Kit, Takara). The following primers were used to amplify β-actin: sense primer 5′-TGGACTTTGAGCAGGAAATG and antisense primer 5′- AATGCCACATGATTCCATAC (17). For the amplification of oncogene and protooncogene products, the following primers were developed: sense primer 5′- CTAACCGGACCGTCTTCATG, located just upstream of the translation initiation site, and antisense primer 5′-TTGAGGTAGTGATTGTCCAG, located at the beginning of exon 2. The final concentration of the primers was 100 nM. cDNA and amplification was done under the following conditions: initial denaturation at 95°C for 5 min, then 33 cycles of denaturation at 95°C for 60 s, annealment at 60°C for 60 s, extension at 72°C for 30 s, followed by a final extension at 72°C for 5 min. Pilot experiments with the β-actin primer set indicated PCR amplification was exponential between cycles 29 and 35 under these PCR conditions. A 10-μl aliquot of the amplification products was fractionated by electrophoresis on an 8.0% polyacrylamide gel in 1X TBE (45 mM Tris-borate, 1 mM EDTA) buffer and visualized after staining with SYBR Green (Invitrogen) and UV transillumination. The gel image was taken with a Gel Logic100 system (Kodak).
All analyses comply with laws of the United States and the Animal Care Guidelines of Ohio University (Animal Care and Use Approval L01–01).
Supplementary Material
Acknowledgments.
We thank the Mexican government for permission to collect these fish; Oscar Rios-Cardenas, Scarlett Tudor, Natalie Dubois, Donelle Robinson, Geoff Baker, Yancey Fernandez, and Mike Nicholson for assistance with collecting fish in the field; Soichi Tanda for assistance in the molecular analyses and insightful comments on this manuscript; and two anonymous reviewers for their helpful comments. This research was funded by a National Institutes of Health National Research Service Award predoctoral fellowship (to A.A.F.) and a National Science Foundation grant (to M.R.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803851105/DCSupplemental.
References
- 1.Graham J. Cancer Selection: A New Theory of Evolution. Lexington VA: Aculeus Press; 1992. [Google Scholar]
- 2.Greaves M. Cancer: The Evolutionary Legacy. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 3.Crespi B, Summers K. Positive selection in the evolution of cancer. Biol Rev. 2006;81:407–424. doi: 10.1017/S1464793106007056. [DOI] [PubMed] [Google Scholar]
- 4.Whitfield LS, Lovell-Badge R, Goodfellow PN. Rapid sequence evolution of the mammalian sex-determining gene. SRY Nature. 1993;364:713–715. doi: 10.1038/364713a0. [DOI] [PubMed] [Google Scholar]
- 5.Tricoli JV, Yao JL, D'Souza SA, Bracken RB. Detection of sex-region Y (SRY) transcripts in human prostate adenocarcinoma and benign prostatic hypertrophy. Genes Chromosomes Cancer. 1993;8:28–33. doi: 10.1002/gcc.2870080106. [DOI] [PubMed] [Google Scholar]
- 6.Kleene KC. Sexual selection, genetic conflict, selfish genes and the atypical patterns of gene expression in spermatogenetic cells. Dev Biol. 2005;277:16–26. doi: 10.1016/j.ydbio.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Summers K, Crespi B. Cadherins in maternal-fetal interactions: Red queen with a green beard? Proc R Soc London B. 2005;272:643–649. doi: 10.1098/rspb.2004.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lala PK, Lee BP, Xu G, Chakraborty C. Human placental trophoblast as an in vitro model for tumor progression. Can J Physiol Pharmacol. 2002;80:142–149. doi: 10.1139/y02-006. [DOI] [PubMed] [Google Scholar]
- 9.Summers K, Crespi B. The androgen receptor and prostate cancer: A role for sexual selection and sexual conflict? Med hypotheses. 2008;70:435–443. doi: 10.1016/j.mehy.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Andersson M. Sexual Selection. Princeton, NJ: Princeton Univ Press; 1994. [Google Scholar]
- 11.Ryan MJ, Keddy-Hector A. Directional patterns of female mate choice and the role of sensory biases. Am Nat. 1992;139:S4–S35. [Google Scholar]
- 12.Meierjohann S, Schartl M. From Mendelian to molecular genetics: The Xiphophorus melanoma model. Trends Genet. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Schartl A, Malitschek B, Kazianis S, Borowsky R, Schartl M. Spontaneous melanoma formation in nonhybrid. Xiphophorus Cancer Res. 1995;55:159–165. [PubMed] [Google Scholar]
- 14.Fernandez AA, Bowser PR. Two cases of non-hybrid melanoma formation in. Xiphophorus nezahualcoyotl. J Fish Biol. 2008;72:292–300. [Google Scholar]
- 15.Weis S, Schartl M. The macromelanophore locus and the melanoma oncogene. Xmrk are separate genetic entities in the genome of Xiphophorus. Genetics. 1998;149:1909–1920. doi: 10.1093/genetics/149.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallman KD. Inheritance of melanophore patterns and sex determination in the Montezuma swordtail, Xiphophorus montezumae cortezi. Rosen. Zoologica. 1971;56:77–94. [Google Scholar]
- 19.Froschauer A, et al. Construction and initial analysis of bacterial artificial chromosome (BAC) contigs from the sex-determining region of the platyfish Xiphophorus maculatus. Gene. 2002;295:247–254. doi: 10.1016/s0378-1119(02)00684-4. [DOI] [PubMed] [Google Scholar]
- 17.Ling S, Kuah MK, Muhammad TST, Kolkovski S, Shu-Chien AC. Effect of dietary HUFA on reproductive performance, tissue fatty acid profile and desaturase and elongase mRNAs in female swordtail Xiphophorus helleri. Aquaculture. 2006;261:204–214. [Google Scholar]
- 18.Schartl M, Wilde B, Hornung U. Triplet repeat variability in the signal peptide sequence of the. Xmrk receptor tyrosine kinase gene in Xiphophorus fish. Gene. 1998;224:17–21. doi: 10.1016/s0378-1119(98)00520-4. [DOI] [PubMed] [Google Scholar]
- 20.Gimenez-Conti I, et al. A proposed classification scheme for Xiphophorus melanomas based on histopathologic analyses. Mar Biotechnol. 2001;3:S100–S106. doi: 10.1007/s10126001-0031-4. [DOI] [PubMed] [Google Scholar]
- 21.Ryan MJ, Hews D, Wagner WE., Jr Sexual selection on alleles that determine body size in the swordtail. Xiphophorus nigrensis. Behav Ecol Sociobiol. 1990;26:231–237. [Google Scholar]
- 22.Morris MR, Batra P, Ryan MJ. Male-male competition and access to females in the swordtail. Xiphophorus nigrensis Copeia. 1992;1992:980–986. [Google Scholar]
- 23.Bisazza A, Vaccari G, Pilastro A. Female mate choice in a mating system dominated by male sexual coercion. Behav Ecol. 2001;12:59–64. [Google Scholar]
- 24.Cummings M, Mollaghan D. Repeatability and consistency of female preference behaviours in a northern swordtail, Xiphophorus nigrensis. Anim Behav. 2006;72:217–224. [Google Scholar]
- 25.Kirkpatrick M, Ryan MJ. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. [Google Scholar]
- 26.Gutiérrez-Rodriguez C, Morris MR, Dubois NS, de Quieroz K. Genetic variation and phylogeography of the swordtail fish. Xiphophorus cortezi (Cyprinodontiformes, Poeciliidae) Mol Phylogenet Evol. 2007;43:111–123. doi: 10.1016/j.ympev.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Resnick DN, Miles DB. In: Ecology and Evolution of Livebearing Fishes (Poeciliidae) Meffe GK Jr, Snelson FF, editors. Englewood Cliffs, NJ: Prentice-Hall; 1989. pp. 125–148. [Google Scholar]
- 28.Morris MR, Ryan MJ. Age at sexual maturity of male Xiphophorus nigrensis in nature. Copeia. 1990;3:747–751. [Google Scholar]
- 29.Endler JA, Basolo AL. Sensory ecology, receiver bias and sexual selection. Trends Ecol Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds JD, Gross MR. Costs and benefits of female mate choice: Is there a lek paradox? Am Nat. 1990;136:230–243. [Google Scholar]
- 31.Westcott DA. Leks of leks: A role for hotspots in lek evolution? Proc R Soc London B. 1994;258:281–286. [Google Scholar]
- 32.Morris MR, Elias JA, Moretz JA. Defining the sexually selected male trait vertical bars in relation to female preference in the swordtail fish. Xiphophorus cortezi (Cyprinodontiformes, Poeciliidae) Ethology. 2001;107:827–837. [Google Scholar]
- 33.Brooks R. Negative genetic correlation between male sexual attractiveness and survival. Nature. 2000;406:67–70. doi: 10.1038/35017552. [DOI] [PubMed] [Google Scholar]
- 34.Franck D, Dikomey M, Schartl M. Selection and the maintenance of a colour pattern polymorphism in the green swordtail (Xiphophorus helleri) Behaviour. 2001;138:467–486. [Google Scholar]
- 35.Kallman KD, Atz JW. Gene and chromosome homology in fishes of the genus. Xiphophorus Zoologica. 1966;51:107–115. [Google Scholar]
- 36.Maynard Smith J, Haigh J. The hitch-hiking effect of a favourable gene. Genet Res. 1974;23:23–35. [PubMed] [Google Scholar]
- 37.Baker RH, Wilkinson GS. Phylogenetic analysis of sexual dimorphism and eye-span allometry in stalk-eyed flies (Diopsidae) Evolution. 2001;55:1373–1385. doi: 10.1111/j.0014-3820.2001.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie MG, et al. Sex and differentiation: Population genetic divergence and sexual dimorphism in Mexican goodeid fish. J Evol Biol. 2007;20:2048–2055. doi: 10.1111/j.1420-9101.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 39.Takahasi KR, Tajima F. Evolution of coadaptation in a two-locus epistatic system. Evolution. 2005;59:2324–2332. [PubMed] [Google Scholar]
- 40.Morris MR. Female preference for trait symmetry in addition to trait size in swordtail fishes. Proc R Soc London B. 1998;1399:907–911. [Google Scholar]
- 41.Morris MR, Casey K. Female swordtail fish prefer symmetrical sexual signal. Anim Behav. 1998;55:33–39. doi: 10.1006/anbe.1997.0580. [DOI] [PubMed] [Google Scholar]
- 42.Morris MR, Nicoletto PF, Hesselman E. A polymorphism in female preference for a polymorphic male trait in the swordtail fish Xiphophorus cortezi. Anim Behav. 2003;65:45–52. [Google Scholar]
- 43.Hoefler CH, Morris MR. A technique for the temporary application and augmentation of pigment patterns in fish. Ethology. 1999;195:431–438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.