Abstract
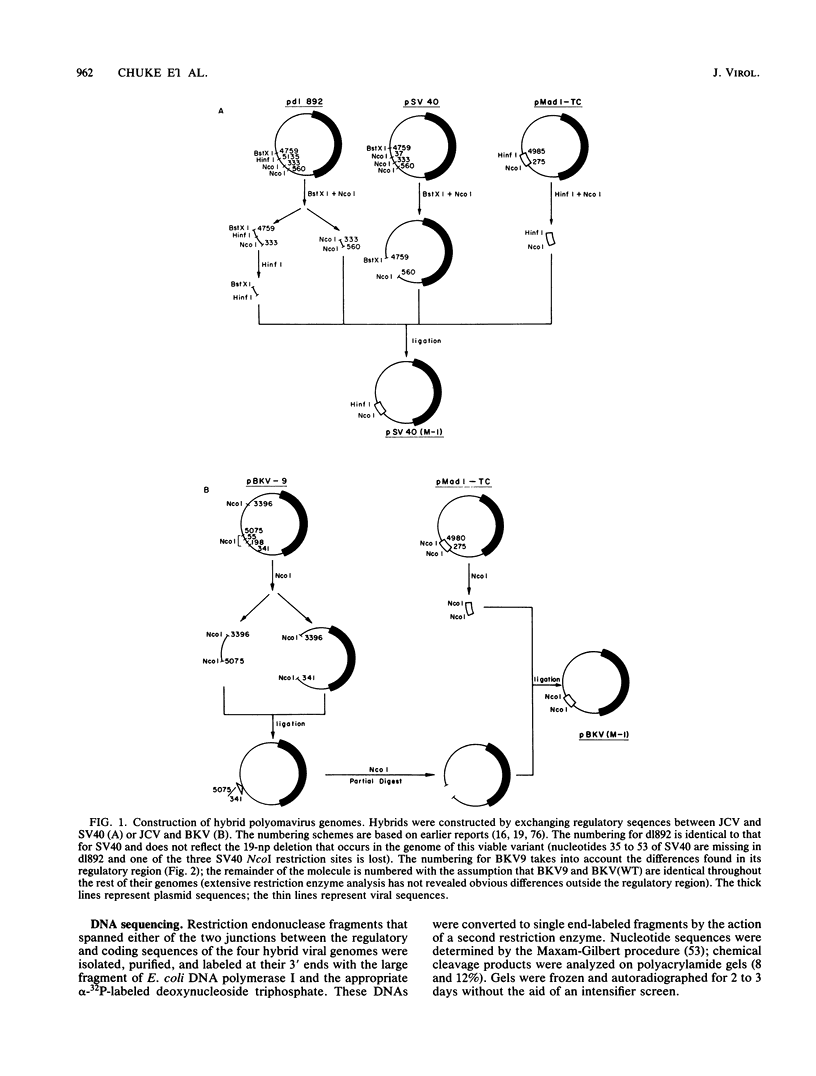
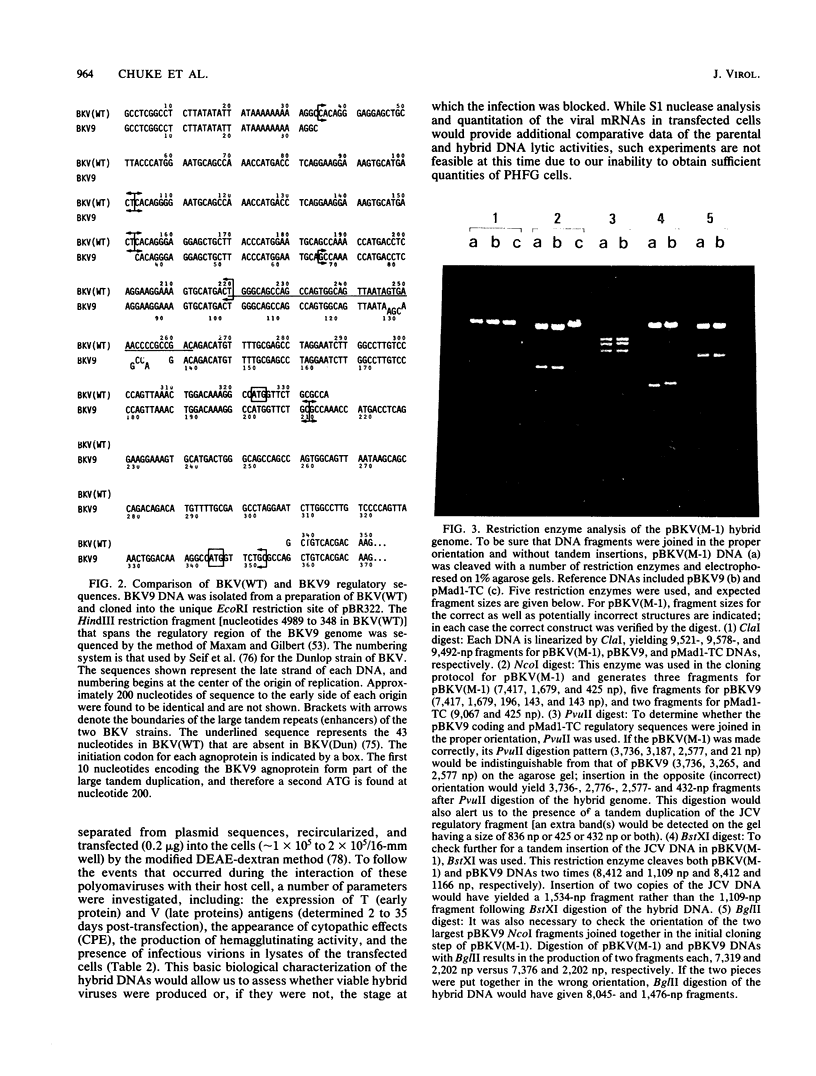
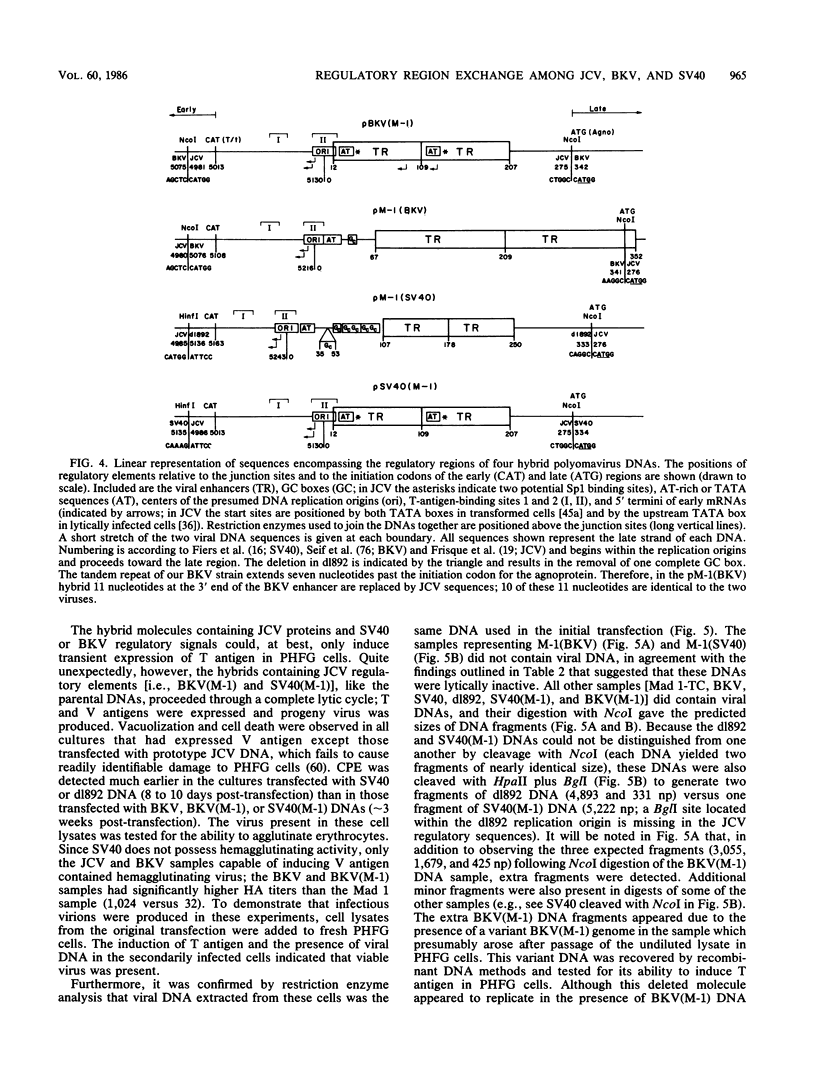
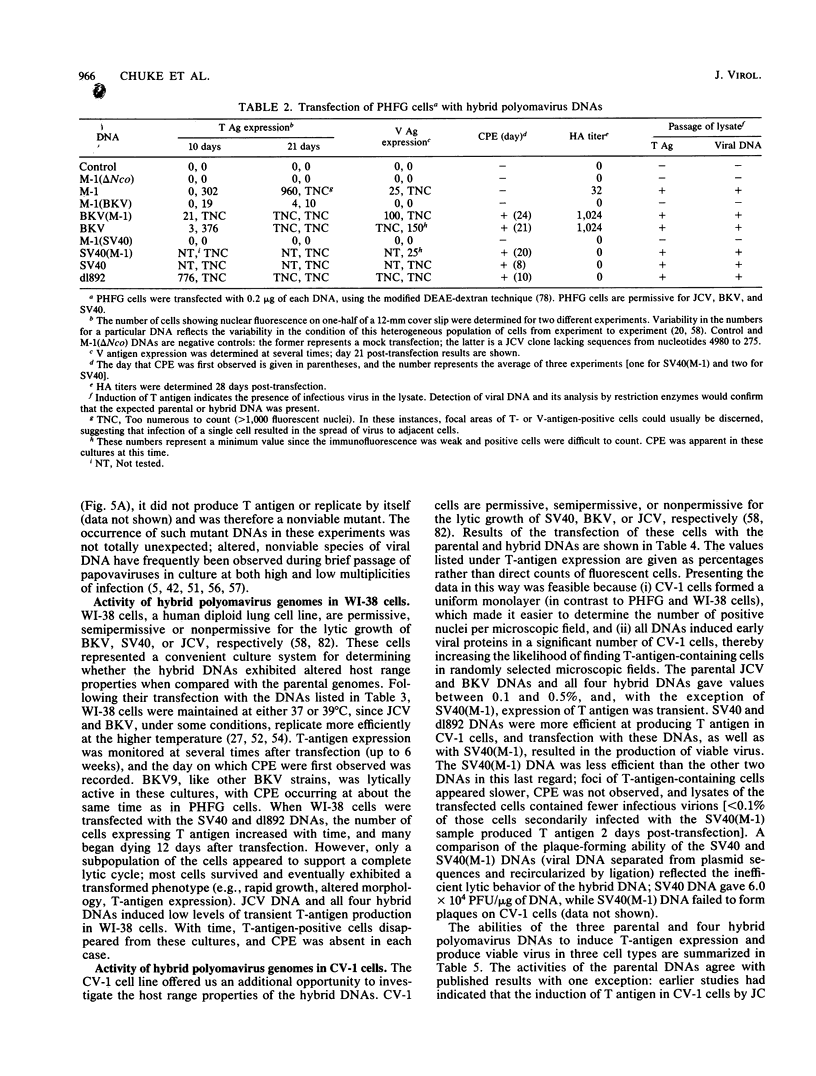
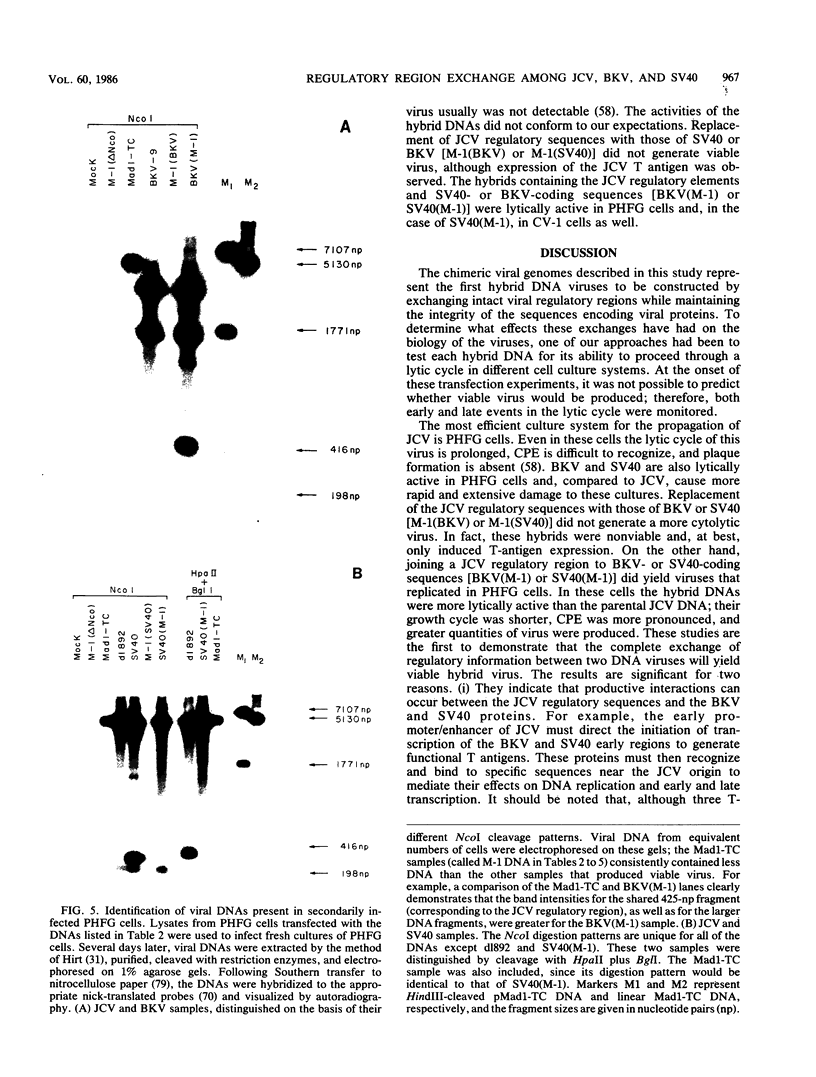
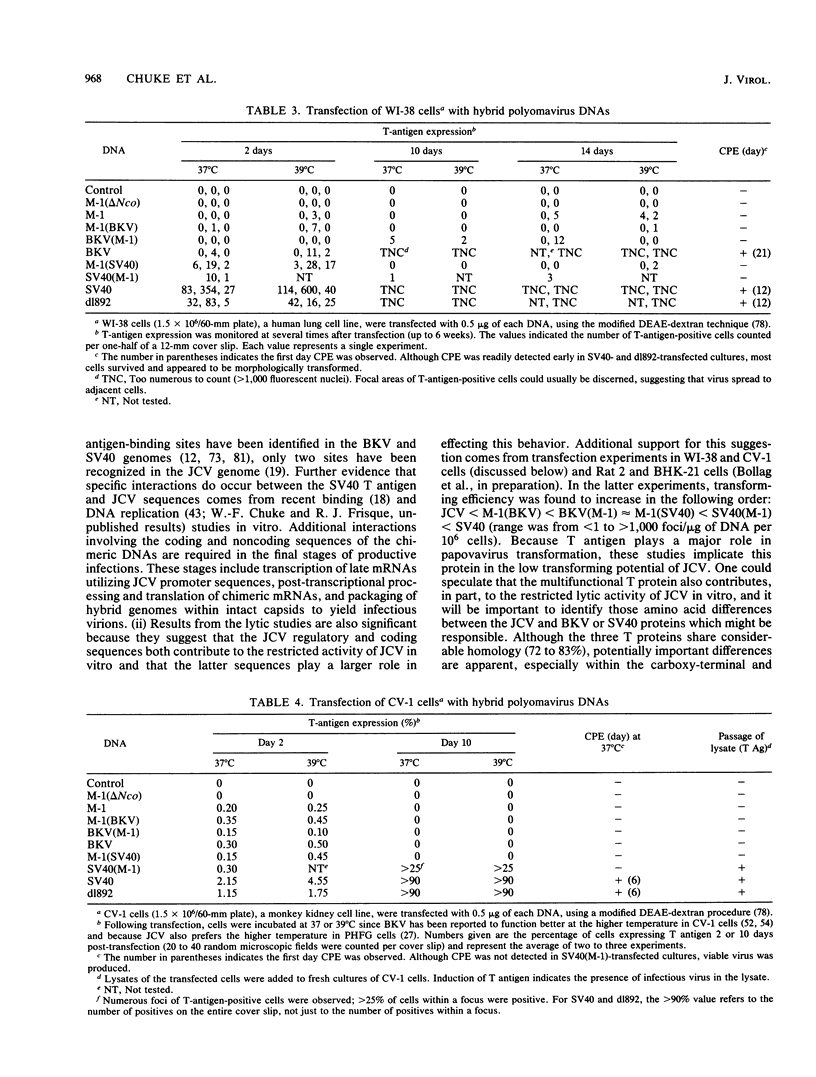
Several studies have suggested that certain unique features of the JC virus (JCV) regulatory region are responsible for the restricted lytic and transforming activities of this virus in vitro. To pursue this possibility, we have constructed hybrid polyomavirus genomes by exchanging the regulatory sequences of JCV, BK virus (BKV), and simian virus 40 (SV40). The host range of JCV was not expanded by the substitution of the BKV or SV40 regulatory signals; such hybrids were nonviable even in primary human fetal glial cells, the sole permissive cell for JCV. However, chimeric DNAs containing JCV regulatory sequences and BKV- or SV40-coding sequences were lytically active, indicating that the BKV and SV40 T proteins were capable of effectively interacting with the JCV replication and transcription signals to yield infectious hybrid viruses. Although JCV regulatory sequences and coding sequences both contributed to the restricted lytic activity of this virus, it appears that the latter sequences, most likely hose encoding the T protein, have a greater influence on this behavior.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Berg P. E., Popovic Z., Anderson W. F. Promoter dependence of enhancer activity. Mol Cell Biol. 1984 Aug;4(8):1664–1668. doi: 10.1128/mcb.4.8.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- Byrne B. J., Davis M. S., Yamaguchi J., Bergsma D. J., Subramanian K. N. Definition of the simian virus 40 early promoter region and demonstration of a host range bias in the enhancement effect of the simian virus 40 72-base-pair repeat. Proc Natl Acad Sci U S A. 1983 Feb;80(3):721–725. doi: 10.1073/pnas.80.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Host species specificity of polyomavirus DNA replication is not altered by simian virus 40 72-base-pair repeats. Mol Cell Biol. 1985 Jun;5(6):1534–1537. doi: 10.1128/mcb.5.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Tornow J., Clark R., Tjian R. Properties of the simian virus 40 (SV40) large T antigens encoded by SV40 mutants with deletions in gene A. J Virol. 1986 Feb;57(2):539–546. doi: 10.1128/jvi.57.2.539-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Martin J. D., Padgett B. L., Walker D. L. Infectivity of the DNA from four isolates of JC virus. J Virol. 1979 Nov;32(2):476–482. doi: 10.1128/jvi.32.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983 Apr;46(1):170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Regulatory sequences and virus-cell interactions of JC virus. Prog Clin Biol Res. 1983;105:41–59. [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Walker D. L. Transformation of primary hamster brain cells with JC virus and its DNA. J Virol. 1980 Jul;35(1):265–269. doi: 10.1128/jvi.35.1.265-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P., Frisque R. J., Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. doi: 10.1073/pnas.78.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Ahrens B. SV40 early mutants that are defective for viral DNA synthesis but competent for transformation of cultured rat and simian cells. Virology. 1982 Nov;123(1):78–92. doi: 10.1016/0042-6822(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Martin J. D., Padgett B. L., Walker D. L. Is progressive multifocal leukoencephalopathy a chronic disease because of defective interfering particles or temperature-sensitive mutants of JC virus? J Virol. 1982 Sep;43(3):1143–1150. doi: 10.1128/jvi.43.3.1143-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Sassone-Corsi P., Chambon P. An enhancer element is located 340 base pairs upstream from the adenovirus-2 E1A capsite. Nucleic Acids Res. 1983 Dec 20;11(24):8747–8760. doi: 10.1093/nar/11.24.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Rentier-Delrue F., Heilman C. A., Law M. F., Chowdhury K., Israel M. A., Takemoto K. K. Cloned human polyomavirus JC DNA can transform human amnion cells. J Virol. 1980 Dec;36(3):878–882. doi: 10.1128/jvi.36.3.878-882.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Selzer G., Salzman N. P. Mapping 5' termini of JC virus early RNAs. J Virol. 1986 May;58(2):651–654. doi: 10.1128/jvi.58.2.651-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Botchan M. Enhanced transformation by a simian virus 40 recombinant virus containing a Harvey murine sarcoma virus long terminal repeat. Mol Cell Biol. 1983 Mar;3(3):325–339. doi: 10.1128/mcb.3.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Martin J. D., Takemoto K. K., Howley P. M. The colinear alignment of the genomes of papovaviruses JC, BK, and SV40. Virology. 1979 Jul 30;96(2):576–587. doi: 10.1016/0042-6822(79)90113-2. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Brockman W. W., Nathans D. Evolutionary variants of simian virus 40: cloned substituted variants containing multiple initiation sites for DNA replication. Virology. 1975 Jul;66(1):53–69. doi: 10.1016/0042-6822(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- Mandl C. W., Frisque R. J. Characterization of cells transformed by the human polyomavirus JC virus. J Gen Virol. 1986 Aug;67(Pt 8):1733–1739. doi: 10.1099/0022-1317-67-8-1733. [DOI] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Genetic and biochemical analysis of transformation-competent, replication-defective simian virus 40 large T antigen mutants. J Virol. 1985 Jan;53(1):120–127. doi: 10.1128/jvi.53.1.120-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Simian virus 40 large T-antigen point mutants that are defective in viral DNA replication but competent in oncogenic transformation. Mol Cell Biol. 1984 Jun;4(6):1125–1133. doi: 10.1128/mcb.4.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee R. F., Nathans D. Simian virus 40 mutant T antigens with relaxed specificity for the nucleotide sequence at the viral DNA origin of replication. J Virol. 1984 Feb;49(2):386–393. doi: 10.1128/jvi.49.2.386-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., King D. M., Slauch J. M., Frisque R. J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985 Jan;53(1):306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., Padgett B. L., Walker D. L. Characterization of tissue culture-induced heterogeneity in DNAs of independent isolates of JC virus. J Gen Virol. 1983 Oct;64(Pt 10):2271–2280. doi: 10.1099/0022-1317-64-10-2271. [DOI] [PubMed] [Google Scholar]
- Mason D. H., Takemoto K. K. Complementation between BK human papovavirus and a simian virus 40 tsA mutant. J Virol. 1976 Mar;17(3):1060–1062. doi: 10.1128/jvi.17.3.1060-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyamura T., Takemoto K. K. Helper function for adenovirus replication in monkey cells by BK human papovavirus. Virology. 1979 Oct 15;98(1):279–282. doi: 10.1016/0042-6822(79)90549-x. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C., Wojcik J. B., Goguen C. A. Effect of the host cell on the generation of defective Simian Virus 40 during undiluted serial passages and persistent infection. Virology. 1981 Jan 30;108(2):525–530. doi: 10.1016/0042-6822(81)90462-1. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Carroll D. Amplification of papovavirus defectives during serial low multiplicity infections. Virology. 1981 Jul 30;112(2):800–803. doi: 10.1016/0042-6822(81)90330-5. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Hodach A. E., Chou S. M. JC Papovavirus in progressive multifocal leukoencephalopathy. J Infect Dis. 1976 Jun;133(6):686–690. doi: 10.1093/infdis/133.6.686. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Messing A., Brinster R. L. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985 Aug 1;316(6027):457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J Virol. 1985 May;54(2):569–575. doi: 10.1128/jvi.54.2.569-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Covey L., Scheller A., Gluzman Y. DNA-binding properties of simian virus 40 T-antigen mutants defective in viral DNA replication. Mol Cell Biol. 1983 Nov;3(11):1958–1966. doi: 10.1128/mcb.3.11.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rieth K. G., Di Chiro G., London W. T., Sever J. L., Houff S. A., Kornblith P. L., McKeever P. E., Buonomo C., Padgett B. L., Walker D. L. Experimental glioma in primates: a computed tomography model. J Comput Assist Tomogr. 1980 Jun;4(3):285–290. doi: 10.1097/00004728-198006000-00001. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N., Kress M., Gruss P., Khoury G. BK viral enhancer element and a human cellular homolog. Science. 1983 Nov 18;222(4625):749–755. doi: 10.1126/science.6314501. [DOI] [PubMed] [Google Scholar]
- Ryder K., DeLucia A. L., Tegtmeyer P. Binding of SV40 a protein to the BK virus origin of DNA replication. Virology. 1983 Aug;129(1):239–245. doi: 10.1016/0042-6822(83)90412-9. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Mutant of simian virus 40 large T-antigen that is defective for viral DNA synthesis, but competent for transformation of cultured rat cells. J Virol. 1982 Jun;42(3):854–864. doi: 10.1128/jvi.42.3.854-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Tornow J., Polvino-Bodnar M., Santangelo G., Cole C. N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985 Feb;53(2):415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982 Jun;42(3):978–985. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Nozawa A., Yuasa Y., Uchida S. Viable deletion mutant of human papovavirus BK that induces insulinomas in hamsters. J Virol. 1979 Dec;32(3):934–942. doi: 10.1128/jvi.32.3.934-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W. A small segment of polyoma virus DNA enhances the expression of a cloned beta-globin gene over a distance of 1400 base pairs. Nucleic Acids Res. 1981 Dec 11;9(23):6251–6264. doi: 10.1093/nar/9.23.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]