Abstract
Ceramide is among a number of potential lipotoxic molecules that are thought to modulate cellular energy metabolism. The heart is one of the tissues thought to become dysfunctional due to excess lipid accumulation. Dilated lipotoxic cardiomyopathy, thought to be the result of diabetes and severe obesity, has been modeled in several genetically altered mice, including animals with cardiac-specific overexpression of glycosylphosphatidylinositol (GPI)-anchored human lipoprotein lipase (LpLGPI). To test whether excess ceramide was implicated in cardiac lipotoxicity, de novo ceramide biosynthesis was inhibited pharmacologically by myriocin and genetically by heterozygous deletion of LCB1, a subunit of serine palmitoyltransferase (SPT). Inhibition of SPT, a rate-limiting enzyme in ceramide biosynthesis, reduced fatty acid and increased glucose oxidation in isolated perfused LpLGPI hearts, improved systolic function, and prolonged survival rates. Our results suggest a critical role for ceramide accumulation in the pathogenesis of lipotoxic cardiomyopathy.
Keywords: serine palmitoyltransferase, lipotoxicity, heart, glucose, fatty acid
Increasing caloric intake and greater body mass index produce a series of diseases due to tissue lipid accumulation. As storage capacity of adipocytes is exceeded, fat begins to infiltrate the liver, skeletal muscle, and heart (1, 2). In addition to triglyceride (TG), these tissues also have increased levels of FAs, fatty acyl CoA, diacylglycerol (DAG), and ceramide (3, 4).
Cardiac ceramide levels are elevated in models of cardiac lipotoxicity due to cardiac overexpression of long-chain acyl CoA synthase (5), PPARα (6), PPARγ (7), and FATP (8). Ceramide is produced by two pathways: 1) condensation of palmitoyl CoA with serine by serine palmitoyltransferase (SPT) (9), and 2) sphingomyelinase hydrolysis of sphingomyelin (SM) (10). Recently, Holland et al. (4) compared the effects of soybean- and lard-based emulsion infusions and suggested that the two types of lipids cause insulin resistance via different mechanisms. Only saturated fat increased tissue ceramide levels. Genetic and pharmacologic inhibition of the de novo ceramide biosynthetic pathway ameliorated insulin resistance.
Aside from ceramide, DAG and/or FA could be responsible for dilated lipotoxic cardiomyopathy. DAG activates protein kinase C in skeletal muscle and aorta, which is associated with insulin resistance (11–13). Excess FAs, especially saturated FA, increase reactive oxygen species (ROS) formation (14), create endoplasmic reticulum stress, and activate apoptotic pathways in several cell lines, including adipocytes and cardiomyocytes (15–17). In addition, high plasma FAs created by infusion of soybean lipid emulsions and heparin into animals activate toll-like receptors and NFκB (18).
We have previously studied mice with cardiomyocyte overexpression of a glycosylphosphatidylinositol (GPI) membrane-anchored form of lipoprotein lipase, denoted LpLGPI, that develop dilated cardiomyopathy (19). These hearts have an accumulation of ceramide. In this report, we show that ceramide accumulation plays a significant role in the progression of the dilated cardiomyopathy seen in LpLGPI mice. Using pharmacologic and genetic methods, we tested whether inhibition of SPT in normal and lipotoxic hearts altered cardiac ceramide concentrations and cardiac substrate utilization. Reduction of ceramide was associated with improved cardiac function, reversal of abnormal substrate use, and improved survival.
MATERIALS AND METHODS
Myriocin treatment of transgenic mice
Generation of LpLGPI transgenic mice has been described previously (19). LpLGPI and wild-type (WT) C57BL/6J mice (8–10 weeks old) were fed either a chow diet (Research Diets, New Brunswick, NJ) or chow mixed with myriocin (Sigma Adrich, St. Louis, MO, prepared by Research Diets)(0.3 mg/kg/day) for 6 weeks. Heterozygous LCB1 knockout (LCB1+/−) and LCB1+/− crossed with LpLGPI transgenic mice were fed a normal chow diet. For survival analyses, the same dose of myriocin was fed to 12 week-old LpLGPI (n = 14) and C57Bl/6J mice (n = 14) for 40 weeks. All animals were maintained on a 12 h light-dark cycle.
AC16 cardiomyocytes
Generation of a human cardiomyocyte cell line, AC16, has been described previously by Davidson et al. (20). Cells were grown in DMEM/F12 media containing 12.5% FBS and incubated at 37°C in an atmosphere containing 5% CO2/95% air. Cardiomyocytes that were 70–80% confluent were switched to DMEM/F12 media containing 1% FBS and treated with various concentrations of palmitic acid conjugated with 2% FA-free BSA in the presence or absence of 1 μM myriocin for 16 h. In other experiments, cells were treated with C6-ceramide for 16 h after incubation in DMEM/F12 containing 2% FA-free BSA. mRNA for RT-PCR was isolated using TRizol (Invitrogen).
Substrate metabolism in isolated working mouse hearts
Cardiac metabolism was measured in hearts isolated from 14 to 16 week-old male WT and LpLGPI mice (n = 5 per genotype). All hearts were prepared and perfused in the working mode, using protocols that have been previously described (21–24).
Heart and plasma lipids
Heart lipids were extracted as described by Folch, Lees, and Sloane Stanley (25). TG and cholesterol concentrations in hearts and plasma were measured using TG and cholesterol enzymatic assay kits (Infinity, Louisville, CO); FFAs were measured using NEFA C kits (Wako Chemicals, Richmond, VA). Tissue lipids were normalized by protein concentration. Cardiac and plasma SM levels were measured enzymatically as described previously (26). Cardiac ceramide and DAG levels were determined using the DAG kinase method (27). Cardiac acyl CoAs were measured by liquid chromatography/tandem mass spectrometry using Perkin Elmer S200 HPLC (Perkin Elmer, Waltham, MA) and API 3000 (Applied Biosystems, Foster City, CA) and analyzed by Analyst 1.4.1 software (Applied Biosystems).
Echocardiography
Two-dimensional echocardiography was performed on conscious 14 to 16 week-old male (n = 10–12 per group) mice (Sonos 5500 system; Philips Medical Systems, Andover, MA) (28). Echocardiographic images were recorded in a digital format. Images were then analyzed off-line by a single observer blinded to the murine genotype (29).
Gene expression
Quantitative real-time PCR was performed with SYBR Green PCR Core Reagents (Applied Biosystems). Incorporation of the SYBR green dye into the PCR products was monitored in real time with an Mx3000 sequence detection system (Stratagene, La Jolla, CA). Samples were normalized against β-actin. The sequences of the primers are provided in supplementary Table I.
Western blots
Isolated heart tissues were homogenized in PBS containing protease inhibitors and phosphatase inhibitors (Roche, Indianapolis, IN). Membrane and cytosolic fractions were separated by ultracentrifugation. Thirty micrograms from each fraction was applied to SDS-PAGE and transferred onto nitrocellulose membranes. GLUT4 and GLUT1 proteins in each fraction were detected by mouse-specific antibodies (Chemicon, Temecula, CA). Thirty micrograms of whole-tissue extracts was applied for Western blot analyses to examine phosphorylated (p) GSK-3β and pAKT, which were detected by mouse-specific antibodies (Cell Signaling, Danvers, MA).
Glucose uptake studies
Basal glucose uptake was measured in hearts following an intravenous administration of 3 μCi of 2-deoxy-D-[1-14C]glucose (PerkinElmer Life Sciences). Blood was collected 30 s and 5, 30, and 60 min following injection. At 60 min, hearts were perfused with PBS, tissues were excised, and radioactive counts were measured.
Histology
Hearts from 6 h-fasted mice were fixed in 10% formalin for 24 h and mounted on paraffin. Midventricular sections were stained with Schiff Reagent (Polyscientific, Bay Shore, NY) to identify glycogen in hearts [periodic acid-Schiff (PAS) staining] (30). The specificity of glycogen staining was confirmed by treating sections with diastase to digest tissue glycogen, followed by regular PAS staining (30). Sectioned heart tissues were stained for DNA fragmentation by a terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining protocol according to the manufacturer's instructions (R and D Systems, Minneapolis, MN).
Glycogen analysis
Hearts were hydrolyzed with 1 M NaOH at 65°C for 2 h, and glycogen was precipitated by 66% cold ethanol. Cardiac glycogen was digested by amyloglucosidase at 45°C for 2 h (31). The produced glucose was measured enzymatically by an AutoGlucose kit (Wako Chemicals).
Statistics
Cardiac metabolism data in isolated working hearts were analyzed by ANOVA, and significance was evaluated by the Fisher Least Protected Squares test. Differences among groups were determined using one-way ANOVA with post hoc Dunnett's t-test. A value of P < 0.05 was regarded as a significant difference.
RESULTS
Myriocin lowers sphingolipids in plasma and heart
During myriocin treatment for 6 weeks, no significant changes in body weight, plasma glucose, cholesterol, TG, and FFA levels were observed (Table 1). Myriocin lowered plasma SM levels significantly in WT and LpLGPI mice.
TABLE 1.
Body and heart weight, glucose, and plasma lipid measurements in WT and LpLGPI mice
| WT | WT-myr | LpLGPI | LpLGPI-myr | |
|---|---|---|---|---|
| Body wt. (g) | 29.8 ± 2.1 | 27.7 ± 1.5 | 29.5 ± 1.5 | 30.6 ± 3.5 |
| Heart wt./body wt. (%) | 0.58 ± 0.02 | 0.53 ± 0.06 | 0.64 ± 0.02a | 0.51 ± 0.02a,b |
| Glucose (mg/dl) | 108 ± 3.9 | 103 ± 6.0 | 102 ± 7.7 | 104 ± 6.4 |
| TG (mg/dl) | 70.5 ± 12.7 | 63.9 ± 4.8 | 78.0 ± 9.5 | 73.5 ± 2.4 |
| Cholesterol (mg/dl) | 69.1 ± 1.75 | 74.5 ± 3.4 | 64.4 ± 4.7 | 74.1 ± 5.5 |
| FFA (mM) | 0.61 ± 0.12 | 0.44 ± 0.13 | 0.51 ± 0.08 | 0.43 ± 0.05 |
| SM (μg/ml) | 24.9 ± 2.3 | 12.1 ± 1.6a | 21.1 ± 5.0 | 9.1 ± 2.0a,b |
LpLGPI, glycosylphosphatidylinositol (GPI)-anchored human lipoprotein lipase; SM, sphingomyelin; myr, myriocin; TG, triglyceride; WT, wild type. Results are given as mean ± SEM, n = 8–10.
P < 0.05 (vs. WT).
P < 0.05 (vs. LpLGPI).
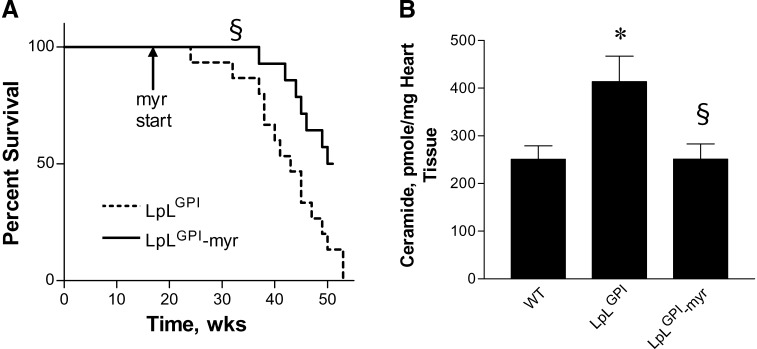
TG and cholesterol levels in mouse hearts were not changed by myriocin treatment or overexpression of LpL (Table 2). SM levels were 45% less in hearts of myriocin-treated LpLGPI than in untreated mice of the same genotype. In contrast, SM levels were not altered in WT mice (Table 2). Cardiac ceramide levels were increased approximately 45% in LpLGPI mouse hearts. Treatment with myriocin reduced ceramide to control levels (Table 2). Myriocin-treated WT mice did not show alteration of cardiac ceramide. This appeared to be due to upregulation of SPT subunits LCB1 and LCB2 as a compensatory mechanism to maintain basal sphingolipid pools (Table 3). DAG was also elevated in LpLGPI hearts, but myriocin had no effect on cardiac DAG levels (Table 2). Acyl CoAs were reduced in LpLGPI hearts, and myriocin treatment restored them to WT levels (Table 2). Thus, myriocin lowered cardiac SM and ceramide without changing TG, cholesterol, and DAG levels. Moreover, the improved hearts had a normalization of the reduced acyl CoA. We assumed that this was a secondary change due to reduced FA oxidation (see below).
TABLE 2.
Cardiac lipid measurement in WT and LpLGPI mice
| WT | WT-myr | LpLGPI | LpLGPI-myr | |
|---|---|---|---|---|
| TG (μg/mg) | 16.8 ± 6.5 | 19.1 ± 9.0 | 18.1 ± 9.5 | 22.2 ± 8.0 |
| Cholesterol (μg/mg) | 4.2 ± 1.0 | 4.2 ± 0.18 | 5.0 ± 1.0 | 5.3 ± 0.7 |
| Fatty acyl CoA (pmol/mg) | 81.4 ± 6.2 | 130.6 ± 4.8a | 41.4 ± 11.2a | 122.1 ± 4.8a,b |
| SM (μg/mg) | 15.2 ± 2.5 | 14.6 ± 2.1 | 22.2 ± 1.9a | 12.2 ± 2.3a,b |
| Ceramide (pmol/mg) | 210.5 ± 11.3 | 216.3 ± 22.6 | 305.9 ± 21.1a | 151.8 ± 9.9a,b |
| DAG (pmol/mg) | 184.4 ± 4.9 | 176.4 ± 8.5 | 233.5 ± 5.6a | 228.0 ± 8.7a |
DAG, diacylglycerol. Results are given as mean ± SEM, n = 8–10.
P < 0.05 (vs. WT).
P < 0.05 (vs. LpLGPI).
TABLE 3.
Expression of SPT subunits and heart failure markers in WT and LpLGPI mice
| 4 month-old |
||||
|---|---|---|---|---|
| Gene | WT | WT-myr | LpLGPI | LpLGPI-myr |
| LCB1 | 1.00 ± 0.14 | 2.19 ± 0.19a | 0.61 ± 0.05a | 1.19 ± 0.19b |
| LCB2 | 1.00 ± 0.14 | 2.32 ± 0.38a | 0.87 ± 0.04 | 1.61 ± 0.22b |
| ANF | 1.00 ± 0.09 | 0.81 ± 0.22 | 1.74 ± 0.20a | 0.47 ± 0.15a,b |
| BNP | 1.00 ± 0.22 | 1.14 ± 0.17 | 2.27 ± 0.45a | 0.51 ± 0.12a,b |
SPT, serine palmitoyltransferase. Results are given as mean ± SEM, n = 8–10.
P < 0.05 (vs. WT).
P < 0.05 (vs. LpLGPI).
Myriocin improves cardiac function in LpLGPI mice and reduces expression of heart failure markers
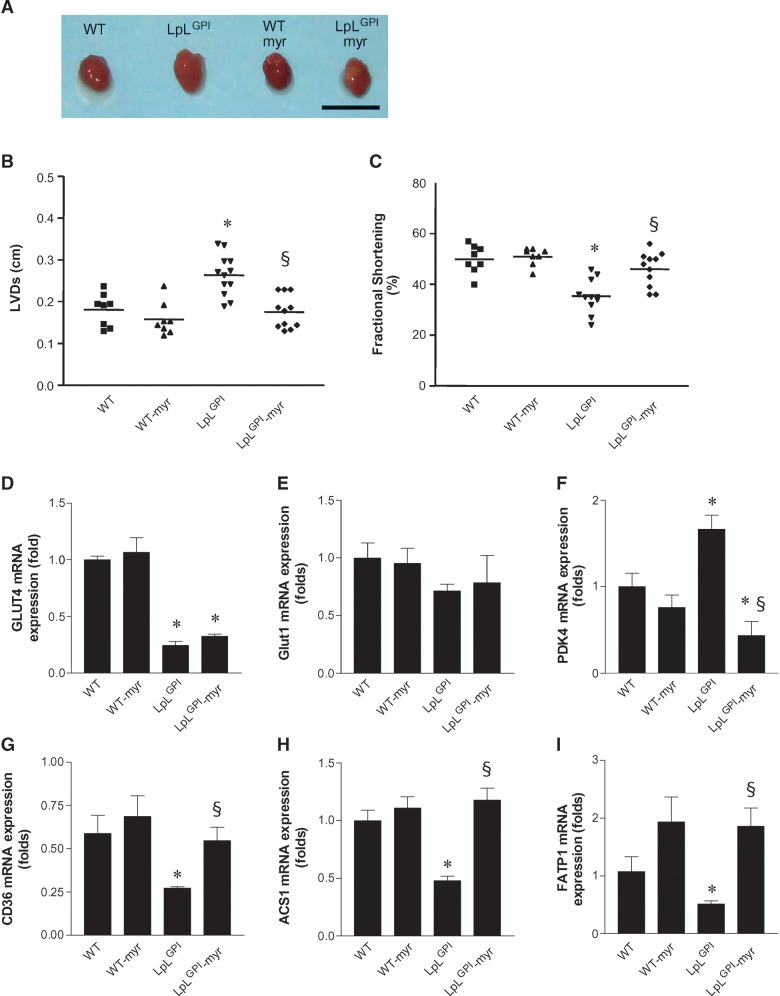
LpLGPI hearts were hypertrophied with an increase in heart/body weight (Table 1; Fig. 1A). Myriocin treatment of these mice returned heart weights to WT levels. Echocardiography also revealed that hearts from chow-fed LpLGPI mice had left ventricular dilatation, increased left ventricular systolic diameter (LVD), and decreased fractional shortening, compared with hearts from chow-fed WT mice, as previously reported (19). The LVDs of myriocin-treated LpLGPI mice were comparable to those of WT mice and were smaller than those of untreated LpLGPI mice (0.18 ± 0.014 cm vs. 0.24 ± 0.014 cm) (Fig. 1B). Reduced fractional shortening in LpLGPI mice was corrected by myriocin (46.1 ± 2.0% in myriocin-LpLGPI vs. 35.5 ± 2.0% in LpLGPI mice) (Fig. 1C).
Fig. 1.
Prevention of cardiomyopathy by myriocin in glycosylphosphotidylinositol (GPI)-anchored human lipoprotein lipase (LpLGPI) hearts and regulation of cardiac gene expression in LpLGPI mice. Eight week-old wild-type (WT) and LpLGPI mice were fed chow or chow with 0.3 mg myriocin/kg/day for 6 weeks. Hearts from mice are shown (A). Chow-fed LpLGPI mouse hearts were enlarged, but hearts from LpLGPI mice fed myriocin-mixed chow were normal size. Left ventricular systolic diameter (LVD) (B) of the heart and fractional shortening (C) were measured by echocardiography as described in Materials and Methods (n = 8–12 each group). mRNA expression in the heart was measured by RT-PCR as described in Materials and Methods. Glucose-metabolizing genes GLUT4 (D), GLUT1 (E), and PDK4 (F). FA transporters CD36 (G), ACS1 (H), and FATP1 (I). Bar indicates 1 cm. *P < 0.05 versus WT; §P < 0.05 versus LpLGPI.
We assessed whether myriocin affected markers of cardiac failure. ANF and BNP gene expression was increased in LpLGPI hearts when compared with WT, and myriocin treatment reduced mRNA levels of these genes even further than those of WT (Table 3). Thus, treatment with myriocin that specifically reduced cardiac ceramide and had no impact on the levels of other major lipids improved cardiac function and reduced gene expression of cardiac failure markers.
Effects of myriocin on cardiac gene expression
We assessed whether myriocin affected expression of metabolic genes in WT and LpLGPI hearts. GLUT4 was downregulated in LpLGPI hearts, and myriocin had no effect (Fig. 1D). GLUT1 expression was not altered by LpL overexpression or myriocin treatment (Fig. 1E). PDK4 mRNA was increased in hearts of LpLGPI mice (Fig. 1F). Increased PDK4 increases phosphorylation of pyruvate dehydrogenase and would be expected to reduce glucose oxidation rates in isolated hearts. Myriocin restored upregulation of PDK4 in LpLGPI hearts to WT levels. Expression levels of genes that regulate FA oxidation, such as PPAR-α, CPT-1, and ACO, were not changed in LpLGPI hearts by myriocin treatment (see supplementary Fig. I). Expression of fatty acid transporters such as CD36, acyl CoA synthase1 (ACS1), and fatty acid transport protein1 (FATP1) were downregulated in LpLGPI hearts (Fig. 1G, H, I). Myriocin restored them to the levels of WT.
Myriocin corrects the increased FA and reduced glucose oxidation in LpLGPI hearts
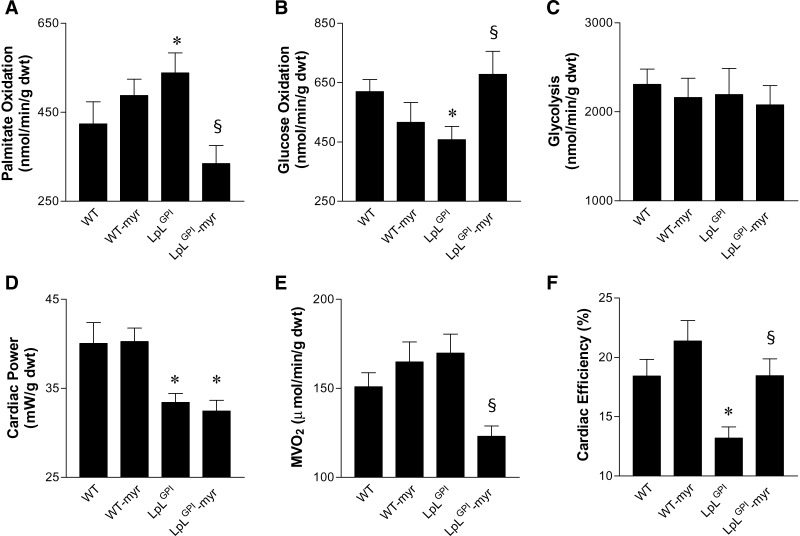
Cardiac metabolism was measured in hearts from 14 to 16 week-old male WT and LpLGPI mice. All hearts were prepared and perfused in the working mode, using previously described protocols (21–24). Palmitate oxidation was increased by 27% and glucose oxidation was decreased by 26% in LpLGPI hearts relative to controls (Fig. 2A, B). Myriocin treatment reduced palmitate oxidation and increased glucose oxidation rates in LpLGPI hearts to the levels of WT (Fig. 2A, B). The average rates of glycolysis were not altered (Fig. 2C). Thus, hearts of LpLGPI mice develop altered substrate utilization with increased reliance on FAs, and myriocin altered substrate utilization by reducing FA oxidation and increasing glucose oxidation in LpLGPI hearts. In isolated working hearts, we did not detect any improvement in cardiac power following treatment of LpLGPI mice with myriocin (Fig. 2D). However, there was a significant reduction in MVO2 (Fig. 2E). This normalized cardiac efficiency (Fig. 2F), which was reduced in hearts from nontreated mice. These data suggest that myriocin enhanced myocardial energetics by maintaining cardiac performance at a lower oxygen cost. The gene expression data suggest that the increase in oxidation rates of exogenous palmitate in LpLGPI hearts might be the consequence of the PDK4-mediated reduction in pyruvate flux, which would reduce glucose oxidation.
Fig. 2.
Cardiac metabolism in isolated working hearts. Cardiac metabolism was measured in hearts isolated from 14 to 16 week-old male WT and LpLGPI mice in the presence or absence of myriocin (n = 5 per genotype). All hearts were prepared and perfused in the working mode. Palmitate oxidation (A) was determined in separate perfused hearts by measuring the amount of 3H2O released from [9,10-3H]palmitate. Glucose oxidation (B) was determined by trapping and measuring 14CO2 released by the metabolism of [14C]glucose, and glycolysis (C) was determined by measuring the amount of 3H2O released from the metabolism of exogenous [5-3H]glucose. Cardiac power (D) was measured in hearts in which palmitate and glucose oxidation rates were determined (n = 5 each group). MVO2 (E) and cardiac efficiency (F) were measured during palmitate oxidation. *P < 0.05 versus WT; §P < 0.05 versus LpLGPI.
In vivo uptake of glucose in WT and myriocin-treated LpLGPI hearts
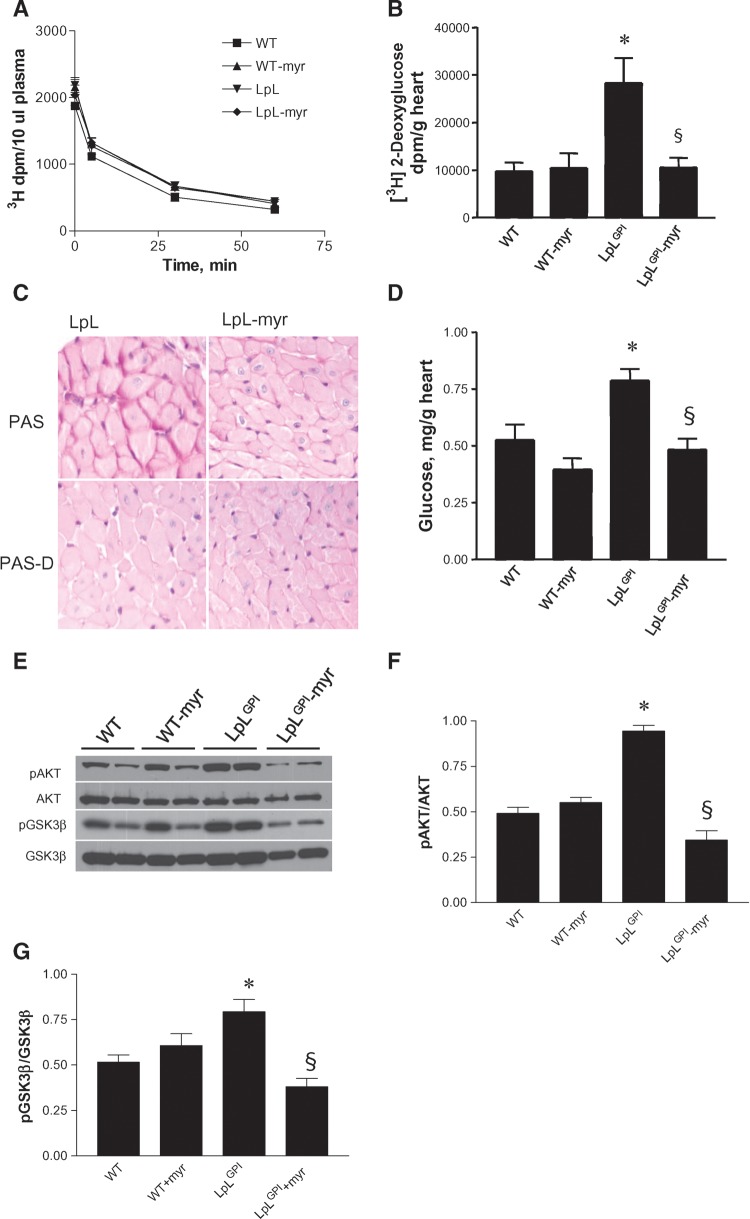
We examined whether in vivo glucose uptake was affected by myriocin using 2-deoxy-d-[1-3H]glucose (2-DG). There was no difference in plasma glucose clearance between WT and LpLGPI mice (Fig. 3A). In contrast to findings in isolated hearts (reduced glucose oxidation and normal rates of glycolysis), in vivo glucose uptake was increased in LpLGPI mouse hearts (Fig. 3B). Myriocin reduced in vivo glucose uptake in LpLGPI hearts to the levels of WT. The estimation of glycolytic rates in isolated hearts is based on the appearance of 3H, which is released as water at the final step in glycolysis, whereas 2-DG measures glucose transport and phosphorylation. If exogenous glucose uptake is increased but the glucose is not being oxidized, more glucose might be converted to glycogen; this would account for the differences between measurements obtained in vivo versus those obtained in isolated hearts.
Fig. 3.
Glucose uptake and glycogen accumulation. Basal glucose uptake was measured in hearts following an intravenous administration of 3 μCi of 2-deoxy-d-glucose (2-DG). Blood was collected 30 s and 5, 30, and 60 min following injection to determine plasma clearance (A) of 2-DG. At 60 min, hearts were perfused with PBS, tissues were excised, and radioactive counts were measured to calculate glucose uptake (n = 6–8 each group) (B). Cardiac glycogen in LpLGPI heart was identified by periodic acid-Schiff (PAS) staining (C) of midventricular cross sections. Alternatively, the sections were treated with diastase to digest cardiac glycogen for comparison (PAS-D). Cardiac glycogen was measured biochemically as described in Materials and Methods (n = 10 each group); glucose released from glycogen hydrolysis is shown in D. PAKT and pGSK-3β in WT and LpLGPI hearts were immunoblotted (E) and quantified by densitometry (F–G). *P < 0.05 versus WT; §P < 0.05 versus LpLGPI.
Histological analysis of heart tissue by PAS staining demonstrated that hearts of LpLGPI mice had greater glycogen stores than did hearts of myriocin-treated WT and LpLGPI mice (Fig. 3C). In contrast, hearts of LpLGPI mice treated with myriocin had a pattern of glycogen staining that was similar to that of WT mouse hearts (Fig. 3C). To confirm this, we biochemically measured cardiac glycogen. Glycogen content in hearts of LpLGPI mice was 49% greater than that in hearts of WT mice (Fig. 3D). Hearts of myriocin-treated LpLGPI mice had glycogen levels similar to those in hearts of WT mice (Fig. 3D). Thus, in hearts of LpLGPI mice, glucose carbons appear to preferentially accumulate as glycogen; myriocin treatment reversed this process.
Phosphorylation of AKT and GSK-3β
To assess whether the AKT/GSK-3β pathway was involved in hypertrophy and glycogen synthesis in hearts of LpLGPI mice, pAKT and its downstream target, pGSK-3β, were examined by Western blot. PAKT and pGSK-3β were elevated in hearts of LpLGPI mice, compared with hearts of WT mice (Fig. 3E–G). Myriocin treatment decreased pAKT and pGSK-3β levels in hearts of LpLGPI mice by 63% and 53%, respectively (Fig. 3E–G). GSK-3β blocks cardiac hypertrophy and inhibits glycogen synthesis. GSK-3β inactivation by phosphorylation would be predicted to promote cardiac hypertrophy and increase glycogen synthesis in the hearts of LpLGPI mice.
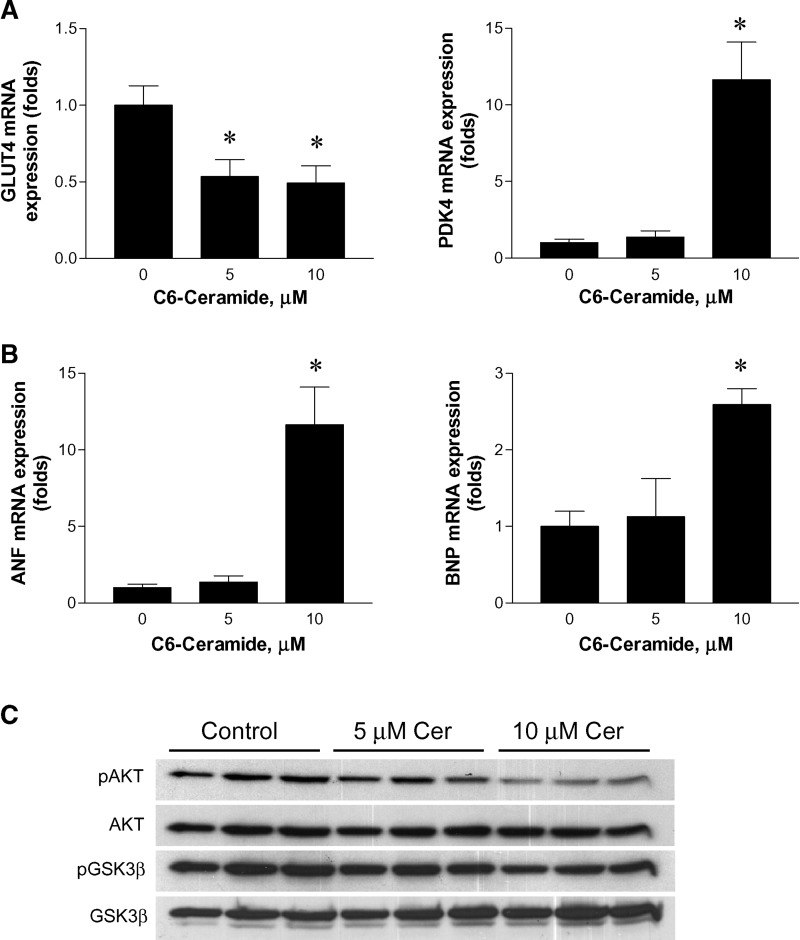
Effects of ceramide on the expression of glucose-metabolizing genes and cardiac failure markers in AC16 human cardiomyocytes
Using recently developed human cardiomyocyte AC16 cells (20), we tried to reproduce conditions associated with lipotoxic cardiomyopathy and then examine whether ceramide alters cardiac substrate utilization in a direct manner, as opposed to metabolic changes being secondary to cardiac dysfunction. To do this, we grew cells in the presence or absence of C6-ceramide and examined the gene expression. Ceramide downregulated GLUT4 expression and upregulated PDK4 (Fig. 4A). Ceramide also upregulated ANF and BNP (Fig. 4B). These changes in gene expression are consistent with the in vivo gene expression profile in LpLGPI mice with elevated cellular levels of ceramide. In contrast, C6-ceramide treatment of AC16 cardiomyocytes decreased pAKT and pGSK-3β in a concentration-dependent manner (Fig. 4C); these decreases were opposite to the in vivo results.
Fig. 4.
Effects of ceramide in AC16 cells. AC16 cardiomyocytes were incubated with C6-ceramide at various concentrations for 16 h. mRNA expression of GLUT4 and PDK4 (A), and ANF and BNP (B) were analyzed by RT-PCR (n = 3). PAKT and pGSK-3β in AC16 cells were immunoblotted (C). *P < 0.05 versus no-treatment control.
Myriocin decreases mortality rates in LpLGPI mice
Myriocin was administered to LpLGPI mice to assess whether improved cardiac function was associated with improved survival of this lipotoxic cardiomyopathic animal model. First, we sought to determine whether ceramide accumulation increased apoptosis in LpLGPI hearts and whether limiting apoptosis could account in part for the beneficial effect of myriocin. However, we did not detect any increase in TUNEL-positive staining in the hearts of WT versus LpLGPI mice at this age (14–16 weeks; see supplementary Fig. II). Thus, lipid-induced apoptosis is not the major factor contributing to cardiac dysfunction at this age. As described previously, LpLGPI mice had early deaths from cardiac dysfunction (19). Myriocin treatment improved survival rates of LpLGPI mice but did not restore survival rates to those of WT mice (Fig. 5A). We checked whether LpLGPI mice might have tachyphylaxis to myriocin by measuring ceramide levels in 6 month-old mice. Cardiac ceramide was elevated even more, 66%, in 6 month-old LpLGPI mice. As in the younger animals, the long-term myriocin treatment reduced the elevated ceramide in LpLGPI hearts to WT levels (Fig. 5B), so tachyphylaxis to myriocin did not occur. These results suggest that although sphingolipid biosynthesis is one of the major factors contributing to the cardiac dysfunction observed in lipotoxic LpLGPI mice, additional factors are likely to contribute as well.
Fig. 5.
Mortality rates and cardiac ceramide levels of LpLGPI mice. LpLGPI mice were fed control or myriocin-mixed chow (n = 14 each group) at the age of 12 weeks. Mortality of the mice was documented over the subsequent 40 weeks (A). Cardiac ceramide levels in 6 month-old mice were measured by liquid chromatography/tandem mass spectrometry (B) (n = 5 each group). *P < 0.05 versus WT; §P < 0.05 versus LpLGPI. The mortality of WT mice has been reported previously (19); >90% of these mice survive over 1 year.
Heterozygous deletion of SPT improves cardiac function of LpLGPI mice
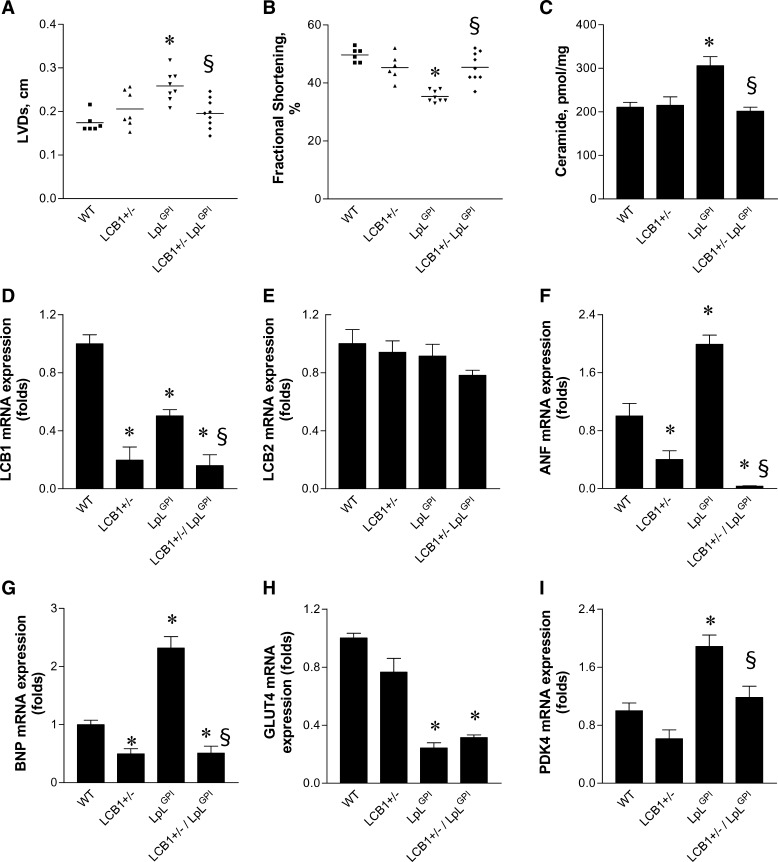
SPT is composed of two different subunits, LCB1 and LCB2. Heterozygous deletion of LCB1 or LCB2 results in reduced hepatic and plasma ceramide levels (32). To exclude nonspecific pharmacological effects of myriocin, we crossed LpLGPI mice with whole-body heterozygous LCB1 knockout mice (LCB1+/−/LpLGPI). Hearts from 14 to 16 week-old LCB1+/−/LpLGPI mice demonstrated improved systolic function and fractional shortening when compared with LpLGPI hearts (Fig. 6A, B). Cardiac ceramide levels in LCB1+/−/LpLGPI mice were decreased to the levels of WT mice (Fig. 6C). Heterozygous deletion of LCB1 downregulated LCB1 mRNA in the heart with intact LCB2 expression (Fig. 6D, E). Downregulation of LCB1 normalized elevated expression levels of ANF, BNP, and PDK4 in LpLGPI hearts, but did not alter GLUT4 expression (Fig. 6F–I). Thus, reducing cardiac ceramide concentrations by heterozygous deletion of an SPT subunit also improved the lipotoxic cardiomyopathy of LpLGPI transgenic mice.
Fig. 6.
Prevention of cardiomyopathy and regulation of cardiac gene expression by heterozygous deletion of LCB1 in LpLGPI hearts. LVDs of the heart (A) and fractional shortening (B) in WT, heterozygous LCB1 knockout (LCB1+/−), LpLGPI, and LCB1+/−/LpLGPI mice (14–16 weeks old) were measured by echocardiography as described in Materials and Methods (n = 6–8 each group). Cardiac ceramide content (C) was measured by diacylglycerol kinase assay followed by TLC and autoradiography (n = 5 per genotype). Gene expression of LCB1 (D), LCB2 (E), ANF (F), BNP (G), GLUT4 (H) and PDK4 (I) was measured by real-time PCR and normalized by β-actin (n = 5 each group). *P < 0.05 versus WT; §P < 0.05 versus LpLGPI.
DISCUSSION
Several lipids are likely to be toxic to tissues. We specifically explored whether ceramide, a lipid associated with cellular apoptosis, abnormal insulin signaling, and inflammation, mediated the lipotoxic phenotype in LpLGPI mice. Our data show the following: 1) SPT inhibition reduced ceramide content of LpLGPI but not WT hearts; 2) abnormal cardiac substrate utilization was corrected; 3) this was associated with improved cardiac function; 4) survival was improved; 5) and a partial genetic deletion of SPT1 also was beneficial.
By selectively altering the ceramide biosynthetic pathway using pharmacologic and genetic methods, we showed that ceramide is a cardiac toxin in the LpLGPI heart. Myriocin, a specific inhibitor of SPT, has been used to reduce ceramide levels in cancer cells (33), in models of atherosclerosis (34, 35), and insulin-resistant models (4). We tested whether myriocin decreased ceramide concentrations in WT and LpLGPI mouse hearts. Plasma ceramide levels were reduced by this treatment. Although myriocin restored cardiac ceramide levels in LpLGPI mice to WT levels, myriocin had no effect on levels in WT mice. This appears to be due to a compensatory activation of SPT in the WT mice, because gene expression of LCB1 and LCB2 was upregulated by myriocin treatment. LpLGPI hearts had reduced SPT expression in the presence of increased tissue ceramide levels. This suggests that excess substrate availability increased cardiac ceramide synthesis. In contrast, myriocin treatment of WT mice did not alter hepatic SPT expression (34). Therefore, either liver ceramide content is unaltered by myriocin or cardiac and liver SPT regulation differ. Despite the lack of change in cardiac levels of cholesterol or FFA, myriocin-treated LpLGPI mice had improved cardiac function. These data implicate ceramide as a basis for cardiac disease.
Lipid analysis of the LpLGPI hearts demonstrated elevated cardiac DAG and ceramide. In contrast, long-chain fatty acyl CoA levels were decreased, probably due to increased cardiac FA oxidation. Reduced conversion of acyl CoAs into ceramide by SPT inhibition might cause increased cardiac acyl CoA pools. However, the SPT reaction utilizes saturated palmitoyl CoA specifically. The fact that not only saturated acyl CoAs but also short-chain and unsaturated acyl CoAs were reduced in LpLGPI hearts (data not shown) suggests that these reductions were secondary to increased cardiac FA oxidation. LpLGPI hearts oxidize more FAs and less glucose, and myriocin treatment reversed cardiac energy metabolism in isolated LpLGPI hearts to that of WT. This was associated with no changes in the usual FA oxidation enzymes (CPT1 and ACO) and no changes in GLUT1 or GLUT4. PDK4, a PPAR-regulated gene that modulates glucose oxidation by allowing pyruvate to enter the tricarboxylic acid cycle, was increased in LpLGPI hearts and reduced by myriocin. Thus, this gene was a better indicator of the metabolic changes found in the isolated perfused hearts. By studying the effects of ceramide on a cardiomyocyte-like cell line, we showed that ceramide alone increased PDK4 expression.
Unlike other organs, our data show that glucose uptake and oxidation are not directly correlated in the heart. Therefore, this mismatch of elevated glucose uptake and reduced oxidation in the LpLGPI heart was because a large percent of cardiac glucose was shunted into glycogen rather than directly oxidized. At first we were surprised to find greater 2-DG uptake in the LpLGPI hearts. However, we demonstrated that these hearts have more stored glycogen and that this was corrected by myriocin treatment. Whether increased glycogen stores were due to a direct effect of ceramide on glycogen synthesis pathways or were secondary to reduced cardiac function cannot be determined from our in vivo studies.
Glycogen synthesis is regulated by the AKT/GSK-3β pathway. Heart failure is associated with increased pGSK-3β, which might lead to increased glycogen synthesis. PAKT is increased in failing hearts of dogs and humans, and cardiac overexpression of AKT caused hypertrophy in mice (36–38). In contrast to studies in adipocytes (39) and skeletal muscle cells (40), we found no evidence that ceramide increases dephosphorylation of AKT via protein phosphatase 2A in lipotoxic hearts. In the failing heart, pAKT production is increased by non-insulin receptor-mediated pathways such as via G-protein-activated phosphatidylinositol 3-kinase γ (41–43). In AC16 cardiomyocytes, ceramide treatment decreased pAKT and pGSK-3β, which is consistent with previous observations in skeletal muscle cells and adipocytes (39, 44, 45). Thus, increased pAKT and GSK-3β in lipotoxic hearts could be due to secondary effects of heart failure. However, the detailed mechanism of AKT/GSK-3β activation in the pathogenesis of cardiac failure is elusive and deserves further investigation.
Although myriocin-treated LpLGPI mice had more-prolonged survival than untreated LpLGPI mice, they still died earlier than did WT mice. Ceramide levels are greater in older mice. However, the myriocin treatment was equally effective in young and older mice. It is possible that the long-term effects of the treatment are suboptimal. Alternatively, additional processes leading to cardiac dysfunction are operative in the LpLGPI mice. Thus, other bioactive lipids, such as DAG, which activates protein kinase C, could contribute to early death of lipid-driven cardiomyopathic mice (46, 47).
To exclude nonspecific pharmacological effects of myriocin, we crossed LpLGPI transgenic mice with LCB1+/− mice that have reduced plasma and hepatic ceramide (32). Heterozygous deletion of the SPT subunit LCB1 normalized ceramide levels and PDK4 expression, improved cardiac function, and reduced the expression of heart failure markers. This pattern mirrors the effect of myriocin treatment on LpLGPI hearts. Interestingly, expression of the cardiac failure markers ANF and BNP was downregulated by pharmacologic and genetic inhibition of SPT even further than those of WT. Thus, both pharmacological inhibition and genetic deletion of SPT improve these lipotoxic hearts. Whether ceramide inhibition will prove to be a common therapeutic modality to correct cardiomyopathy in other models of lipotoxicity requires further investigation.
Increased accumulation of cardiac lipids is associated with dilated cardiomyopathy, and this is true both for humans with obesity and diabetes (48–50) and in several animal models (5, 8, 51). One major issue is whether increased oxidation of lipids and generation of ROS or effects of toxic lipids are pathogenic. Although conversion of hearts from use of FA to glucose as energy is beneficial in the setting of oxygen deficiency, i.e., ischemia, it is unclear whether disproportionate FA oxidation is harmful in other settings. It has been difficult to isolate the possible causes of lipotoxic cardiomyopathy, because genetic and pharmacologic interventions associated with increased FA oxidation also modulate lipid uptake and augment cardiomyocyte lipid accumulation. However, several genetic alterations have clearly linked lipid accumulation, rather than lipid oxidation, to cardiac dysfunction. Two genetic models of dilated cardiomyopathy, cardiac and muscle LpL overexpression on the PPARα knockout background (52) and cardiac-specific PPARδ knockout (53), are associated with reduced FA oxidation and cardiac lipid accumulation. Moreover, cardiomyopathy due to transgenic overexpression of PPARα is corrected by reduction of FA uptake by cross onto the CD36 knockout background, despite no reduction in FA oxidation (54). Thus, toxic lipids, rather than excess FA oxidation, are likely to lead to heart dysfunction.
Excess lipid associated with dysfunctional tissues and organs is becoming a common problem in obesity. Islet cell, liver, and cardiac manifestations of lipotoxicity include type 2 diabetes, nonalcoholic steatohepatitis, and dilated cardiomyopathies. The underlying pathophysiology is likely to be similar in these three conditions. Other investigators have implicated ceramide as a toxic lipid (3, 4). By using a specific inhibitor of ceramide production and genetic methods, we provide evidence that this lipid is responsible, at least in part, for one form of dilated lipotoxic cardiomyopathy. The mechanisms behind myriocin's therapeutic benefit were examined and suggest that correction of defective cardiac energetics is involved. These studies provide a model for developing pharmacologic interventions for an increasingly important cause of human disease.
Supplementary Material
Acknowledgments
The authors thank Drs. Gerald Shulman and Gary Cline at Yale University for helpful discussion and assistance regarding fatty acyl CoA analysis by LC/MS/MS.
Abbreviations
DAG, diacylglycerol
2-DG, 2-deoxy-d-glucose
GPI, glycosylphosphatidylinositol
LCB1+/−, heterozygous LCB1 knockout
LVD, left ventricular systolic diameter
LpL, lipoprotein lipase
ROS, reactive oxygen species
SM, sphingomyelin
SPT, serine palmitoyltransferase
TG, triglyceride
WT, wild type
Published, JLR Papers in Press, May 30, 2008.
Footnotes
This study was supported by National Institutes of Health Grants HL-73029 and HL-45095 (T-S.P., I.J.G.), and HL-70525 and HL-73167 to E.D.A. from the National Heart, Lung, and Blood Institute.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and one table.
References
- 1.Zhou Y. T., P. Grayburn, A. Karim, M. Shimabukuro, M. Higa, D. Baetens, L. Orci, and R. H. Unger. 2000. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA. 97 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molavi B., N. Rasouli, and P. A. Kern. 2006. The prevention and treatment of metabolic syndrome and high-risk obesity. Curr. Opin. Cardiol. 21 479–485. [DOI] [PubMed] [Google Scholar]
- 3.Shimabukuro M., M. Higa, Y. T. Zhou, M. Y. Wang, C. B. Newgard, and R. H. Unger. 1998. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273 32487–32490. [DOI] [PubMed] [Google Scholar]
- 4.Holland W. L., J. T. Brozinick, L. P. Wang, E. D. Hawkins, K. M. Sargent, Y. Liu, K. Narra, K. L. Hoehn, T. A. Knotts, A. Siesky, et al. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5 167–179. [DOI] [PubMed] [Google Scholar]
- 5.Chiu H. C., A. Kovacs, D. A. Ford, F. F. Hsu, R. Garcia, P. Herrero, J. E. Saffitz, and J. E. Schaffer. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finck B. N., X. Han, M. Courtois, F. Aimond, J. M. Nerbonne, A. Kovacs, R. W. Gross, and D. P. Kelly. 2003. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc. Natl. Acad. Sci. USA. 100 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son N. H., T. S. Park, H. Yamashita, M. Yokoyama, L. A. Huggins, K. Okajima, S. Homma, M. J. Szabolcs, L. S. Huang, and I. J. Goldberg. 2007. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Invest. 117 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu H. C., A. Kovacs, R. M. Blanton, X. Han, M. Courtois, C. J. Weinheimer, K. A. Yamada, S. Brunet, H. Xu, J. M. Nerbonne, et al. 2005. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 96 225–233. [DOI] [PubMed] [Google Scholar]
- 9.Hanada K. 2003. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta. 1632 16–30. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson A., and R. D. Duan. 2006. Absorption and lipoprotein transport of sphingomyelin. J. Lipid Res. 47 154–171. [DOI] [PubMed] [Google Scholar]
- 11.Griffin M. E., M. J. Marcucci, G. W. Cline, K. Bell, N. Barucci, D. Lee, L. J. Goodyear, E. W. Kraegen, M. F. White, and G. I. Shulman. 1999. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 48 1270–1274. [DOI] [PubMed] [Google Scholar]
- 12.Xia P., T. Inoguchi, T. S. Kern, R. L. Engerman, P. J. Oates, and G. L. King. 1994. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 43 1122–1129. [DOI] [PubMed] [Google Scholar]
- 13.Avignon A., K. Yamada, X. Zhou, B. Spencer, O. Cardona, S. Saba-Siddique, L. Galloway, M. L. Standaert, and R. V. Farese. 1996. Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes. 45 1396–1404. [DOI] [PubMed] [Google Scholar]
- 14.Listenberger L. L., X. Han, S. E. Lewis, S. Cases, R. V. Farese, Jr., D. S. Ory, and J. E. Schaffer. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo W., S. Wong, W. Xie, T. Lei, and Z. Luo. 2007. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 293 E576–E586. [DOI] [PubMed] [Google Scholar]
- 16.Wei Y., D. Wang, F. Topczewski, and M. J. Pagliassotti. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291 E275–E281. [DOI] [PubMed] [Google Scholar]
- 17.Borradaile N. M., K. K. Buhman, L. L. Listenberger, C. J. Magee, E. T. Morimoto, D. S. Ory, and J. E. Schaffer. 2006. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol. Biol. Cell. 17 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H., M. V. Kokoeva, K. Inouye, I. Tzameli, H. Yin, and J. S. Flier. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagyu H., G. Chen, M. Yokoyama, K. Hirata, A. Augustus, Y. Kako, T. Seo, Y. Hu, E. P. Lutz, M. Merkel, et al. 2003. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson M. M., C. Nesti, L. Palenzuela, W. F. Walker, E. Hernandez, L. Protas, M. Hirano, and N. D. Isaac. 2005. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 39 133–147. [DOI] [PubMed] [Google Scholar]
- 21.Belke D. D., T. S. Larsen, E. M. Gibbs, and D. L. Severson. 2000. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 279 E1104–E1113. [DOI] [PubMed] [Google Scholar]
- 22.Belke D. D., T. S. Larsen, E. M. Gibbs, and D. L. Severson. 2001. Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am. J. Physiol. Endocrinol. Metab. 280 E420–E427. [DOI] [PubMed] [Google Scholar]
- 23.Belke D. D., T. S. Larsen, G. D. Lopaschuk, and D. L. Severson. 1999. Glucose and fatty acid metabolism in the isolated working mouse heart. Am. J. Physiol. 277 R1210–R1217. [DOI] [PubMed] [Google Scholar]
- 24.Mazumder P. K., B. T. O'Neill, M. W. Roberts, J. Buchanan, U. J. Yun, R. C. Cooksey, S. Boudina, and E. D. Abel. 2004. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 53 2366–2374. [DOI] [PubMed] [Google Scholar]
- 25.Folch J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- 26.Hojjati M. R., and X. C. Jiang. 2006. Rapid, specific, and sensitive measurements of plasma sphingomyelin and phosphatidylcholine. J. Lipid Res. 47 673–676. [DOI] [PubMed] [Google Scholar]
- 27.Perry D. K., A. Bielawska, and Y. A. Hannun. 2000. Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol. 312 22–31. [DOI] [PubMed] [Google Scholar]
- 28.Takuma S., K. Suehiro, C. Cardinale, T. Hozumi, H. Yano, J. Shimizu, S. Mullis-Jansson, R. Sciacca, J. Wang, D. Burkhoff, et al. 2001. Anesthetic inhibition in ischemic and nonischemic murine heart: comparison with conscious echocardiographic approach. Am. J. Physiol. Heart Circ. Physiol. 280 H2364–H2370. [DOI] [PubMed] [Google Scholar]
- 29.Wang C. Y., S. P. Mazer, K. Minamoto, S. Takuma, S. Homma, M. Yellin, L. Chess, A. Fard, S. L. Kalled, M. C. Oz, et al. 2002. Suppression of murine cardiac allograft arteriopathy by long-term blockade of CD40-CD154 interactions. Circulation. 105 1609–1614. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura A., Y. Toyoda, T. Murakami, H. Yoshizato, Y. Ando, and N. Fujitsuka. 2005. Glycogen depletion in intrafusal fibres in rats during short-duration high-intensity treadmill running. Acta Physiol. Scand. 185 41–50. [DOI] [PubMed] [Google Scholar]
- 31.Ranalletta M., H. Jiang, J. Li, T. S. Tsao, A. E. Stenbit, M. Yokoyama, E. B. Katz, and M. J. Charron. 2005. Altered hepatic and muscle substrate utilization provoked by GLUT4 ablation. Diabetes. 54 935–943. [DOI] [PubMed] [Google Scholar]
- 32.Hojjati M. R., Z. Li, and X. C. Jiang. 2005. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta. 1737 44–51. [DOI] [PubMed] [Google Scholar]
- 33.Chalfant C. E., K. Rathman, R. L. Pinkerman, R. E. Wood, L. M. Obeid, B. Ogretmen, and Y. A. Hannun. 2002. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J. Biol. Chem. 277 12587–12595. [DOI] [PubMed] [Google Scholar]
- 34.Park T. S., R. L. Panek, S. B. Mueller, J. C. Hanselman, W. S. Rosebury, A. W. Robertson, E. K. Kindt, R. Homan, S. K. Karathanasis, and M. D. Rekhter. 2004. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 110 3465–3471. [DOI] [PubMed] [Google Scholar]
- 35.Hojjati M. R., Z. Li, H. Zhou, S. Tang, C. Huan, E. Ooi, S. Lu, and X. C. Jiang. 2005. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 280 10284–10289. [DOI] [PubMed] [Google Scholar]
- 36.Haq S., G. Choukroun, H. Lim, K. M. Tymitz, F. del Monte, J. Gwathmey, L. Grazette, A. Michael, R. Hajjar, T. Force, et al. 2001. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 103 670–677. [DOI] [PubMed] [Google Scholar]
- 37.Ananthakrishnan R., G. W. Moe, M. J. Goldenthal, and J. Marin-Garcia. 2005. Akt signaling pathway in pacing-induced heart failure. Mol. Cell. Biochem. 268 103–110. [DOI] [PubMed] [Google Scholar]
- 38.Shiojima I., K. Sato, Y. Izumiya, S. Schiekofer, M. Ito, R. Liao, W. S. Colucci, and K. Walsh. 2005. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 115 2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratford S., K. L. Hoehn, F. Liu, and S. A. Summers. 2004. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279 36608–36615. [DOI] [PubMed] [Google Scholar]
- 40.Chavez J. A., and S. A. Summers. 2003. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 419 101–109. [DOI] [PubMed] [Google Scholar]
- 41.DeBosch B., I. Treskov, T. S. Lupu, C. Weinheimer, A. Kovacs, M. Courtois, and A. J. Muslin. 2006. Akt1 is required for physiological cardiac growth. Circulation. 113 2097–2104. [DOI] [PubMed] [Google Scholar]
- 42.Nienaber J. J., H. Tachibana, S. V. Naga Prasad, G. Esposito, D. Wu, L. Mao, and H. A. Rockman. 2003. Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J. Clin. Invest. 112 1067–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oudit G. Y., Z. Kassiri, M. P. Patel, M. Chappell, J. Butany, P. H. Backx, R. G. Tsushima, J. W. Scholey, R. Khokha, and J. M. Penninger. 2007. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 75 29–39. [DOI] [PubMed] [Google Scholar]
- 44.Chavez J. A., T. A. Knotts, L. P. Wang, G. Li, R. T. Dobrowsky, G. L. Florant, and S. A. Summers. 2003. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 278 10297–10303. [DOI] [PubMed] [Google Scholar]
- 45.Summers S. A., L. A. Garza, H. Zhou, and M. J. Birnbaum. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18 5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Listenberger L. L., and J. E. Schaffer. 2002. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc. Med. 12 134–138. [DOI] [PubMed] [Google Scholar]
- 47.Carlsson C., L. A. Borg, and N. Welsh. 1999. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology. 140 3422–3428. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S., J. V. Adrogue, L. Golfman, I. Uray, J. Lemm, K. Youker, G. P. Noon, O. H. Frazier, and H. Taegtmeyer. 2004. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 18 1692–1700. [DOI] [PubMed] [Google Scholar]
- 49.Peterson L. R., P. Herrero, K. B. Schechtman, S. B. Racette, A. D. Waggoner, Z. Kisrieva-Ware, C. Dence, S. Klein, J. Marsala, T. Meyer, et al. 2004. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 109 2191–2196. [DOI] [PubMed] [Google Scholar]
- 50.McGavock J. M., I. Lingvay, I. Zib, T. Tillery, N. Salas, R. Unger, B. D. Levine, P. Raskin, R. G. Victor, and L. S. Szczepaniak. 2007. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 116 1170–1175. [DOI] [PubMed] [Google Scholar]
- 51.Finck B. N., J. J. Lehman, T. C. Leone, M. J. Welch, M. J. Bennett, A. Kovacs, X. Han, R. W. Gross, R. Kozak, G. D. Lopaschuk, et al. 2002. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nohammer C., F. Brunner, G. Wolkart, P. B. Staber, E. Steyrer, F. J. Gonzalez, R. Zechner, and G. Hoefler. 2003. Myocardial dysfunction and male mortality in peroxisome proliferator-activated receptor alpha knockout mice overexpressing lipoprotein lipase in muscle. Lab. Invest. 83 259–269. [DOI] [PubMed] [Google Scholar]
- 53.Cheng L., G. Ding, Q. Qin, Y. Huang, W. Lewis, N. He, R. M. Evans, M. D. Schneider, F. A. Brako, Y. Xiao, et al. 2004. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 10 1245–1250. [DOI] [PubMed] [Google Scholar]
- 54.Yang J., N. Sambandam, X. Han, R. W. Gross, M. Courtois, A. Kovacs, M. Febbraio, B. N. Finck, and D. P. Kelly. 2007. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 100 1208–1217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.