Abstract
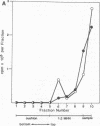
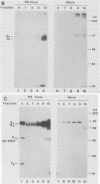
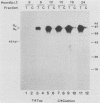
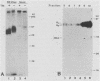
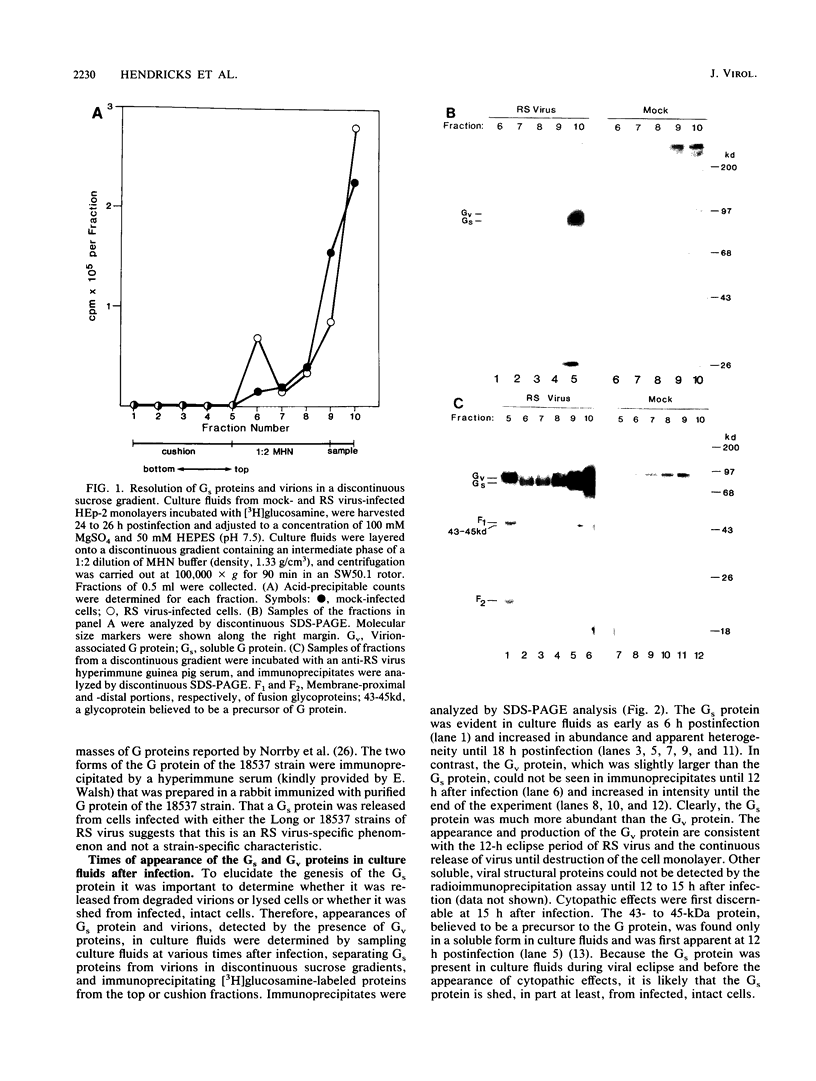
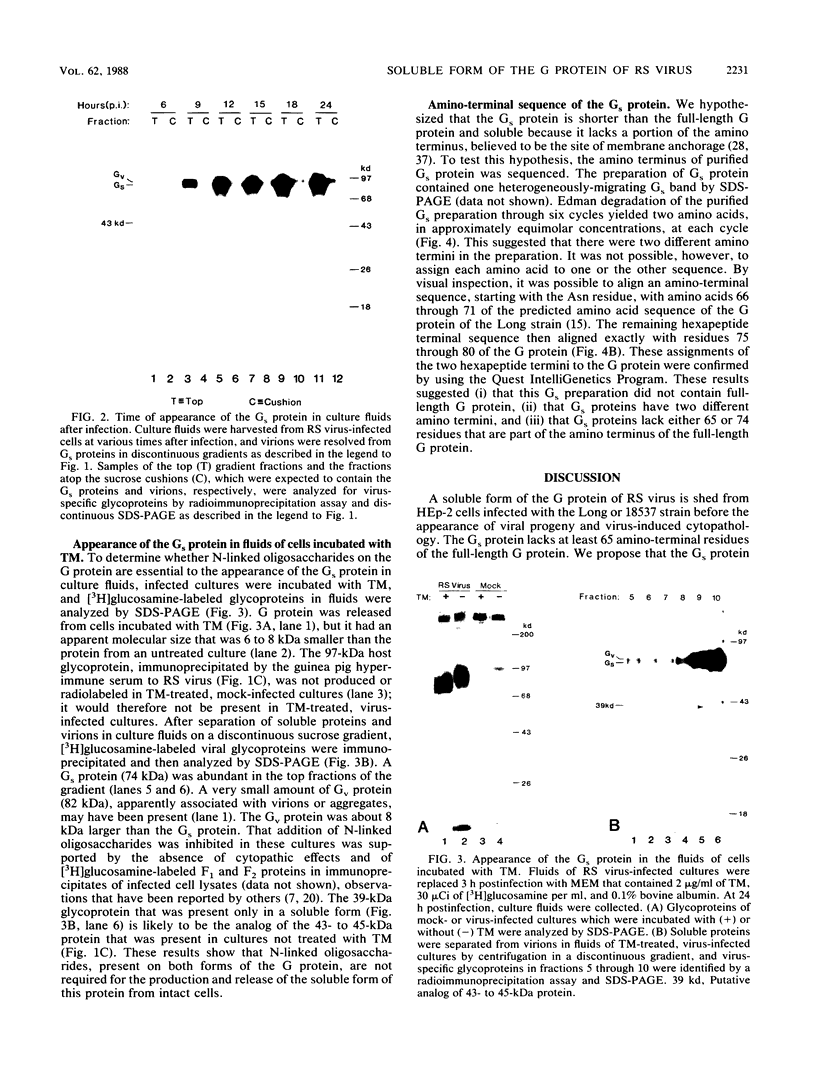
A soluble form of the G glycoprotein, the attachment protein, of respiratory syncytial virus is shed from infected HEp-2 cells. The Gs proteins of the Long and 18537 strains have apparent molecular sizes of 82 and 71 kilodaltons, respectively, 6 to 9 kilodaltons smaller than the virion-associated forms (Gv). The Gs protein of the Long strain was further characterized. Approximately one in six of all of the radiolabeled G molecules in these cultures at 24 h postinfection was present as the Gs protein. The Gs protein was clearly evident in culture fluids at 6 h postinfection, but the Gv protein could not be discerned until 12 h after infection, an observation that is consistent with the 12-h eclipse period for respiratory syncytial virus. Therefore, the Gs protein is shed, in part at least, from intact, infected cells and before the appearance of progeny virus. The appearance of a smaller Gs protein (74 kilodaltons) in fluids of infected calls which were incubated with tunicamycin shows that addition of N-linked oligosaccharides is not required for the genesis and shedding of the Gs protein. Sequencing of the amino terminus of purified Gs protein revealed two different termini, whose generations are consistent with cleavages of the full-length G protein between amino acids 65 and 66 and between residues 74 and 75. This result suggests that the Gs protein is present in two different forms which lack the proposed intracytoplasmic and transmembrane domains of the full-length G protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Langlois A. J., Schäfer W. Polypeptides of mammalian oncornaviruses. IV. Structural components of murine leukemia virus released as soluble antigens in cell culture. Virology. 1975 Dec;68(2):550–555. doi: 10.1016/0042-6822(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Characterization of the soluble glycoprotein released from vesicular stomatitis virus-infected cells. J Virol. 1983 Jan;45(1):80–90. doi: 10.1128/jvi.45.1.80-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. B., Ben-Porat T., Whitley R. J., Kaplan A. S. Purification and characterization of proteins excreted by cells infected with herpes simplex virus and their use in diagnosis. Virology. 1978 Dec;91(2):234–242. doi: 10.1016/0042-6822(78)90372-0. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Olmsted R. A., Spriggs M. K., Johnson P. R., Buckler-White A. J. Gene overlap and site-specific attenuation of transcription of the viral polymerase L gene of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5134–5138. doi: 10.1073/pnas.84.15.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. The envelope-associated 22K protein of human respiratory syncytial virus: nucleotide sequence of the mRNA and a related polytranscript. J Virol. 1985 Apr;54(1):65–71. doi: 10.1128/jvi.54.1.65-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Wunner W. H., Varrichio A. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology. 1983 Jan 30;124(2):330–337. doi: 10.1016/0042-6822(83)90349-5. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Dapolito G., Cote P. J., Jr, Gerin J. L. Kinetics of synthesis of respiratory syncytial virus glycoproteins. J Gen Virol. 1985 Sep;66(Pt 9):1983–1990. doi: 10.1099/0022-1317-66-9-1983. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J Gen Virol. 1983 Apr;64(Pt 4):825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. IV. The oligosaccharides of the glycoproteins. J Gen Virol. 1985 Mar;66(Pt 3):417–432. doi: 10.1099/0022-1317-66-3-417. [DOI] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. V. The kinetics of glycoprotein synthesis. J Gen Virol. 1985 Jun;66(Pt 6):1241–1247. doi: 10.1099/0022-1317-66-6-1241. [DOI] [PubMed] [Google Scholar]
- Hendricks D. A., Baradaran K., McIntosh K., Patterson J. L. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J Gen Virol. 1987 Jun;68(Pt 6):1705–1714. doi: 10.1099/0022-1317-68-6-1705. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Spriggs M. K., Olmsted R. A., Collins P. L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Jeffries B. C., Pyles G., Reid J. L., Chanock R. M., Parrott R. H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973 Sep;98(3):216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Lambert D. M., Pons M. W. Respiratory syncytial virus glycoproteins. Virology. 1983 Oct 15;130(1):204–214. doi: 10.1016/0042-6822(83)90128-9. [DOI] [PubMed] [Google Scholar]
- Levine S., Klaiber-Franco R., Paradiso P. R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987 Sep;68(Pt 9):2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. The membrane-spanning segment of invariant chain (I gamma) contains a potentially cleavable signal sequence. Cell. 1986 Sep 26;46(7):1103–1112. doi: 10.1016/0092-8674(86)90710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Synthesis and distribution of vesicular stomatitis virus-specific polypeptides in the absence of progeny production. Virology. 1977 Aug;81(1):37–47. doi: 10.1016/0042-6822(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Markoff L., Lai C. J. Sequence of the influenza A/Udorn/72 (H3N2) virus neuraminidase gene as determined from cloned full-length DNA. Virology. 1982 Jun;119(2):288–297. doi: 10.1016/0042-6822(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Markoff L., Lin B. C., Sveda M. M., Lai C. J. Glycosylation and surface expression of the influenza virus neuraminidase requires the N-terminal hydrophobic region. Mol Cell Biol. 1984 Jan;4(1):8–16. doi: 10.1128/mcb.4.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Mufson M. A., Sheshberadaran H. Structural differences between subtype A and B strains of respiratory syncytial virus. J Gen Virol. 1986 Dec;67(Pt 12):2721–2729. doi: 10.1099/0022-1317-67-12-2721. [DOI] [PubMed] [Google Scholar]
- Paramyxovirdae. Intervirology. 1978;10(3):137–152. doi: 10.1159/000148979. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Satake M., Coligan J. E., Elango N., Norrby E., Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985 Nov 11;13(21):7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976 Nov;75(1):130–144. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Markoff L. J., Lai C. J. Cell surface expression of the influenza virus hemagglutinin requires the hydrophobic carboxy-terminal sequences. Cell. 1982 Sep;30(2):649–656. doi: 10.1016/0092-8674(82)90261-6. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Brandriss M. W., Schlesinger J. J. Purification and characterization of the respiratory syncytial virus fusion protein. J Gen Virol. 1985 Mar;66(Pt 3):409–415. doi: 10.1099/0022-1317-66-3-409. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983 Jul;47(1):171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol. 1984 Apr;65(Pt 4):761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Belshe R. B., Kim H. W., Van Voris L. P., Chanock R. M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982 Jul;37(1):397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]