Abstract
Background and Aims
Genome size and chromosome numbers are important cytological characters that significantly influence various organismal traits. However, geographical representation of these data is seriously unbalanced, with tropical and subtropical regions being largely neglected. In the present study, an investigation was made of chromosomal and genome size variation in the majority of Curcuma species from the Indian subcontinent, and an assessment was made of the value of these data for taxonomic purposes.
Methods
Genome size of 161 homogeneously cultivated plant samples classified into 51 taxonomic entities was determined by propidium iodide flow cytometry. Chromosome numbers were counted in actively growing root tips using conventional rapid squash techniques.
Key Results
Six different chromosome counts (2n = 22, 42, 63, >70, 77 and 105) were found, the last two representing new generic records. The 2C-values varied from 1·66 pg in C. vamana to 4·76 pg in C. oligantha, representing a 2·87-fold range. Three groups of taxa with significantly different homoploid genome sizes (Cx-values) and distinct geographical distribution were identified. Five species exhibited intraspecific variation in nuclear DNA content, reaching up to 15·1 % in cultivated C. longa. Chromosome counts and genome sizes of three Curcuma-like species (Hitchenia caulina, Kaempferia scaposa and Paracautleya bhatii) corresponded well with typical hexaploid (2n = 6x = 42) Curcuma spp.
Conclusions
The basic chromosome number in the majority of Indian taxa (belonging to subgenus Curcuma) is x = 7; published counts correspond to 6x, 9x, 11x, 12x and 15x ploidy levels. Only a few species-specific C-values were found, but karyological and/or flow cytometric data may support taxonomic decisions in some species alliances with morphological similarities. Close evolutionary relationships among some cytotypes are suggested based on the similarity in homoploid genome sizes and geographical grouping. A new species combination, Curcuma scaposa (Nimmo) Škorničk. & M. Sabu, comb. nov., is proposed.
Key words: Chromosome number, Curcuma, cytology, DNA C-value, flow cytometry, genome size, India, intraspecific variation, polyploidy, taxonomy
INTRODUCTION
The genus Curcuma L. (Zingiberaceae) contains many taxa of economic, medicinal, ornamental and cultural importance, turmeric (C. longa L.) probably being the best known. It is found throughout south and south-east Asia with a few species extending to China, Australia and the South Pacific. The highest diversity is concentrated in India and Thailand, with at least 40 species in each area, followed by Burma, Bangladesh, Indonesia and Vietnam. Due to the lack of a comprehensive taxonomic revision, there is still little consensus on the number of species that should be recognized. Recent estimates vary from about 50 (Smith, 1981) to 80 (Larsen et al., 1998) and 100 species (Sirirugsa, 1996), although Škorničková et al. (2004) suggested that their number will probably reach 120 in the near future in connection with detailed botanical exploration of India and south-east Asia.
Several taxonomic and biological problems have hindered satisfactory systematic treatment of the genus. Original descriptions of many Curcuma species are vague and inaccurate, and type specimens are often lacking or fragmentary. Proper preservation of Curcuma specimens is extremely difficult, exacerbating the limited amount of type material, leading to ambiguous name assignment and usage. In addition, high intra- and interpopulation variation has led to debate concerning species concepts and boundaries. As a result, one species has often been described repeatedly under different names whereas the same name has been applied to different taxonomic entities.
Some species may hybridize in the wild and the crosses may become naturalized (Škorničková and Sabu, 2005b; Škorničková et al., 2007). Frequent cultivation of Curcuma spp. and targeted selection of peculiar morphotypes have further contributed to taxonomic complexity of the group. Moreover, polyploidy has played a significant role in evolution and diversification of various members of Zingiberaceae (e.g. Mukherjee, 1970; Lim, 1972a,b; Poulsen, 1993; Chen and Chen, 1984; Takano, 2001; Takano and Okada, 2002), including Curcuma (Prana et al., 1978; Apavatjrut et al., 1996; Joseph et al., 1999; Ardiyani, 2002; Sirisawad et al., 2003). It is well documented, in both plants and animals, that an increase in ploidy level is commonly associated with blurring of morphological boundaries between taxa (see Stace, 2000).
The occurrence of different ploidy levels in Curcuma was highlighted in early cytological studies (e.g. Suguira, 1931, 1936; Raghavan and Venkatasubban, 1943; Venkatasubban, 1946; Chakravorti, 1948; Sharma and Bhattacharya, 1959), and 20 different somatic chromosome numbers have been reported (Table 1). Considering the widely accepted basic chromosome number x = 21 (Ramachandran, 1961; Prana, 1977; Islam, 2004), this chromosomal variation roughly corresponds to three euploid levels (2x, 3x and 4x) plus several aneuploids. However, many of the records should be treated with caution due to potential errors in chromosome counting as well as taxonomic ambiguity of the analysed material, which is rarely documented by herbarium vouchers. Such a high basic chromosome number is likely to be secondary, further complicating accurate inference of ploidy level and its evolution within the genus.
Table 1.
A synopsis of published chromosome counts and genome sizes in the genus Curcuma
| No. of chromosomes |
|||||
|---|---|---|---|---|---|
| Species | n | 2n | Genome size (pg)* | Origin of plant material | Reference |
| subgen. Curcuma K.Schum. | |||||
| C. aeruginosa Roxb. | 63 | Indonesia | Prana (1977, 1978) | ||
| 63 | Thailand | Apavatjrut et al. (1996) | |||
| 63 | India | Joseph et al. (1999) | |||
| 63 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 63 | Indonesia | Ardiyani (2002) | |||
| 28–35 | 63 | Thailand | Sirisawad et al. (2003) | ||
| 63, 84 | 3·203–5·302 | Bangladesh | Islam (2004) | ||
| C. amada Roxb | 42 | India | Raghavan and Venkat. (1943) | ||
| 42 | India | Chakravorti (1948) | |||
| 42 | India | Raghavan and Arora (1958) | |||
| 42 | India | Sharma and Bhattacharya (1959) | |||
| 42 | India | Ramachandran (1961, 1969) | |||
| 40 | 4·234/4C | India | Das et al. (1999) | ||
| 42 | 2·132 | Bangladesh | Islam (2004) | ||
| C. amarissima Roscoe | 63 | 3·289 | Bangladesh | Islam (2004) | |
| C. angustifolia Roxb. | 42 | India | Chakravorti (1948) | ||
| 42 | India | Sharma and Bhattacharya (1959) | |||
| 42 | 2·121–2·141 | Bangladesh | Islam (2004) | ||
| C. aromatica Salisb. | 42 | India | Raghavan and Venkat (1943) | ||
| 42 | India | Chakravorti (1948) | |||
| 63, 86 | India | Ramachandran (1961, 1969) | |||
| 84 | India | Nambiar et al. (1982) | |||
| 63 | China | Chen and Chen (1984) | |||
| 42 | India | Sarkar (1990) | |||
| 63 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 63 | 3·184 | Bangladesh | Islam (2004) | ||
| C. attenuata Wall. ex Baker | 42 | 84 | Thailand | Apavatjrut et al. (1996) | |
| 42 | 84 | Thailand | Sirisawad et al. (2003) | ||
| C. australasica Hook.f | 2·153–1·181 | Bangladesh | Islam (2004) | ||
| C. aurantiaca Zijp | 42 | Indonesia | Prana (1977, 1978) | ||
| 21 | Pen. Malaysia | Beltran and Kam (1984) | |||
| 21 | 42 | Thailand | Sirisawad et al. (2003) | ||
| C. brog Valeton | 63, 64, 70 | Indonesia | Prana (1977) | ||
| 63, 64 | Indonesia | Prana (1978) | |||
| C. caesia Roxb. | 22 | 3·12/4C | India | Das et al. (1999) | |
| 63 | India | Joseph et al. (1999) | |||
| 63 | 3·333 | Bangladesh | Islam (2004) | ||
| C. colorata Valeton | 62, 63 | Indonesia | Prana (1977, 1978) | ||
| C. comosa Craib | 42 | India | Joseph et al. (1999) | ||
| C. cf. comosa Roxb. | 63 | Thailand | Paisooksantivatana and Thepsen (2001) | ||
| C. decipiens Dalzell | 21 | 42 | India | Ramachandran (1961, 1969) | |
| C. elata Roxb. | 63 | Thailand | Apavatjrut et al. (1996) | ||
| 28–35 | 63 | Thailand | Sirisawad et al. (2003) | ||
| 63 | 3·181 | Bangladesh | Islam (2004) | ||
| C. haritha Mangaly & M. Sabu | 42 | India | Joseph et al. (1999) | ||
| C. heyneana Valeton & Zijp | 63 | Indonesia | Prana (1977, 1978) | ||
| 63 | Indonesia | Ardyiani (2002) | |||
| C. kwangsiensis S.G.Lee & C.F.Liang | 42 | 84 | China | Chen et al. (1988) | |
| C. latifolia Roscoe | 63 | 3·435 | Bangladesh | Islam (2004) | |
| C. longa L. | 64 | Unknown | Sugiura (1931, 1936) | ||
| 62 | India | Raghavan and Venkat (1943) | |||
| 62, 63, 64 | India | Chakravorti (1948) | |||
| 32 | Unknown | Sato (1948) | |||
| 62, 93 | India | Sharma and Bhattacharya (1959) | |||
| 63 | India | Ramachandran (1961, 1969) | |||
| 63 | Indonesia | Prana (1977, 1978) | |||
| 48 | 5·1–5·26/4C | India | Das et al. (1999) | ||
| 63 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 63 | 3·275 | Bangladesh | Islam (2004) | ||
| 48 | 4·30–8·84/4C | India | Nayak et al. (2006) | ||
| C. malabarica Velay., Mural. & Amalraj | 42 | India | Joseph et al. (1999) | ||
| C. mangga Valeton & Zijp | 42 | Indonesia | Prana (1977, 1978) | ||
| 63 | Indonesia | Ardyiani (2002) | |||
| C. neilgherrensis Wight | 42 | India | Chakravorti (1948) | ||
| 42 | India | Ramachandran (1961, 1969) | |||
| C. aff. oligantha Trimen | 42 | Thailand | Eksomtramage et al. (2002) | ||
| 40 | Thailand | Saensouk and Chantaranothai (2003) | |||
| C. petiolata Roxb. | 64 | India | Venkatasubban (1946) | ||
| 42 | Indonesia | Prana (1977, 1978) | |||
| 42 | Thailand | Apavatjrut et al. (1996) | |||
| 21 | 42 | Thailand | Sirisawad et al. (2003) | ||
| 42 | 2·142 | Bangladesh | Islam (2004) | ||
| C. cf. petiolata Roxb. | 42 | Thailand | Paisooksantivatana and Thepsen (2001) | ||
| C. phaeocaulis Valeton | 62, 63, 64 | Indonesia | Prana (1977, 1978) | ||
| C. purpurascens Blume | 63 | Indonesia | Prana (1977, 1978) | ||
| C. raktakanta Mangaly & M.Sabu | 63 | India | Joseph et al. (1999) | ||
| 21 | 42 | Thailand | Sirisawad et al. (2003) | ||
| C. roscoeana Wall. | 21 | 42 | Thailand | Apavatjrut et al. (1996) | |
| 42 | Thailand | Eksomtramage et al. (1996a,b) | |||
| 42 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| C. rubescens Roxb. | 28–35 | 63 | Thailand | Sirisawad et al. (2003) | |
| 63 | 2·204 | Bangladesh | Islam (2004) | ||
| 42 | Bangladesh | Islam (2004) | |||
| C. sessilis Gage | 84 | Thailand | Saensouk et al. (1998) | ||
| 46, 92 | Thailand | Saensouk and Chantaranothai (2003) | |||
| C. soloensis Valeton | 63 | Indonesia | Prana (1977, 1978) | ||
| C. viridiflora Roxb. | 42 | 2·164–2·173 | Bangladesh | Islam (2004) | |
| C. wenyujin Y.H.Chen & C.Ling | 63 | China | Chen and Chen (1984) | ||
| C. zanthorrhiza Roxb. | 63 | Indonesia | Prana (1977, 1978) | ||
| 63 | China | Chen and Chen (1984) | |||
| 63 | Thailand | Apavatjrut et al. (1996) | |||
| 63 | Indonesia | Ardiyani (2002) | |||
| 28–35 | 63 | Thailand | Sirisawad et al. (2003) | ||
| 63 | 3·285 | Bangladesh | Islam (2004) | ||
| 1·30/1C | Origin unknown | Bharathan et al. (1994) | |||
| C. zedoaria (Christm.) Roscoe | 64 | India | Venkatasubban (1946) | ||
| 63, 64 | India | Chakravorti (1948) | |||
| 63 | India | Ramachandran (1961, 1969) | |||
| 66 | India | Sharma (1970) | |||
| 63, 64, 66 | Indonesia | Prana (1977, 1978) | |||
| 66 | India | Chatterjee et al. (1989) | |||
| 63 | Thailand | Apavatjrut et al. (1996) | |||
| 42 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 63 | Indonesia | Ardiyani (2002) | |||
| 28–35 | 63 | Thailand | Sirisawad et al. (2003) | ||
| 63 | 3·321 | Bangladesh | Islam (2004) | ||
| subgen. Hitcheniopsis (Baker) K.Schum. | |||||
| C. alismatifolia Gagnep. | 16 | 32 | Thailand | Apavatjrut et al. (1996) | |
| 32 | Thailand | Saensouk et al. (1998) | |||
| 32 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 32 | Thailand | Saensouk and Chantaranothai (2003) | |||
| 16 | 32 | Thailand | Sirisawad et al. (2003) | ||
| C. gracillima Gagnep. | 24 | Thailand | Saensouk et al. (1998) | ||
| 24 | Thailand | Saensouk and Chantaranothai (2003) | |||
| 16 | 32 | Thailand | Sirisawad et al. (2003) | ||
| C. cf. gracillima Gagnep. | 40 | Thailand | Paisooksantivatana and Thepsen (2001) | ||
| C. harmandii Gagnep. | 10 | 20 | Thailand | Eksomtramage et al. (1996a,b) | |
| 20 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 10 | 20 | Thailand | Sirisawad et al. (2003) | ||
| C. parviflora Wall. | 14, 17, 18, 28 | 28, 34, 36 | Thailand | Apavatjrut et al. (1996) | |
| 32 | Thailand | Weerapakdee and Krasaechai (1997) | |||
| 30 | Thailand | Saensouk et al. (1998) | |||
| 42 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 32 | Thailand | Eksomtramage et al. (2002) | |||
| 30 | Thaialnd | Saensouk and Chantaranothai (2003) | |||
| 16 | 32 | Thailand | Sirisawad et al. (2003) | ||
| 12, 14, 17, 18, 28 | 24, 28, 34, 36, 56 | Thailand | Sirisawad et al. (2003) | ||
| C. rhabdota Sirirugsa & M.F.Newman | 24 | Thailand | Eksomtramage et al. (2002) | ||
| 12 | 24 | Thailand | Sirisawad et al. (2003) | ||
| C. thorelii Gagnep. | 36 | Thailand | Eksomtramage and Boontum (1995) | ||
| 17 | 34 | Thailand | Apavajrut et al. (1996) | ||
| 36 | Thailand | Paisooksantivatana and Thepsen (2001) | |||
| 38? | Thailand | Ardiyani (2002) | |||
| 34 | Thailand | Saensouk and Chantaranothai (2003) | |||
| 17 | 34 | Thailand | Sirisawad et al. (2003) | ||
* Genome sizes expressed in 2C-values unless indicated otherwise.
In addition to chromosome numbers and ploidy levels, genome size (nuclear DNA content) data also provide information useful in various fields of plant biology, including systematics, evolution and conservation (Bennett and Leitch, 2005b). In plants holoploid genome sizes (or 1C-values) vary strikingly, ranging from about 0·065 to 127·4 pg. Genome size variation has significant consequences at cellular, tissue and organismal levels and also influences phenological and ecological behaviour. Despite its usefulness in understanding plant evolution and diversification, genome size variation in Curcuma is not well documented, and estimates for only a few species have been published (Table 1). Bharathan et al. (1994) determined by flow cytometry 1C = 1·30 pg in C. zanthorrhiza and Das et al. (1999) used cytophotometry to study genome size in C. amada (4C = 3·120 pg), C. caesia (4C = 4·234 pg) and C. longa (4C = 5·100–5·263 pg). Curcuma longa was also investigated by Nayak et al. (2006), who observed 4C-values ranging from 4·30 to 8·84 pg in 17 varieties. In addition, flow-cytometric nuclear DNA amounts for 16 taxa (including the above-mentioned species and one undetermined sample) from Bangladesh are given in the unpublished PhD thesis of Islam (2004).
As a part of ongoing comprehensive taxonomic revision of Curcuma in India (see Škorničková et al., 2003a,b, 2004, 2007; Škorničková and Sabu, 2005a,b,c), the present study aimed to provide a detailed survey of chromosomal and genome size variation in the majority of known Indian species. In particular, we address whether genome size and chromosome numbers can be used as taxonomically informative markers for species delimitation and whether they can elucidate the taxonomic position of four species with Curcuma-like morphological traits often placed in separate genera (Hitchenia caulina, Kaempferia scaposa, Paracautleya bhatii and Stahlianthus involucratus). The issue of basic chromosome number and the origin of the polyploid taxa are also discussed in the light of the findings.
The infrageneric classification (subgenera Curcuma and Hitcheniopsis) generally followed the treatment of Schumann (1904), with some modifications. In particular, C. petiolata and C. roscoeana were included in subgenus Curcuma based on the presence of two floral epigynous glands (derived from gynopleural nectaries). This hitherto neglected character (well developed in subgenus Curcuma but absent in subgenus Hitcheniopsis) seems to be pivotal for the updated subgeneric delimitation, better reflecting the current state of knowledge than did previous taxonomic concepts (J. Leong-Škorničková et al., unpubl. res.).
MATERIAL AND METHODS
Plant materials
In total, 161 individual plants belonging to 51 taxonomic entities were included in the study, 46 Curcuma species (31 assigned to species, 15 determined only tentatively or undetermined), one natural hybrid and four species often classified into separate but related genera: Hitchenia caulina, Kaempferia scaposa, Paracautleya bhatii and Stahlianthus involucratus. The number of individuals per species varied from one to 16. Multiple samples were available for 26 species, whereas 25 taxa (12 Curcuma species, eight undetermined taxa, four species from related genera and one hybrid) were represented by a single plant accession.
Owing to difficulties surrounding systematic treatment of the genus, specific names were assigned only to plant samples perfectly matching the species description. The remaining samples were left unnamed in order to avoid misleading information resulting from unambiguous identification. All plants were collected in the wild on the Indian peninsula, often at or near the locus classicus, during 2000–2004 (Table 2) and are being grown at the Calicut University Botanical Garden, Kerala, India (11 °35′N, 70 °45′E, 50 m a.s.l.). Geographical positions of the collection localities are shown in Fig. 1. Herbarium vouchers are deposited in CALI, with duplicates in MH and SING; incomplete sets are also kept in CAL, K and PR; vouchers of C. oligantha from Sri Lanka are deposited in PDA and SING. In addition, a large collection of photographic documentation of living material (including details of flower morphology) is available for each accession (see Fig. 5).
Table 2.
2C nuclear DNA content with standard error, mean value for a species, intraspecific variation, 1C-value expressed in DNA picograms and megabase pairs (1 pg = 978 Mbp), somatic chromosome number (2n), ploidy level, homoploid genome size (Cx-value, determined as 2C DNA amount/ploidy level) and its mean for a species, internal standard used, locality, species distribution pattern, and a field accession number for 161 Indian plants belonging to 51 taxa of Curcuma and related genera.
| Group/species | Field no. | 2C-value (pg)±s.e. | Mean 2C-value (pg)* | Intraspecific variation (%) | 1C-value (pg) | 1C-value (Mbp) | 2n† | Ploidy level (x) or DNA ploidy level | 1Cx-value (pg) | Mean Cx-value (pg)* | Internal standard‡ | Locality | Distribution pattern§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DIPLOID (x = 11) | |||||||||||||
| C. vamana M. Sabu & Mangaly | 84156 | 1·663±0·008 | 1·66u | 0·83 | 813 | 22! | 2 | 0·83 | 0·83b | L | Kerala, Trichur Dt. | SW | |
| HEXAPLOIDS–GROUP I | |||||||||||||
| C. amada Roxb. | 71421 | 1·882±0·002 | 1·86st | 3·6 | 0·94 | 920 | 6 | 0·31 | 0·31lmnopqrs | G | W. Bengal, Kolkata | NE & E | |
| 71472 | 1·854±0·007 | 0·93 | 907 | 42 | 6 | 0·31 | L | W. Bengal, Darjeeling Dt. | |||||
| 73440 | 1·816±0·002 | 0·91 | 888 | 6 | 0·30 | L | Bihar, Bhagalpur Dt. | ||||||
| 73482 | 1·873±0·004 | 0·94 | 916 | 6 | 0·31 | G | W. Bengal, Kolkata | ||||||
| 73484 | 1·875±0·006 | 0·94 | 917 | 6 | 0·31 | G | W. Bengal, Kolkata | ||||||
| C. aromatica Salisb. s.l.–sp. 1 | 73423 | 1·900±0·008 | 1·86st | 3·5 | 0·95 | 929 | 42 | 6 | 0·32 | 0·31lmnopqrs | L | Sri Lanka, Kegalle Dt. | SW & SL |
| 84109 | 1·846±0·006 | 0·92 | 903 | 42 | 6 | 0·31 | L | Kerala, Kollam Dt. | |||||
| 84114 | 1·880±0·006 | 0·94 | 919 | 6 | 0·31 | G | Kerala, Kollam Dt. | ||||||
| 84123 | 1·876±0·001 | 0·94 | 917 | 6 | 0·31 | G | Kerala, Wynad Dt. | ||||||
| 84123-II | 1·881±0·004 | 0·94 | 920 | 6 | 0·31 | G | Kerala, Wynad Dt. | ||||||
| 84170 | 1·865±0·006 | 0·93 | 912 | 6 | 0·31 | G | Kerala, Idukki Dt. | ||||||
| 84183 | 1·841±0·001 | 0·92 | 900 | 6 | 0·31 | G | Kerala, Idukki Dt. | ||||||
| 84183A | 1·836±0·009 | 0·92 | 898 | 6 | 0·31 | G | Kerala, Idukki Dt. | ||||||
| 84183B | 1·838±0·002 | 0·92 | 899 | 6 | 0·31 | G | Kerala, Idukki Dt. | ||||||
| C. mangga Valeton & Zijp | 84101 | 1·807±0·001 | 1·83t | 3·2 | 0·90 | 884 | 6 | 0·30 | 0·31mnopqrs | G | Kerala, Ernakulam Dt. | SW | |
| 84115 | 1·862±0·008 | 0·93 | 911 | 6 | 0·31 | L | Kerala, Kollam Dt. | ||||||
| 84149 | 1·851±0·002 | 0·93 | 905 | 6 | 0·31 | G | Kerala, Trichur Dt. | ||||||
| 84150 | 1·804±0·002 | 0·90 | 882 | 42 | 6 | 0·30 | G | Kerala, Trichur Dt. | |||||
| C. montana Roxb. | 71484 | 1·816±0·006 | 1·79tu | 6·1 | 0·91 | 888 | 42! | 6 | 0·30 | 0·30rs | L | Jharkhand, Ranchi Dt. | E & C |
| 73419 | 1·854±0·008 | 0·93 | 907 | 42! | 6 | 0·31 | L | Jharkhand, Paschim Singhbum Dt. | |||||
| 73425 | 1·823±0·004 | 0·91 | 891 | 6 | 0·30 | G | Orissa, Koraput Dt. | ||||||
| 73430 | 1·786±0·008 | 0·89 | 873 | 6 | 0·30 | L | Orissa, Koraput Dt. | ||||||
| 73433 | 1·806±0·004 | 0·90 | 883 | 6 | 0·30 | G | Jharkhand, Paschim Singhbum Dt. | ||||||
| 73433-II | 1·778±0·002 | 0·89 | 869 | 6 | 0·30 | G | Jharkhand, Paschim Singhbum Dt. | ||||||
| 73437 | 1·774±0·001 | 0·89 | 867 | 6 | 0·30 | G | Jharkhand, Paschim Singhbum Dt | ||||||
| 73456 | 1·763±0·004 | 0·88 | 862 | 6 | 0·29 | G | Jharkhand, Ranchi Dt. | ||||||
| 73471 | 1·767±0·004 | 0·88 | 864 | 6 | 0·29 | G | Chhattisgarh, Jagdalpur Dt. | ||||||
| 73473 | 1·782±0·004 | 0·89 | 871 | 6 | 0·30 | G | Chhattisgarh, Jagdalpur Dt. | ||||||
| 73474 | 1·774±0·008 | 0·89 | 867 | 6 | 0·30 | G | Chhattisgarh, Jagdalpur Dt. | ||||||
| 73479 | 1·748±0·002 | 0·87 | 855 | 6 | 0·29 | G | Chhattisgarh, Bilaspur Dt. | ||||||
| C. prakasha S. Tripathi | 71441 | 1·877±0·003 | 1·87st | 4·5 | 0·94 | 918 | ca 42! | 6 | 0·31 | 0·31klmnopqr | L | Meghalaya, Ribhoi Dt. | NE |
| 71442 | 1·847±0·005 | 0·92 | 903 | 6 | 0·31 | L | Meghalaya, Ribhoi Dt. | ||||||
| 71450 | 1·918±0·009 | 0·96 | 938 | 6 | 0·32 | G | Meghalaya, S. Garo Hills Dt. | ||||||
| 71462 | 1·893±0·002 | 0·95 | 926 | 42! | 6 | 0·32 | G | Meghalaya, E. Garo Hills Dt. | |||||
| 71463 | 1·835±0·007 | 0·92 | 897 | 6 | 0·31 | G | Meghalaya, E. Garo Hills Dt. | ||||||
| 73406 | 1·872±0·001 | 0·94 | 915 | 6 | 0·31 | G | Meghalaya, E. Khasi Hills Dt. | ||||||
| C. roscoeana Wall. | 73309 | 1·962±0·005 | 1·96s | 0·98 | 959 | 42 | 6 | 0·33 | 0·33k | G | Andaman Isls. M. Andaman | ANI | |
| C. rubescens Roxb. | 71454 | 1·889±0·005 | 1·87st | 2·5 | 0·94 | 924 | 6 | 0·31 | 0·31lmnopqrs | L | Meghalaya, E. Garo Hills Dt. | NE | |
| 71457 | 1·843±0·004 | 0·92 | 901 | ca 42 | 6 | 0·31 | L | Meghalaya, E. Garo Hills Dt. | |||||
| C. rubrobracteata Škorničk., M. Sabu & Prasanthk. | 86241 | 1·836±0·008 | 1·84st | 0·92 | 898 | 42! | 6 | 0·31 | 0·31mnopqrs | L | Mizoram, Lawngtlai Dt. | NE | |
| C. sp. ‘sulphurea’ | 86231 | 1·853±0·002 | 1·85st | 0·93 | 906 | 42! | 6 | 0·31 | 0·31lmnopqrs | G | Mizoram, Lunglei Dt. | NE | |
| C. sp. ‘repens’ | 71456 | 1·834±0·007 | 1·83st | 0·92 | 897 | 42! | 6 | 0·31 | 0·31mnopqrs | L | Meghalaya, E. Garo Hills Dt. | NE | |
| HEXAPLOIDS–GROUP II | |||||||||||||
| C. angustifolia Roxb. | 73449 | 2·148±0·003 | 2·15r | 2·3 | 1·07 | 1050 | ca 42 | 6 | 0·36 | 0·36j | G | Uttaranchal, Dehra Dun Dt. | N & E |
| 73452 | 2·158±0·007 | 1·08 | 1055 | 42 | 6 | 0·36 | G | Uttaranchal, Tehri Gharwal Dt. | |||||
| 73452-II | 2·162±0·007 | 1·08 | 1057 | 6 | 0·36 | G | Uttaranchal, Tehri Gharwal Dt. | ||||||
| 73454 | 2·145±0·001 | 1·07 | 1049 | 6 | 0·36 | G | Uttaranchal, Tehri Gharwal Dt. | ||||||
| 73465 | 2·114±0·004 | 1·06 | 1034 | 6 | 0·35 | G | Jharkhand, Sahibganj Dt. | ||||||
| 73480 | 2·153±0·006 | 1·08 | 1053 | 6 | 0·36 | G | Chhattisgarh, Bilaspur Dt. | ||||||
| C. aurantiaca Zijp | 73455 | 2·223±0·008 | 2·20qr | 3·1 | 1·11 | 1087 | 6 | 0·37 | 0·37hij | G | Kerala, Kozhikode Dt. | SW | |
| 84108 | 2·156±0·007 | 1·08 | 1054 | 42 | 6 | 0·36 | G | Kerala, Kollam Dt. | |||||
| 77019 | 2·206±0·004 | 1·10 | 1079 | 42 | 6 | 0·37 | G | Kerala, Kozhikode Dt. | |||||
| C. cannanorensis R. Ansari, V. J. Nair & N. C. Nair | 84144 | 2·330±0·007 | 2·33op | 0·0 | 1·17 | 1139 | 42! | 6 | 0·39 | 0·39efg | G | Kerala, Kannur Dt. | SW |
| 84164 | 2·331±0·005 | 1·17 | 1140 | 42! | 6 | 0·39 | B | Karnataka, Udupi Dt. | |||||
| C. decipiens Dalzell | 73445 | 2·363±0·010 | 2·35op | 2·8 | 1·18 | 1156 | 6 | 0·39 | 0·39e | B | Maharashtra, Sindudurg Dt. | W | |
| 84179 | 2·305±0·003 | 1·15 | 1127 | 6 | 0·38 | G | Maharashtra, Sindudurg Dt. | ||||||
| 84179 | 2·370±0·007 | 1·19 | 1159 | 42 | 6 | 0·40 | B | Maharashtra, Sindudurg Dt. | |||||
| C. inodora Blatt. | 73403 | 2·290±0·006 | 2·29pq | 1·15 | 1120 | 42! | 6 | 0·38 | 0·38efg | G | Maharashtra, Thane Dt. | W | |
| C. karnatakensis Amalraj, Velay. & Mural | 84163 | 2·340±0·012 | 2·34op | 1·17 | 1144 | 42! | 6 | 0·39 | 0·39ef | B | Karnataka, Uttar Kannad Dt. | SW | |
| C. kudagensis Velay., V. S. Pillai & Amalraj | 84152 | 2·287±0·007 | 2·29pq | 1·14 | 1118 | 42! | 6 | 0·38 | 0·38efgh | B | Karnataka, Kodagu Dt. | SW | |
| C. neilgherrensis Wt. | 73490 | 2·251±0·011 | 2·29pq | 3·8 | 1·13 | 1101 | 6 | 0·38 | 0·38efg | G | Tamil Nadu, Nilgiris Dt. | S & SW | |
| 84157 | 2·336±0·001 | 1·17 | 1142 | 6 | 0·39 | G | Kerala, Wyanad Dt. | ||||||
| 84174 | 2·297±0·005 | 1·15 | 1123 | 42 | 6 | 0·38 | B | Tamil Nadu, Nilgiris Dt. | |||||
| 84181 | 2·273±0·001 | 1·14 | 1111 | 6 | 0·38 | G | Kerala, Wyanad Dt. | ||||||
| C. pseudomontana J. Graham | 73401 | 2·225±0·005 | 2·25pqr | 2·4 | 1·11 | 1088 | 6 | 0·37 | 0·38fghi | G | Maharashtra, Pune Dt. | W | |
| 73402 | 2·279±0·010 | 1·14 | 1114 | 42! | 6 | 0·38 | B | Maharashtra, Pune Dt. | |||||
| C. reclinata Roxb. | 73477 | 2·313±0·002 | 2·29pq | 3·9 | 1·16 | 1131 | 42! | 6 | 0·39 | 0·38efgh | G | Chhattisgarh, Bilaspur Dt. | C |
| 73467 | 2·226±0·008 | 1·11 | 1089 | 6 | 0·37 | G | Madhya Pradesh, Hoshangabad Dt. | ||||||
| 73469 | 2·292±0·003 | 1·15 | 1121 | 6 | 0·38 | G | Madhya Pradesh, Satna Dt. | ||||||
| 73469 | 2·309±0·007 | 1·15 | 1129 | 6 | 0·38 | G | Madhya Pradesh, Satna Dt. | ||||||
| HEXAPLOIDS–GROUP III | |||||||||||||
| C. coriacea Mangaly & M. Sabu | 73447 | 2·603±0·006 | 2·60lm | 1·30 | 1273 | 42! | 6 | 0·43 | 0·43c | B | Kerala, Idukki Dt. | SW | |
| C. mutabilis Škorničk., M. Sabu & Prasanthk. | 84145 | 2·492±0·004 | 2·49mn | 1·25 | 1219 | 42! | 6 | 0·42 | 0·42d | B | Kerala, Malappuram Dt. | SW | |
| C. sp. ‘aff. prakasha’ | 71443 | 2·446±0·005 | 2·45no | 1·22 | 1196 | 42! | 6 | 0·41 | 0·41d | B | Meghalaya, Ribhoi Dt. | NE | |
| C. sp. | 73417 | 2·501±0·001 | 2·50mn | 1·25 | 1223 | 42! | 6 | 0·42 | 0·42d | L | Assam, Bongaigaon Dt. | NE | |
| NONAPLOIDS | |||||||||||||
| C. aeruginosa Roxb. | 71431 | 2·825±0·013 | 2·86efg | 3·2 | 1·41 | 1381 | 63 | 9 | 0·31 | 0·32klmn | L | Assam, Bongaigaon Dt. | S |
| 84119 | 2·808±0·012 | 1·40 | 1373 | 9 | 0·31 | L | Kerala, Ernakulam Dt. | ||||||
| 84130 | 2·898±0·003 | 1·45 | 1417 | 9 | 0·32 | G | Kerala, Kozhikode Dt. | ||||||
| 86102 | 2·876±0·005 | 1·44 | 1406 | 9 | 0·32 | G | Kerala, Kottayam Dt. | ||||||
| 86354 | 2·889±0·005 | 1·44 | 1413 | 9 | 0·32 | G | Andaman Isls., S. Andaman | ||||||
| C. aromatica Salisb. s.l.–sp. 2 | 71460 | 2·863±0·014 | 2·83efghi | 1·7 | 1·43 | 1400 | 9 | 0·32 | 0·31klmnopq | L | Meghalaya, E. Garo Hills Dt. | NE | |
| 73410 | 2·844±0·001 | 1·42 | 1391 | 9 | 0·32 | G | Meghalaya, E. Khasi Hills Dt. | ||||||
| 71445 | 2·815±0·012 | 1·41 | 1377 | 9 | 0·31 | L | Meghalaya, E. Khasi Hills Dt. | ||||||
| 71447 | 2·822±0·013 | 1·41 | 1380 | 63 | 9 | 0·31 | L | Meghalaya, E. Khasi Hills Dt. | |||||
| 71453 | 2·820±0·012 | 1·41 | 1379 | 9 | 0·31 | L | Meghalaya, E. Garo Hills Dt. | ||||||
| C. aromatica Salisb. s.l.–sp. 3 | 71486 | 2·694±0·007 | 2·68jkl | 0·9 | 1·35 | 1317 | 9 | 0·30 | 0·30rs | G | Jharkhand, Devghar Dt. | E | |
| 71488 | 2·686±0·002 | 1·34 | 1313 | 9 | 0·30 | G | Jharkhand, Dumka Dt. | ||||||
| 71491 | 2·678±0·007 | 1·34 | 1310 | 9 | 0·30 | G | Jharkhand, Dumka Dt. | ||||||
| 71492 | 2·671±0·012 | 1·34 | 1306 | 9 | 0·30 | G | Jharkhand, Dumka Dt. | ||||||
| C. caesia Roxb. | 71418 | 2·825±0·011 | 2·82efghi | 4·9 | 1·41 | 1381 | 9 | 0·31 | 0·31klmnopq | G | Unknown, cultivated at CUBG | NE & E | |
| 71439 | 2·823±0·016 | 1·41 | 1380 | ca 63 | 9 | 0·31 | G | Meghalaya, Ribhoi Dt. | |||||
| 71439A | 2·830±0·011 | 1·42 | 1384 | 9 | 0·31 | L | Meghalaya, Ribhoi Dt. | ||||||
| 71439B | 2·782±0·007 | 1·39 | 1360 | 9 | 0·31 | L | Meghalaya, Ribhoi Dt. | ||||||
| 71451 | 2·893±0·008 | 1·45 | 1415 | 9 | 0·32 | G | Meghalaya, S. Garo Hills Dt. | ||||||
| 71459 | 2·892±0·005 | 1·45 | 1414 | 9 | 0·32 | L | Meghalaya, E. Garo Hills Dt. | ||||||
| 71469 | 2·759±0·008 | 1·38 | 1349 | 9 | 0·31 | L | W. Bengal, Darjeeling Dt. | ||||||
| 71490 | 2·825±0·004 | 1·41 | 1381 | 9 | 0·31 | G | Jharkhand, Pakur Dt. | ||||||
| 86222 | 2·788±0·011 | 1·39 | 1363 | 9 | 0·31 | G | Mizoram, Lunglei Dt. | ||||||
| C. codonantha Škorničk., M. Sabu & Prasanthk. | 73319 | 2·838±0·010 | 2·84efgh | 1·42 | 1388 | 63! | 9 | 0·32 | 0·32klmnop | G | Andaman Isls., N. Andaman | ANI | |
| C. elata complex | |||||||||||||
| C. elata Roxb. | 86321 | 2·853±0·014 | 2·85efg | 1·43 | 1395 | 9 | 0·32 | 0·32klmno | L | Andaman Isls., S. Andaman | ANI | ||
| C. latifolia Roscoe | 73321 | 2·801±0·005 | 2·80efghij | 1·40 | 1370 | 9 | 0·31 | 0·31lmnopqrs | G | Andaman Isls., M. Andaman | ANI | ||
| C. sp. ‘elata-latifolia’ | 71419 | 2·853±0·010 | 2·91e | 3·1 | 1·43 | 1395 | 9 | 0·32 | 0·32kl | L | Uttaranchal, Dehra Dun Dt. | N, E & NE | |
| 71423 | 2·941±0·012 | 1·47 | 1438 | 63 | 9 | 0·33 | L | W. Bengal, Jalpaiguri Dt. | |||||
| 71440 | 2·905±0·006 | 1·45 | 1421 | 9 | 0·32 | G | Meghalaya, Ribhoi Dt. | ||||||
| 71448 | 2·889±0·012 | 1·44 | 1413 | 9 | 0·32 | L | Meghalaya, S. Garo Hills Dt. | ||||||
| 71461 | 2·884±0·008 | 1·44 | 1410 | 9 | 0·32 | G | Meghalaya, E. Garo Hills Dt. | ||||||
| 71471 | 2·925±0·008 | 1·46 | 1430 | 9 | 0·33 | L | W. Bengal, Darjeeling Dt. | ||||||
| 71477 | 2·940±0·004 | 1·47 | 1438 | 9 | 0·33 | G | W. Bengal, Darjeeling Dt. | ||||||
| 71483 | 2·930±0·008 | 1·47 | 1433 | 9 | 0·33 | G | Uttaranchal, Dehra Dun Dt. | ||||||
| 73412 | 2·942±0·005 | 1·47 | 1439 | 9 | 0·33 | G | W. Bengal, Jalpaiguri Dt. | ||||||
| C. ferruginea Roxb. | 71479 | 2·805±0·005 | 2·80efghijk | 2·5 | 1·40 | 1372 | 9 | 0·31 | 0·31lmnopqrs | G | W. Bengal, S. 24-Prghanas Dt. | E & ANI | |
| 73320 | 2·814±0·001 | 1·41 | 1376 | 9 | 0·31 | G | Andaman Isls., N. Andaman | ||||||
| 73320-II | 2·818±0·008 | 1·41 | 1378 | 9 | 0·31 | G | Andaman Isls., N. Andaman | ||||||
| 86334B | 2·749±0·006 | 1·37 | 1344 | 9 | 0·31 | G | Andaman Isls., M. Andaman | ||||||
| C. leucorhiza Roxb. | 71489 | 2·714±0·013 | 2·70jkl | 1·0 | 1·36 | 1327 | 9 | 0·30 | 0·30qrs | L | Jharkhand, Dumka Dt. | E | |
| 71493 | 2·688±0·005 | 1·34 | 1314 | 9 | 0·30 | G | Jharkhand, Pakur Dt. | ||||||
| 73441 | 2·692±0·015 | 1·35 | 1316 | 63! | 9 | 0·30 | L | Bihar, Bhagalpur Dt. | |||||
| C. longa L. | 71420 | 2·621±0·011 | 2·71ijkl | 15·1 | 1·31 | 1282 | 9 | 0·29 | 0·30pqrs | L | W. Bengal, Kolkata | S, C, E & NE | |
| 71422 | 2·580±0·007 | 1·29 | 1262 | 63 | 9 | 0·29 | L | W. Bengal, Kolkata | |||||
| 71433 | 2·817±0·008 | 1·41 | 1378 | 63 | 9 | 0·31 | L | Assam, Bongaigaon Dt. | |||||
| 71436 | 2·603±0·012 | 1·30 | 1273 | 9 | 0·29 | L | Assam, Dispur Dt. | ||||||
| 71473 | 2·603±0·011 | 1·30 | 1273 | 9 | 0·29 | L | W. Bengal, Darjeeling Dt. | ||||||
| 71480 | 2·653±0·004 | 1·33 | 1297 | 9 | 0·29 | G | W. Bengal, S. 24-Parghanas Dt. | ||||||
| 71487 | 2·969±0·005 | 1·48 | 1452 | 9 | 0·33 | G | Jharkhand, Devgar, Dt. | ||||||
| 73303 | 2·751±0·004 | 1·38 | 1345 | 9 | 0·31 | G | Andaman Isls., N. Andaman | ||||||
| 73411 | 2·715±0·002 | 1·36 | 1328 | 9 | 0·30 | G | Meghalaya, Ribhoi Dt. | ||||||
| 73478 | 2·766±0·001 | 1·38 | 1353 | 9 | 0·31 | G | Chhattisgarh, Bilaspur Dt. | ||||||
| 84127 | 2·677±0·002 | 1·34 | 1309 | 9 | 0·30 | G | Kerala, Wyanad Dt. | ||||||
| 84154 | 2·665±0·004 | 1·33 | 1303 | 9 | 0·30 | G | Kerala, Palghat Dt. | ||||||
| 84160 | 2·656±0·007 | 1·33 | 1299 | 9 | 0·30 | G | Kerala, Wyanad Dt. | ||||||
| 86221 | 2·841±0·006 | 1·42 | 1389 | 9 | 0·32 | G | Mizoram, Lunglei Dt. | ||||||
| 86221-II | 2·846±0·006 | 1·42 | 1392 | 9 | 0·32 | G | Mizoram, Lunglei Dt. | ||||||
| 86221-III | 2·829±0·004 | 1·41 | 1383 | 9 | 0·31 | G | Mizoram, Lunglei Dt. | ||||||
| C. zanthorrhiza Roxb. | 73302 | 2·896±0·003 | 2·88ef | 2·9 | 1·45 | 1416 | 9 | 0·32 | 0·32klm | G | Andaman Isls., N. Andaman | S & ANI | |
| 84107 | 2·901±0·007 | 1·45 | 1419 | 9 | 0·32 | G | Kerala, Kollam Dt. | ||||||
| 84166 | 2·895±0·003 | 1·45 | 1416 | 9 | 0·32 | G | Kerala, Idukki Dt. | ||||||
| 84182 | 2·820±0·015 | 1·41 | 1379 | 63 | 9 | 0·31 | L | Kerala, Idukki Dt. | |||||
| 84182A | 2·839±0·003 | 1·42 | 1388 | 9 | 0·32 | G | Kerala, Idukki Dt. | ||||||
| C. sp. ‘fucata’ | 71430 | 2·805±0·013 | 2·80efghij | 1·40 | 1372 | 63! | 9 | 0·31 | 0·31lmnopqrs | L | Assam, Bongaigaon Dt. | NE | |
| C. sp. ‘man-and’ | 86306 | 2·804±0·006 | 2·76fghijk | 3·1 | 1·40 | 1371 | 63! | 9 | 0·31 | 0·31mnopqrs | L | Andaman Isls., S. Andaman | ANI |
| 86313 | 2·721±0·009 | 1·36 | 1331 | 9 | 0·30 | G | Andaman Isls., S. Andaman | ||||||
| C. sp. ‘picta’ | 71452 | 2·803±0·010 | 2·81efghijk | 3·7 | 1·40 | 1371 | 9 | 0·31 | 0·31lmnopqrs | L | Meghalaya, S. Garo Hills Dt. | NE & SL | |
| 71464 | 2·782±0·014 | 1·39 | 1360 | 9 | 0·31 | L | Meghalaya, E. Garo Hills Dt. | ||||||
| 71465 | 2·753±0·009 | 1·38 | 1346 | 9 | 0·31 | L | Assam, Kokrajar Dt. | ||||||
| 73422 | 2·854±0·004 | 1·43 | 1396 | 9 | 0·32 | G | Sri Lanka, Kegalle Dt. | ||||||
| C. sp. ‘roxburgh’ | 71434 | 2·717±0·012 | 2·72hijkl | 1·36 | 1329 | ca 63! | 9 | 0·30 | 0·30opqrs | L | Assam, Bongaigaon Dt. | NE | |
| C. sp. ‘tikhur’ | 73476 | 2·735±0·001 | 2·74ghijk | 1·37 | 1337 | 9 | 0·30 | 0·30nopqrs | G | Chhattisgarh, Bilaspur Dt. | C | ||
| C. sp. ‘aff. zanthorrhiza’ | 73420 | 2·672±0·005 | 2·67kl | 0·5 | 1·34 | 1307 | 9 | 0·30 | 0·30s | G | Jharkhand, Paschim Singhbum Dt | E | |
| 73420A | 2·663±0·013 | 1·33 | 1302 | 9 | 0·30 | G | Jharkhand, Paschim Singhbum Dt | ||||||
| 73420B | 2·676±0·011 | 1·34 | 1309 | 9 | 0·30 | G | Jharkhand, Paschim Singhbum Dt | ||||||
| 11-PLOID | |||||||||||||
| C. oligantha Trimen | 73325 | 4·755±0·026 | 4·76a | 2·38 | 2325 | 77!! | 11 | 0·43 | 0·43c | B | Sri Lanka, Badulla Dt. | SL | |
| cf. 12-PLOID | |||||||||||||
| C. sp. ‘ranchi’ | 71485 | 3·713±0·005 | 3·71c | 1·86 | 1816 | > ca 70! | ≤ 12 | ≥ 0·31 | 0·31lmnopqrs | G | Jharkhand, Ranchi Dt. | E | |
| 15-PLOIDS | |||||||||||||
| C. raktakanta Mangaly & M. Sabu | 73414 | 4·614±0·010 | 4·57b | 5·1 | 2·31 | 2256 | 15 | 0·31 | 0·30nopqrs | G | Assam, Bongaigaon Dt. | SW & NE | |
| 71432 | 4·413±0·017 | 2·21 | 2158 | 105!! | 15 | 0·29 | L | Assam, Bongaigaon Dt. | |||||
| 84120 | 4·637±0·012 | 2·32 | 2267 | 15 | 0·31 | L | Kerala, Ernakulam Dt. | ||||||
| 84120 | 4·637±0·012 | 2·32 | 2267 | 105!! | 15 | 0·31 | G | Kerala, Ernakulam Dt. | |||||
| 84148 | 4·638±0·003 | 2·32 | 2268 | 15 | 0·31 | G | Kerala, Trichur Dt. | ||||||
| HYBRID | |||||||||||||
| C. angustifolia × montana | 73480B | 1·903±0·005 | 1·90st | 0·95 | 931 | 6 | 0·32 | 0·32klmno | G | Chhattisgarh, Bilaspur Dt. | C | ||
| RELATED GENERA | |||||||||||||
| Hitchenia caulina (J. Graham) Baker (= C. caulina J. Graham) | 84178 | 2·245±0·011 | 2·24pqr | 1·12 | 1098 | 42! | 6 | 0·37 | 0·37ghi | B | Maharashtra, Satara Dt. | W | |
| Kaempferia scaposa (Nimmo) Benth. ( = C. scaposa (Nimmo) Škorničk. & M. Sabu, comb. nov.) | 77029 | 2·333±0·007 | 2·33op | 1·17 | 1141 | 42! | 6 | 0·39 | 0·39efg | G | Goa, N. Goa Dt. | W | |
| Paracautleya bhatii R. M. Sm. ( = C. bhatii (R.M.Sm.) Škorničk. & M. Sabu) | 73446 | 2·181±0·003 | 2·18qr | 1·09 | 1067 | 42! | 6 | 0·36 | 0·36ij | G | Karnataka, Udupi Dt. | SW | |
| Stahlianthus involucratus (King ex Baker) Craib ex Loes. | 71449 | 3·114±0·003 | 3·11d | 1·56 | 1523 | (22) | 2 | 1·56 | 1·56 a | G | Meghalaya, S. Garo Hills Dt. | NE |
* Letters indicate group of taxa that are not significantly different at α = 0·05.
† Chromosome numbers determined in the present work, ! new species count, !! new count for a genus. Chromosome numbers taken from the literature are given in parentheses.
‡ B, Bellis perennis L. (2C = 3·96 pg); G, Glycine mas ‘Polanka’ (2C = 2·5 pg, primary reference standard); L, Solanum lycopersicum ‘Stupnické polní tyčkové rané’ (2C = 2·21 pg).
§ ANI, Andaman Islands; C, Central India; E, Eastern India; N, Northern India; S, Southern India; SL, Sri Lanka; W, Western India.
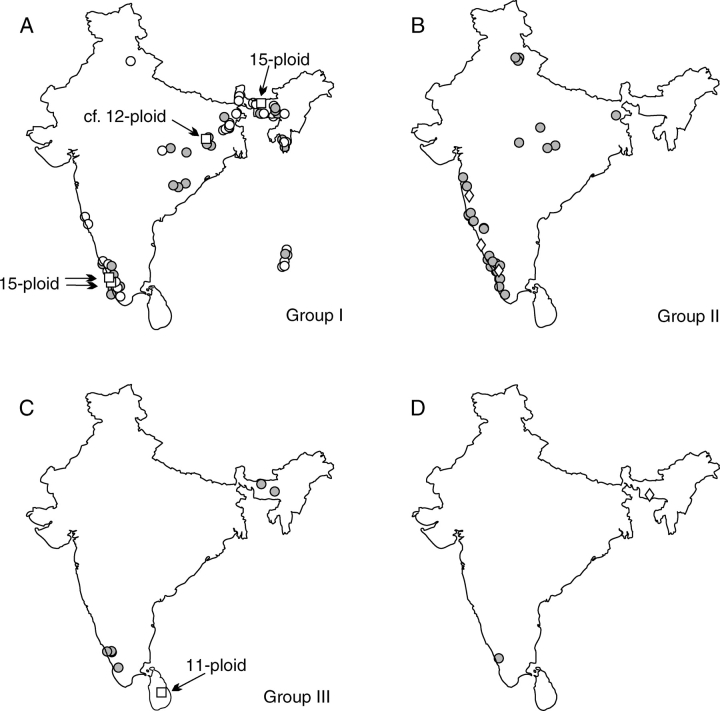
Fig. 1.
Geographical origin of the plant samples analysed. (A) Species from genome group I (1Cx = 0·30–0·33 pg) and x = 7; (B) species from genome group II (1Cx = 0·36–0·39 pg) and x = 7; (C) species from genome group III (1Cx = 0·41–0·43 pg) and x = 7; (D) species with 1Cx > 0·83 pg and x = 11 (circle, Curcuma vamana; diamond, Stahlianthus involucratus). Symbol explanation (unless otherwise indicated): closed circles, hexaploids; open circles, nonaploids; open diamonds, Curcuma-like species often placed into separate genera; open squares, high polyploids designated by a corresponding ploidy level.
Fig. 5.

Phenotypic diversity of Curcuma species included in the study. (A) Curcuma vamana (accession number 84156); (B) C. cannanorensis (no. 84144); (C) C. raktakanta (no. 84120). (D) C. roscoeana (no. 73309); (E) C. oligantha (no. 73325); (F) C. ( = Paracautleya) bhatii (no. 73446); (G) C. ( = Hitchenia) caulina (no. 84178); (H) C. kudagensis (no. 84152); (I) C. ( = Kaempferia) scaposa (no. 77029). Individual plates not to scale.
Genome size estimation
Nuclear DNA C-values ( = holoploid genome sizes) and Cx-values ( = monoploid genome sizes) were estimated using propidium iodide flow cytometry (FCM). Sample preparation generally followed the two-step procedure originally described by Otto (1990). Plants were cultivated for at least 1 year under homogeneous conditions. About 1 cm2 of young and intact fresh leaf tissue and internal standard was co-chopped in a sandwich-like arrangement with a sharp razor blade in 1 mL of ice-cold Otto I buffer (0·1 m citric acid, 0·5 % Tween 20). The nuclear suspension was filtered through a nylon mesh (42-μm pore size) and centrifuged at 150g for 5 min. The supernatant was then removed and nuclei were gently resuspended in 100 µL of fresh Otto I buffer. After incubation (15 min at room temperature), 1 mL of Otto II buffer (0·4 m Na2HPO4.12H2O) supplemented with propidium iodide (at a final concentration 50 µL mL–1), RNase IIA (50 µL mL–1) and 2-mercaptoethanol (2 µL mL–1) was added. The samples were incubated for 30 min at room temperature, after which fluorescence intensity of 5000 particles was recorded on a Partec Cyflow instrument (Partec GmbH, Münster, Germany) equipped with a 532-nm solid-state laser (Cobolt Samba 100 mW, Cobolt, Sweden). Each plant was re-analysed at least three times on different days and only histograms with peaks of approximately the same height were accepted. If between-day variation (max./min. value) exceeded 2 %, the outlying value was discarded and the sample re-measured. Glycine max ‘Polanka’ (2C = 2·50 pg; Doležel et al., 1994) was selected as a primary internal reference standard. Solanum lycopersicum L. ‘Stupické polní tyčkové rané’ (2C = 2·11 pg) and Bellis perennis L. (2C = 3·96 pg) were used as secondary reference standards for Curcuma samples with low and high genome sizes, respectively, in order to minimize standard-to-sample peak ratio and thus avoid potential non-linearity of FCM measurements. Genome sizes of secondary reference standards were calibrated against the primary one, based on nine replications on different days. The total number of flow cytometric measurements for Curcuma was 678.
Chromosome counts
Chromosome numbers were counted in actively growing root tips of the cultivated plants. Samples were pretreated with a saturated solution of p-dichlorbenzene (3 h, room temperature), fixed in a 3:1 mixture of ethanol and acetic acid (4 h, 4 °C), macerated in 1:1 hydrochloric acid/ethanol (30 s, room temperature) and immediately squashed in a drop of lactopropionic orceine. The number of chromosomes was determined in 5–10 complete well-spread mitotic plates using a Carl-Zeiss Jena NU microscope equipped with an Olympus Camedia C-2000 Z camera.
Statistical analysis
Statistical analyses were performed in the SAS 8·1 statistical package (SAS Institute, Cary, NC, USA). Between-species differences in genome size were tested using GLM (general linear models) because of unbalanced data design, and Tukey's procedure was applied to compare mean values. The Spearman-rank correlation coefficient (CORR procedure) was used to test whether mean genome size of the taxa was related to the geographical location of the populations.
RESULTS
Chromosome counts and ploidy levels
Chromosome numbers together with inferred ploidy levels for the majority of the studied taxa (84 %) are given in Table 2. In total, 50 plants (i.e. nearly one-third of all accessions) were analysed karyologically. Forty-one taxa (including undetermined samples) yielded definite chromosome numbers, a preliminary count was obtained for one tentatively determined specimen (C. sp. ‘ranchi’ with 2n > 70), and a single chromosome record referring to Stahlianthus involucratus was taken from the literature (as Kaempferia involucrata; Bisson et al., 1968). Six different chromosome numbers were identified (i.e. 2n = 22, 42, 63, >70, 77 and 105). The majority of these are multiples of x = 7, which may be regarded as a genuine basic chromosome number. Consequently, plants with 42, 63, 77 and 105 somatic chromosomes correspond to hexaploids, nonaploids, 11-ploids and 15-ploids, respectively.
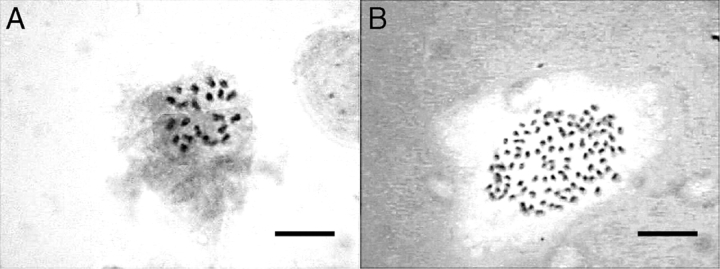
New chromosome numbers were found in two species, C. oligantha (2n = 77) and C. raktakanta (2n = 105). Both also represent new generic records and the latter is the highest chromosome number so far determined in Zingiberaceae. Micrographs documenting metaphase chromosomes in C. vamana (2n = 22) and C. raktakanta (2n = 105) are shown in Fig. 2.
Fig. 2.
Chromosome complements of (A) C. vamana (species with the smallest number of chromosomes) and (B) C. raktakanta (species with the highest number of chromosomes) showing 22 and 105 somatic chromosomes, respectively. Scale bars = 10 µm. In (A), two micrographs were taken at different focal planes and computer-merged in order to achieve sufficient image sharpness.
Genome size variation
Table 2 summarizes the results for 161 samples belonging to 51 taxa of Curcuma and related genera. The majority of genome size estimates represent novel records; previous C-values were available for only ten taxa.
Flow cytometric analyses yielded high-resolution histograms (Fig. 3). Coefficients of variation (CVs) of G0/G1 peaks ranged from 0·89 to 5·93 % (mean 2·60) for Curcuma samples and from 1·13 to 6·54 % (mean 2·98) for the reference standard. An arbitrary threshold of 3·0 % was not exceeded in 72 and 62 % of Curcuma and internal standard runs, respectively. Between-day fluctuation in FCM measurements due to instrument instability or non-identical sample preparation was negligible, with standard error of the mean ranging from 0·02 to 0·55 % of the estimated 2C-value. Reliability of determined DNA amounts was repeatedly confirmed in simultaneous FCM analyses, which gave two distinct peaks even in Curcuma samples with only small differences in genome size (see Fig. 3B).
Fig. 3.
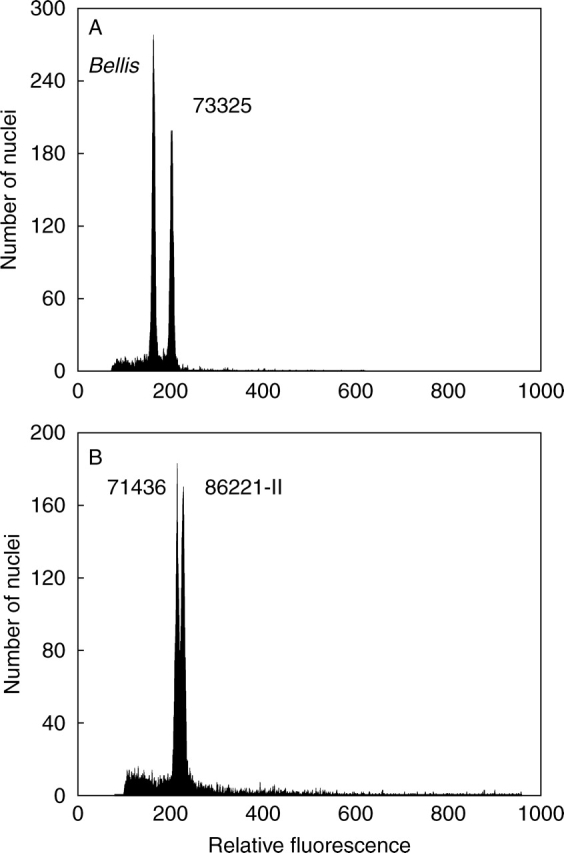
(A) Representative flow cytometric histogram documenting genome size determination in Curcuma oligantha (accession number 73325) using Bellis perennis as internal reference standard. (B) Flow cytometric evidence for genome size variation in Curcuma longa [simultaneous analysis of accessions 71436 (2C = 2·60 pg) and 86221-II (2C = 2·85 pg)]. Plant nuclei were isolated, stained with propidium iodide and measured simultaneously.
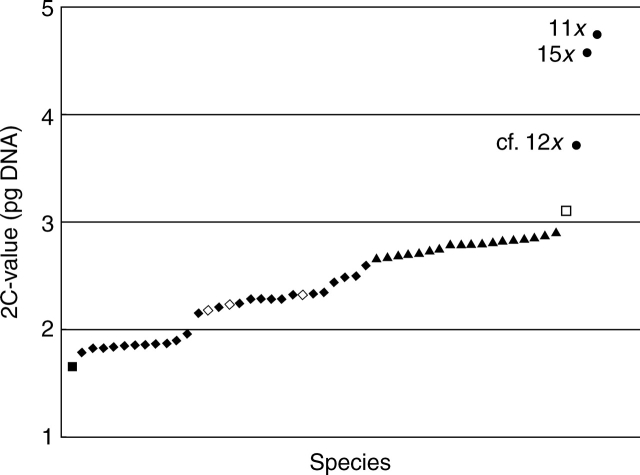
Mean 2C-values varied from 1·66 pg in diploid C. vamana (2n = 2x = 22) to 4·76 pg in C. oligantha (2n = 11x = 77), a 2·87-fold range (Fig. 4). Homoploid genome sizes ranged from 0·30 pg in several species to 1·56 pg in Stahlianthus involucratus, a 5·25-fold range. Most of the species with more than one accession showed low intraspecific genome size variation (3·4 % on average). However, differences between maximum and minimum C-values that exceeded 4 % were observed in five species (C. prakasha, C. caesia, C. raktakanta, C. montana and C. longa – arranged by increasing percentage variation) reaching 15·1 % in C. longa (Fig. 3B).
Fig. 4.
2C-value distribution (DNA pg means) for 51 taxa investigated. Symbol explanation: squares, diploids (2n = 2x = 22); diamonds, hexaploids (2n = 6x = 42); triangles, nonaploids (2n = 9x = 63); circles, higher polyploids designated by a corresponding ploidy level. Closed symbols, Curcuma species; open symbols, closely related taxa often placed into separate genera.
The value of FCM data for taxonomic purposes (species delineation) seems to be rather limited owing to the small number of species-specific genome sizes (see Table 2). Nonetheless, there are some species alliances with phenotypic similarities in which nuclear DNA amounts may provide a clue for accurate determination, C. cannanorensis (2C = 2·33 pg) – C. oligantha (2C = 4·76 pg) being a representative example (Fig. 5). In addition, genome size reflected morphological variation in the C. aromatica complex, in which three taxonomic entities with distinct DNA amounts are currently recognized (Table 2). The genome size of a putative hybrid between C. angustifolia and C. montana was close to the mean of those of the parental species.
Hexaploid Curcuma species showed marked variation in homoploid genome sizes, amounting to 45%. This variation was discontinuous and three groups of taxa with significantly different Cx-values (P < 0·0001, n = 24) could be distinguished (Table 3). These groups corresponded well to the geographical origin of the samples (Fig. 1). Higher polyploids mirrored this pattern and split into a genome group I (1Cx = 0·30–0·32 pg) and a genome group III (1Cx > 0·40 pg) cluster. To gain closer insights into the genome size variation, relationships between Cx-values and geographical locations of all samples with x = 7 were examined. A significant negative correlation was found for both latitude (Spearman r = –0·50, P = 0·0004, n = 49) and longitude (r = –0·63, P < 0·0001, n = 49).
Table 3.
Summary of chromosome counts and genome size estimates for species of Curcuma and related genera determined in the present study
| Ploidy level or DNA ploidy | No. of chromosomes (2n) | No. of taxa/no. of individuals | 2C-value range (pg) | 1Cx-value range (pg) |
|---|---|---|---|---|
| Curcuma | ||||
| 2x | 22 | 1/1 | 1·66 | 0·83 |
| 6x, group I | 42 | 10/42 | 1·79–1·96 | 0·30–0·33 |
| 6x, group II | 42 | 10/27 | 2·15–2·35 | 0·36–0·39 |
| 6x, group III | 42 | 4/4 | 2·45–2·60 | 0·41–0·43 |
| 9x | 63 | 18/75 | 2·67–2·91 | 0·30–0·32 |
| 11x | 77 | 1/1 | 4·76 | 0·43 |
| cf. 12x | >70 | 1/1 | 3·71 | (≥0·31) |
| 15x | 105 | 1/5 | 4·57 | 0·30 |
| Related genera | ||||
| 2x | 22 | 1/1 | 3·11 | 1·56 |
| 6x | 42 | 3/3 | 2·18–2·33 | 0·36–0·39 |
As diploids were found to possess a different basic chromosome number than polyploids (i.e. 11 vs. 7), potential changes in homoploid genome size with respect to ploidy level were difficult to assess. Nevertheless, average DNA content per chromosome in two diploid species (C. vamana and Stahlianthus involucratus) exceeded the corresponding values in polyploid taxa.
With the exception of Stahlianthus involucratus, genome sizes of other species often placed in separate genera (Hitchenia caulina, Kaempferia scaposa, and Paracautleya bhatii; Fig. 5) fitted well into the range of C-values of hexaploid curcumas (they also shared the same number of chromosomes).
DISCUSSION
Comparison of chromosome numbers with previous investigations
Our karyological analyses revealed six different chromosome counts, 2n = 22, 42, 63, >70, 77 and 105. The somatic numbers 42 and 63 predominated and the other counts were each encountered in one taxon only (2n = 22 in C. vamana, 2n > 70 in an undetermined sample from eastern India, 2n = 77 in C. oligantha and 2n = 105 in C. raktakanta; Figs 2 and 5). The last two records represent new chromosome numbers for the genus Curcuma, and 2n = 105 is the highest somatic count recorded so far for the family Zingiberaceae. No intraspecific variation in the chromosome number was detected. Nevertheless, it should be noted that chromosome counts were only analysed in more than one individual for eight taxa only.
A targeted search for karyological data in Curcuma showed that chromosome numbers have so far been published for 40 identified species and four taxa of unclear identity (see Table 1), 17 of which were also included in our study. Generally, there was agreement between the data sets, although some discrepancies occurred. In particular, several different chromosome counts for the same species have been reported in some cases, but this was not confirmed in our study. Moreover, previous numbers showed evidence of aneuploidy, a phenomenon not encountered by us. Such incongruities may be explained either by existence of karyological variation not sampled in the present study, species misidentification or a broader species concept adopted in earlier studies; lack of herbarium vouchers, however, precludes verification of the taxonomic identity of previously studied plant material in many cases.
For example, two different numbers had previously been published for C. rubescens (2n = 42 and 2n = 63; Islam, 2004), but only the former was confirmed in our study. We believe that the plants with 63 chromosomes belonged to another species because C. rubescens was originally described to produce both lateral and central inflorescences by Roxburgh (1810), a feature common in hexaploids but unknown in any nonaploid species. A parallel situation appears to exist in C. mangga. Again, we found only 42 chromosomes in this species, but found 2n = 63 in an undetermined, though closely related, specimen (accession no. 86306). We have some doubts about the degree of chromosomal variation in C. aromatica (published 2n = 42, 63, 84 and 86). Current taxonomic investigations revealed that this is a complex group composed of several species that possess either 42 or 63 chromosomes according to the present results. Despite representative sampling, attempts to find other chromosome numbers within the C. aromatica aggregate were unsuccessful, either using direct karyological counting or using indirect FCM measurements. Finally, there are apparent discrepancies in previous chromosome numbers for C. malabarica (2n = 42) and C. raktakanta (2n = 63; both published by Joseph et al., 1999). The counts here based on material from the type localities showed uniformly 2n = 105. These taxa should be treated as one species (C. raktakanta) as also indicated by morphology. Considering the discrepancies, it is likely that genuine C. malabarica and C. raktakanta were not involved in the study of Joseph et al. (1999).
In addition to euploid chromosome numbers, several apparently aneuploid counts (i.e. 62, 64, 66, etc.) have been reported (see Table 1). Prana (1977) claimed that this phenomenon may be associated with abnormal mitosis occasionally encountered in Indonesian polyploid curcumas. Daughter cells with unequal number of chromosomes may be formed in this way and lead to incidence of aneuploidy and/or chromosomal chimaeras, especially in plants with predominantly vegetative reproduction. However, no aneuploids were found in the present study. Although we do not a priori reject such an option (e.g. intraspecific variation in genome size observed in some Curcuma spp. may potentially refer to chromosomal heterogeneity), several records of aneuploidy are suspicious and should be interpreted with caution until reliably confirmed. Karyological investigation of Curcuma spp. is rather challenging and errors may easily be introduced due to both relatively high number and small size (mostly 0·5–2 µm) of chromosomes (Ramachandran, 1961; Apavatjrut, 1996; Joseph et al., 1999; Sirisawad et al., 2003). It should also be noted that majority of doubtful counts were published several decades ago and have not been recorded since.
Basic chromosome number and ploidy level variation
Since the pioneering karyological surveys, there has been continuous dispute concerning the basic chromosome number in Curcuma. Investigating 25 Zingiberaceae species, including three curcumas, Raghavan and Venkatasubban (1943) were the first to report x = 21. Sato (1948) suggested x = 8 in his early work on C. longa, but later claimed that two basic numbers (x = 7 and x = 8) occur in the genus (Sato, 1960). Further support for x = 21 appeared in the study of Ramachandran (1961), who observed regular bivalent formation during meiosis in C. decipiens (2n = 42) and a high frequency of trivalents in C. longa (under the name C. domestica) with 2n = 63. In line with Venkatasubban (1946), Ramachandran considered x = 21 a secondary number possibly derived from a combination of x = 9 and x = 12, which are common in several genera of Zingiberaceae. Yet another basic chromosome number, x = 16, has been proposed and adopted by some researchers (e.g. Sharma and Bhattacharya, 1959). However, x = 21 became the most commonly accepted basic chromosome number in Curcuma (Ramachandran, 1961; Prana, 1977; Prana et al., 1978; Ardiyani, 2002; Islam, 2004).
It should be noted that the lower alternative, x = 7, is not in conflict with the large majority of published somatic counts. Actually, this value fits better with the range of chromosome numbers currently known (see our prime count 2n = 77). We therefore believe that x = 7 should be considered a primary basic chromosome number, at least for the majority of Indian Curcuma species (belonging to the subgenus Curcuma). Grant (1982) regarded x = 7 and 10 as the most common basic chromosome numbers in monocots so the present findings are in concordance with a large body of evidence. Following this calculation, species investigated here correspond to 6x, 9x, 11x and 15x cytotypes. In addition, dodecaploid (2n = 84) plants from subgenus Curcuma were reported by earlier researchers (Table 1).
It appears that there are some Curcuma species in which somatic chromosome numbers (2n = 20, 22, 24, 32, 34, 36 and 38) do not fit into the series based on x = 7. However, they all belong to the subgenus Hitcheniopsis, which encompasses mainly Thai species such as C. alismatifolia, C. gracillima, C. harmandii, C. parviflora, C. rhabdota and C. thorelii (see Table 1), and which differs markedly in morphological traits from the nominate subgenus. From the present species set, only C. vamana (2n = 22) belongs to subgenus Hitcheniopsis. We are convinced that this species is only distantly related to the other investigated taxa (subgenus Curcuma), and its basic chromosome number is most plausibly x = 11, as also documented for several other genera of Zingiberaceae (e.g. Kaempferia, Zingiber and Renealmia).
It is plausible that both subgenera Curcuma and Hitcheniopsis represent independent evolutionary units with distinct basic chromosome numbers. In addition, x = 7 may be considered a subgenus-specific cytological marker for the former.
Inter- and intraspecific variation in genome size
The taxa involved in the present study differed 2·87-fold in their holoploid genome sizes (2C = 1·66–4·76 pg). This range is slightly below the mean for other plant genera (3·28-fold based on data from the Plant DNA C-values database; Bennett and Leitch, 2005a). There was no clear gap in nuclear DNA C-values between hexaploid and nonaploid plants and a major discontinuity in these cytotypes actually occurred between two groups of hexaploids (Fig. 4). These results indicate that DNA content alone is often insufficient to distinguish between 6x and 9x cytotypes, and FCM measurements should always be accompanied by chromosome counts. The C-values of diploid and high polyploid taxa were more distinct, but again FCM measurements alone may not be an accurate indicator of ploidy level, as illustrated by 11-ploid C. oligantha and 15-ploid C. raktakanta with reverse total amounts of nuclear DNA (Fig. 4).
By contrast, homoploid genome sizes formed three well-defined clusters with significantly different values, namely a genome group I (1Cx = 0·30–0·33 pg; 29 taxa), a genome group II (1Cx = 0·36–0·39; 13 taxa) and a genome group III (1Cx = 0·41–0·43 pg; five taxa). We assume that these groups may have different evolutionary histories, as also supported by their distinct distribution patterns (see below).
Intraspecific genome size variation was low in most taxa in which multiple individuals were analysed. However, values above 4 % (i.e. beyond potential instrumental fluctuation) were encountered in five species. When C. longa with about 1·15-fold divergence in C-value is excluded, the extent of intraspecific variation was comparable in all cytotypes: hexaploids, 0·0–6·1 %; nonaploids, 0·5–4·9 %; and a 15-ploid, 5·1%. Intraspecific variation in nuclear DNA content has been controversial since the early studies on genome size. This debate has been fuelled by numerous early reports of intraspecific variation, many of which were dismissed in subsequent investigations using the best practice methodology (Greilhuber, 2005). Several sources of artefactual variation have been identified: (1) instrumental or methodological errors; (2) disturbing effects of secondary metabolites with potential seasonal fluctuation (e.g. Walker et al., 2006); (3) differences in measurements among different laboratories (Doležel et al., 1998); and (4) taxonomic heterogeneity of the material under investigation (Murray, 2005). As a result, the concept of genome size stability has gained broader support. Examples have nonetheless been accumulated in recent years of some genome size variation in FCM assays despite meticulous methodology (Obermayer and Greilhuber, 2005; Šmarda and Bureš, 2006). Several Curcuma species are known to contain, particularly in rhizomes, phenolic secondary metabolites such as curcumins (Lubis, 1968; Prana, 1977). Although phenolics may bias genome size estimates (Greilhuber, 1998; Walker et al., 2006), we believe that our FCM measurements are accurate for several reasons. Low coefficients of variation were achieved, which are not compatible with the presence of interfering metabolites. Nuclear suspensions were clear, lacking any coatings of debris that might indicate a negative effect of metabolites (Loureiro et al., 2006). Repeated measurements of the same sample yielded nearly identical genome size values (average difference 0·8 %) even if particular FCM analyses were performed in different years. Between-plant differences remained stable also in studies using DAPI (an AT-selective fluorochrome less sensitive to secondary metabolites than the intercalating propidium iodide). Also, a narrow species concept was adopted to guarantee taxonomic homogeneity of the plant material. Moreover, co-processed samples with different genome sizes always gave two distinct peaks, which is the most convincing evidence for genuine differences in DNA content (Greilhuber, 2005).
The reasons for intraspecific genome size variation in Curcuma remain unknown. Aneuploidy or presence of B-chromosomes may lead to heterogeneity in nuclear DNA amount, but this explanation seems rather unlikely as only euploid numbers were revealed in our study and, more importantly, two accessions of C. longa with more than 9 % genome size variation possessed the same number of chromosomes (see Table 2). Plausibly, intraspecific variation may be related to a long-term cultivation and targeted selection of desirable genotypes in several Curcuma species, C. longa in particular. India is responsible for around 90 % of turmeric production worldwide, and this species has been widely used in India since Vedic times. Perhaps the variation may have adaptive value, as previously documented in another crop, Zea mays (Rayburn and Auger, 1990). Murray (2005) argued that intraspecific genome size variation may indicate incipient speciation; the blurred species boundaries in several Curcuma alliances may support such a hypothesis. A third explanation takes into account heterochromatic polymorphism. In vegetatively propagating lines, strains with smaller and larger telomeric or centromeric heterochromatin regions may occur (J. Greilhuber, University of Vienna, Austria, pers. comm.) leading to greater genome sizes.
Comparison of genome sizes in Curcuma with those in other members of Zingiberaceae and other monocots
More than three-quarters of the taxa (39 out of 51) in the present study possessed very small genomes defined as 1C ≤ 1·40 pg (Leitch et al., 1998), whereas the remaining 12 taxa had small genomes (i.e. 1C = 1·41–3·50 pg). Low nuclear DNA content in Curcuma spp. is in line with previous records for other members of Zingiberaceae and related families. The Plant DNA C-values database (Bennett and Leitch, 2005a) includes measurements for three members of Zingiberaceae with 1C-values varying from 1·30 pg in Curcuma zantorrhiza (misspelled as C. xanthorrhiza) to 6·03 pg in Zingiber officinale. Very small genomes were also revealed in all but one (Lowiaceae with 1C = 3·55 pg) families from the order Zingiberales, including Marantaceae (14 species, 1C = 0·33–0·68 pg), Musaceae (six species, 1C = 0·58–0·61 pg), Heliconiaceae (1C = 0·45 pg), Strelitziaceae (1C = 0·58 pg), Cannaceae (1C = 0·72 pg) and Costaceae (1C = 1·00 pg).
Although only one Curcuma record is included in the Plant DNA C-values database, more genome size estimates have been published (Table 1). Das et al. (1999) used Feulgen densitometry to analyse three Curcuma spp.: C. caesia (2n = 22, 4C = 3·120 pg), C. amada (2n = 40, 4C = 4·234 pg) and C. longa (2n = 48, 4C = 5·100–5·263 pg). Curcuma longa was also investigated by Nayak et al. (2006) who reported 4C-values from 4·30 to 8·84 pg (i.e. 2·06-fold variation) in 17 cultivars. However, both these studies come from the same laboratory and we believe the results should be treated with caution. First, chromosome counts have not been confirmed by other researchers. Moreover, the authors used hot hydrolysis, which is strongly discouraged owing to the very short optimum treatment that is difficult to control (Greilhuber, 2005). No fixative solution was specified, and if a standard acetic acid/alcohol fixation was applied to plant samples rich in phenolics, stoichiometry of the Feulgen staining was likely to be distorted (the so-called ‘self-tanning’ effect; Greilhuber, 1986, 1988). Feulgen methodology is also not recommended for conventional chromosome counting in Curcuma as it does not allow good spreading of the root tips issue on slides (Islam, 2004) and gives rather pale staining. By contrast, the FCM data published for Curcuma spp. collected in Bangladesh by Islam (2004) seem to be accurate, at least from the methodological point of view. Unfortunately, genome size estimates were not linked to a particular herbarium voucher, which lessens their value. We had the opportunity to examine several well-prepared herbarium specimens prepared from Islam's living material and encountered some misidentifications (e.g. for C. aeruginosa and C. zedoaria).
In comparison with Islam's work (Islam, 2004), our estimates for ten species in common were on average 14 % lower, whereas we determined genome size to be about 11 % higher in C. zantorrhiza than that reported by Bharathan et al. (1994). Plausibly, different reference standards could be responsible for such divergences (i.e. Raphanus sativus – Islam, 2004; chicken red blood cells – Bharathan, 1994).
Species distribution and origin of high polyploids with respect to homoploid genome size
Excluding the distantly related C. vamana, species with the lowest number of chromosomes (2n = 6x = 42) clustered in three groups with significantly different Cx-values (Table 3), which also showed a distinct geographical pattern (Fig. 1). Group I (1Cx = 0·30–0·33 pg) contained ten taxa, the majority of which occurred in north-east India, group II (1Cx = 0·36–0·39 pg) contained ten taxa with a centre of distribution in the Western Ghats (west and south-west India) and Central India and group III (1Cx = 0·41–0·43 pg) contained four taxa occurring either in south-west or north-east India. A link between genome size and geographical location was also confirmed by correlation analysis, which revealed highly significant negative relationships between Cx-values and both latitude and longitude of the localities.
Homoploid genome sizes of all but one (C. oligantha) high-polyploid taxa matched perfectly the Cx-values of hexaploids from group I. This allows us to hypothesize that these hexaploids have played a key role in the evolution of polyploidy in Indian Curcuma spp. Nonaploid cytotypes probably originated by a fusion of reduced and unreduced gametes of hexaploids, either within or between species, giving rise to auto- or allopolyploids. Within Zingiberaceae, regular production of gametes with the somatic number of chromosomes has been reported in the genus Globba (Takano and Okada, 2002), which shows similar ploidy composition to Curcuma. Both nonaploid (unreduced gamete) and hexaploid (reduced and unreduced gamete) plants were probably involved in the genesis of rare dodecaploid and 15-ploid taxa. The vast majority of nonaploid species undergo asexual propagation via rhizome branching and produce a high percentage of aborted pollen grains (Prana, 1977; Nasir Uddin, 2000). It is plausible, however, that even a small fraction of viable pollen may eventually lead to the formation of higher polyploids. The origin of 11-ploid C. oligantha cannot be explained with certainty given the current stage of knowledge. Homoploid genome size (1Cx = 0·43 pg) of this species fits in the range of a genome group III.
Genome size and chromosome numbers as supportive markers for delimitation of Curcuma species
Chromosome numbers and/or ploidy levels have long been utilized as an efficient taxonomic marker, helping to delimit boundaries between various taxonomic categories or reveal cryptic taxa (Stace, 2000). In addition, the last decade has seen significant progress in the application of FCM to exploit differences in nuclear DNA content, which can in some cases be used as a supportive marker for distinguishing between closely related taxa at both heteroploid and homoploid levels (e.g. Mahelka et al., 2005).
As Curcuma is a taxonomically challenging group, the usefulness of genome size and chromosome counts as supportive markers for taxon determination was assessed. Although the low C-value variation (2·87-fold) and the small number of species-specific DNA amounts reduce their utility, some alliances with morphological similarities have been identified in which cytogenetic data may nonetheless support taxonomic decisions. For example, C. oligantha (described from Sri Lanka) and C. cannanorensis (described from Kerala, western coast of South India) have traditionally be merged as one taxon due to their overall phenotypic resemblance (Bhat, 1987; Mangaly and Sabu, 1993; Sabu, 2006). However, the present examination of living plants in natural conditions revealed constant, though minor, morphological divergences (see Fig. 5), along with differences in ecological requirements. Distinctiveness of both species was further corroborated by distinct chromosome numbers (2n = 77 in the former, 2n = 42 in the latter). Similarly, it was found that C. aromatica is a species complex comprising three distinct morphotypes with non-overlapping genome sizes, including hexaploids (2C = 1·86 pg) from south India, nonaploids (2C = 2·83 pg) from north-east India and nonaploids (2C = 2·68 pg) from eastern India. Taxon descriptions and clarification of the nomenclatural issues are in progress. Curcuma reclinata (2C = 2·29 pg), C. decipiens (2C = 2·35 pg) and C. inodora (2C = 2·29 pg) are difficult to define morphologically and have more or less overlapping distributions. Cytogenetic data do not provide reliable data for species-level determination and we believe that they should be merged as one taxon (i.e. C. reclinata Roxb.).
Taxonomic position of Curcuma-like species placed into separate genera
The taxonomic position of some species with a Curcuma-like morphology has long been discussed. Traditionally, they have been placed into separate genera such as Hitchenia, Kaempferia, Paracautleya and Stahlianthus. However, a phylogenetic analysis of the tribe Zingibereae based on internally transcribed spacers and trnL–trnF gene sequences (Ngamriabsakul et al., 2004) did not support their independent generic status as these taxa were nested within the Curcuma complex. Our molecular analyses (T. Fér et al., Charles University, Prague, Czech Republic, unpubl. res.) based on trnL–trnF gene sequences of a number of Indian taxa, including Hitchenia caulina, Kaempferia scaposa and Paracautleya bhatii, reveal a similar situation.
One aim of the present study was to assess whether ploidy and genome size data provide additional information useful for taxonomic decision-making. Genome sizes of the three controversial species (Hitchenia caulina: 2C = 2·25 pg, Kaempferia scaposa: 2C = 2·33 pg and Paracautleya bhatii: 2C = 2·18 pg) were found to match hexaploid Curcuma taxa from the genome group II. In addition, they all had the hexaploid number of chromosomes (2n = 42) and similar geographical distribution in the Western Ghats, a biodiversity hotspot for Curcuma. These lines of evidence together with a lack of clear-cut morphological traits support inclusion of the taxa in question in a broadly defined Curcuma (see Škorničková and Sabu, 2005a, for the latest circumscription of the genus).
Although valid name combinations in Curcuma already exist for both Hitchenia caulina (originally described as C. caulina) and Paracautleya bhatii (C. bhatii, in Škorničková and Sabu, 2005a), Kaempferia scaposa has never been included in Curcuma, perhaps due to the absence of pouches formed by the bracts and its pure white flowers with extremely long floral tubes (Fig. 5). However, the presence of pouches is neither a unique nor a universal feature for the genus Curcuma (Kress et al., 2002; Škorničková and Sabu, 2005a) and the distinct flower morphology and colour may represent an adaptation to night pollination, as also observed in another ginger species with nocturnal anthesis, Leptosolena haenkei (Funakoshi et al., 2005). Transfer of Kaempferia scaposa to the genus Curcuma requires a new name combination, which is proposed below.
By contrast, Stahlianthus involucratus possesses unique holoploid and homoploid genome sizes (1C = 1Cx = 1·56 pg), dissimilar to any other species involved in the current study. The diploid number of chromosomes (2n = 22) was shared only with south Indian C. vamana, but this species has a different Cx-value and overall morphology. We therefore keep this taxon in a separate genus at this point, but targeted investigation of Thai and south-east Asian members of the genera Curcuma and Kaempferia is necessary to arrive at a final decision concerning the validity of this genus.
Curcuma scaposa (Nimmo) Škorničk. & M.Sabu, comb. nov.
Basionym Hedychium scaposum Nimmo, in Graham, Cat. Pl. Bombay 205 (1839);≡Monolophus scaposus (Nimmo) Dalzell, Hooker's J. Bot. Kew Gard. Misc. 2: 143 (1850);≡Kaempferia scaposa (Nimmo) Benth. Gen. Pl. 3: 642 (1883).
Type: [India, Maharashtra], Western Ghats, Lonavlie, September 1878, G. King s.n. (Neotype: BM!, designated here. Isoneotype: K!).
ACKNOWLEDGEMENTS
A long-term stay of J.L.-Š. at the Calicut University, Kerala, India, was supported by fellowships from the Indian Council of Cultural Relationships and the Ministry of Education, Youth and Sport of the Czech Republic. We thank the authorities of the Ministry of Environment and Forests, Government of India, and the Botanical Survey of India for granting collecting permits and permits to access Indian herbaria. We are also grateful to Johann Greilhuber (Vienna) for critical comments on intraspecific genome size variation, and Ilia Leitch (Kew) for providing some literature. We thank the handling editor Michael Fay (Kew) and two anonymous reviewers for useful remarks on the manuscript. This project was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (project no. B6407401), the Department of Science and Technology, Government of India (Order No. SP/SO/A-20/99 dt. 9·11·2001), the European Commission's Research Infrastructure Action via the SYNTHESYS Project (GB-TAF-606), the Ministry of Education, Youth and Sport of the Czech Republic (MSM 0021620828) and the Academy of Sciences of the Czech Republic (AV0Z60050516).
LITERATURE CITED
- Apavatjrut P, Sirisawad T, Sirirugsa P, Voraurai P, Suwanthada C. Studies on chromosome number of seventeen Thai Curcuma species. Proceedings of 2nd National Conference on Flower and Ornamental Plant; 1996. pp. 86–99. [Google Scholar]
- Ardiyani M. Systematic study of Curcuma L.: turmeric and its allies. Scotland: University of Edinburgh; 2002. PhD thesis. [Google Scholar]
- Beltran IC, Kam YK. Cytotaxonomic studies in the Zingiberaceae. Notes from the Royal Botanic Garden Edinburgh. 1984;41:541–559. [Google Scholar]
- Bennett MD, Leitch IJ. Plant DNA C-values Database (Release 4·0, Oct. 2005) 2005a. http://www.kew.org/genomesize/homepage. (accessed 5 January, 2007).
- Bennett MD, Leitch IJ. Nuclear DNA amounts in angiosperms: progress, problems, and prospects. Annals of Botany. 2005b;95:45–90. doi: 10.1093/aob/mci003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharathan G, Lambert G, Galbraith DW. Nuclear DNA content of monocotyledons and related taxa. American Journal of Botany. 1994;81:381–386. [Google Scholar]
- Bhat KG. Curcuma oligantha Trimen (Zingiberaceae) – a new record for India. Indian Journal of Forestry. 1987;10:66–68. [Google Scholar]
- Bisson S, Guillemet S, Hamel J-L. Contribution a l'étude caryo-taxonomique des Scitaminées. Memoires du Museum National d'Histoire Naturelle. Série B. Botanique. 1968;18:59–145. [Google Scholar]
- Chakravorti AK. Multiplication of chromosome numbers in relation to speciation in Zingiberaceae. Science and Culture. 1948;14:137–140. [Google Scholar]
- Chen ZY, Chen SJ. A report on chromosome numbers of Chinese Zingiberaceae. Guihaia. 1984;4:13–18. [Google Scholar]
- Chen ZY, Chen SJ, Huang XX, Huang SF. A report on chromosome numbers on Chinese Zingiberaceae (5) Guihaia. 1988;8:143–147. [Google Scholar]
- Das AB, Rai S, Das P. Karyotype analysis and cytophotometric estimation of nuclear DNA content in some members of the Zingiberaceae. Cytobios. 1999;97:23–33. [Google Scholar]
- Doležel J, Doleželová M, Novák FJ. Flow cytometric estimation of nuclear DNA amount in diploid bananas (Musa acuminata and M. balbisiana) Biologia Plantarum. 1994;36:351–357. [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, Meister A, Lysák MA, Nardi L, Obermayer R. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany. 1998;82(Suppl. A):17–26. [Google Scholar]
- Eksomtramage L, Boontum K. Chromosome counts of Zingiberaceae. Songklanakarin Journal of Science and Technology. 1995;17:291–297. [Google Scholar]
- Eksomtramage L., Sirirugsa P, Mayakul S. Chromosome numbers of some Thai Zingiberaceae. Songklanakarin Journal of Science and Technology. 1996a;18:153–159. [Google Scholar]
- Eksomtramage L, Sirirugsa P, Mayakul S. Chromosome counts of Thai Zingiberaceae. In: Wu TL, Wu QG, Chen ZY, editors. Proceedings of the 2nd Symposium on the Family Zingiberaceae; Guangzhou: Zhongshan University Press Press; 1996b. pp. 107–111. [Google Scholar]
- Eksomtramage L, Sirirugsa P, Jivanit P, Maknoi C. Chromosome counts of some zingiberaceous species from Thailand. Songklanakarin Journal of Science and Technology. 2002;24:311–319. [Google Scholar]
- Funakoshi H, Kress JW, Škorničková J, Liu A, Inoue K. Return from the lost: rediscovery of the presumed extinct Leptosolena (Zingiberaceae) in the Philippines and its phylogenetic placement in gingers. Acta Phtyotaxonomica et Geobotanica. 2005;56:41–53. [Google Scholar]
- Grant V. Periodicities in the chromosome numbers of the angiosperms. Botanical Gazette. 1982;143:379–389. [Google Scholar]
- Greilhuber J. Severely distorted Feulgen DNA amounts in Pinus (Coniferophytina) after nonadditive fixations as a result of meristematic self-tanning with vacuole contents. Canadian Journal of Genetics and Cytology. 1986;28:409–415. [Google Scholar]
- Greilhuber J. ‘Self-tanning’ – a new and important source of stoichiometric error in cytophotometric determination of nuclear DNA content in plants. Plant Systematics and Evolution. 1988;158:87–96. [Google Scholar]
- Greilhuber J. Intraspecific variation in genome size: a critical reassessment. Annals of Botany. 1998;82(Suppl. A):27–35. [Google Scholar]
- Greilhuber J. Intraspecific variation in genome size in angiosperms: identifying its existence. Annals of Botany. 2005;95:91–98. doi: 10.1093/aob/mci004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MA. Genetic diversity of the genus Curcuma in Bangladesh and further biotechnological approaches for in vitro regeneration and long-term conservation of C. longa germplasm. Germany: University of Hannover; 2004. PhD thesis. [Google Scholar]
- Joseph R, Joseph T, Joseph J. Karyomorphological studies in the genus Curcuma Linn. Cytologia. 1999;64:313–317. [Google Scholar]
- Kress WJ, Prince LM, Williams KJ. The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. American Journal of Botany. 2002;89:1682–1696. doi: 10.3732/ajb.89.10.1682. [DOI] [PubMed] [Google Scholar]
- Larsen K, Lock JM, Maas H, Maas PJM. Zingiberaceae. In: Kubitzki K., editor. The families and genera of vascular plants. vol. 4. Berlin, Springer-Verlag: 1998. pp. 474–495. [Google Scholar]
- Leitch IJ, Chase MW, Bennett MD. Phylogenetic analysis of DNA C-values provides evidence for a small ancestral genome size in flowering plants. Annals of Botany. 1998;82(Suppl. A):85–94. [Google Scholar]
- Lim SN. Cytogenetics and taxonomy of the genus Globba L. (Zingiberaceae) in Malaya. II. Cytogenetics. Notes Royal Botanic Gardens Edinburgh. 1972a;31:271–284. [Google Scholar]
- Lim SN. Cytogenetics and taxonomy of the genus Globba L. (Zingiberaceae) in Malaya. IV. Distribution in relation to polyploidy. Gardens' Bulletin Singapore. 1972b;26:115–126. [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. Flow cytometric and microscopic analysis of the effect of tannic acid on plant nuclei and estimation of DNA content. Annals of Botany. 2006;98:515–527. doi: 10.1093/aob/mcl140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubis I. Phenolic compounds of Curcuma. Annales Bogoriensis. 1968;4:219–225. [Google Scholar]
- Mahelka V, Suda J, Jarolímová V, Trávníček P, Krahulec F. Genome size discriminates between closely related taxa Elytrigia repens and E. intermedia (Poaceae: Triticeae) and their hybrid. Folia Geobotanica. 2005;40:367–384. [Google Scholar]
- Mangaly JK, Sabu M. A taxonomic revision of the South Indian species of Curcuma Linn. (Zingiberaceae) Rheedea. 1993;3:139–171. [Google Scholar]
- Mukherjee I. Chromosome studies of some species of Hedychium. Botanical Magazine Tokyo. 1970;83:237–241. [Google Scholar]
- Murray BG. When does intraspecific C-value variation become taxonomically significant? Annals of Botany. 2005;95:119–125. doi: 10.1093/aob/mci007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar MC, Pillai PKT, Sharma YN. Seedling propagation in turmeric (Curcuma aromatica Salisb.) Journal of Plantation Crops. 1982;10:81–85. [Google Scholar]
- Nasir Uddin S. The origin of turmeric: the domestication of an important ginger spice. Scotland: University of Edinburgh; 2000. MSc thesis. [Google Scholar]
- Nayak S, Naik PK, Acharya LK, Pattnaik AK. Detection and evaluation of genetic variation in 17 promising cultivars of turmeric (Curcuma longa L.) using 4C nuclear DNA content and RAPD markers. Cytologia. 2006;71:49–55. [Google Scholar]
- Ngamriabsakul C, Newman MF, Cronk QCB. The phylogeny of tribe Zingibereae (Zingiberaceae) based on ITS (nrDNA) and trnL-F (cpDNA) sequences. Edinburgh Journal of Botany. 2004;57:39–61. [Google Scholar]
- Obermayer R, Greilhuber J. Does genome size in Dasypyrum villosum vary with fruit colour? Heredity. 2005;95:91–95. doi: 10.1038/sj.hdy.6800696. [DOI] [PubMed] [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Crissman HA, Darzynkiewicz Z, editors. Methods in cell biology. vol. 33. New York: Academic Press; 1990. pp. 105–110. [DOI] [PubMed] [Google Scholar]
- Paisooksantivatana Y, Thepsen O. Phenetic relationship of some Thai Curcuma species (Zingiberaceae) based on morphological, palynological and cytological evidence. Thai Journal of Agricultural Science. 2001;34:47–57. [Google Scholar]
- Poulsen AD. Two new species of Boesenbergia (Zingiberaceae) from Borneo. Nordic Journal of Botany. 1993;13:289–294. [Google Scholar]
- Prana MS. Studies on some Indonesian Curcuma species. England: University of Birmingham; 1977. PhD thesis. [Google Scholar]
- Prana MS, Sastrapradja S, Hawkes JG, Lubis I. A cytological study of some Indonesian Curcuma species. Journal of Root Crops. 1978;4:31–35. [Google Scholar]
- Raghavan RS, Arora CM. Chromosome numbers in Indian medicinal plants. II. Proceedings of Indian Academy of Sciences Series B; 1958. pp. 352–358. [Google Scholar]
- Raghavan TS, Venkatasubban KR. Cytological studies in the family Zingiberaceae with special reference to chromosome number and cyto-taxonomy. Proceedings of Indian Academy of Sciences Series B. 1943;17:118–132. [Google Scholar]
- Ramachandran K. Chromosome numbers in the genus Curcuma Linn. Current Science. 1961;30:194–196. [Google Scholar]
- Ramachandran K. Chromosome numbers in Zingiberaceae. Cytologia. 1969;34:213–221. [Google Scholar]
- Rayburn AL, Auger JA. Nuclear DNA-content variation in the ancient indigenous races of Mexican maize. Acta Botanica Neerlandica. 1990;39:197–202. [Google Scholar]
- Roxburgh W. Descriptions of several of the monandrous plants of India. Asiatick Researches. 1810;11:318–362. [Google Scholar]
- Sabu M. Zingiberaceae and Costaceae of South India. Feroke: Indian Association of Angiosperm Taxonomy; 2006. [Google Scholar]
- Saensouk S, Chantaranothai P. The family Zingiberaceae in Phu Phan National Park. In: Chantaranothai P, Larsen K, Sirirugsa P, Simpson D, editors. Proceedings of the 3rd Symposium on the family Zingiberaceae; Khon Kaen: Applied Taxonomic Research Centre, Khon Kaen University; 2003. pp. 16–25. [Google Scholar]
- Saensouk S, Sumonthip B, Luangpirom A. Chromosome numbers of some Zingiberaceae in Phu Phan National Park. In: Saensouk S, Chantaranothai P, editors. 24th Congress on Science and Technology of Thailand; 19–21 October 1998; Queen Sirikit Botanical Garden; 1998. 2003. [Google Scholar]
- Sarkar AK. Cytological investigation of certain members of Zingiberaceae Lindl. to ascertain their taxonomic affinities. Proceedings of Indian Science Congress. 1990;77:150. [Google Scholar]
- Sato D. Karyotype and systematics of Zingiberales. Japanese Journal of Genetics. 1948;23:44–45. [Google Scholar]
- Sato D. Scientific Papers of the College of General Education. Vol. 10. University of Tokyo; 1960. The karyotype analysis in Zingiberales with special reference to the protokaryotype and stable karyotype; pp. 225–243. [Google Scholar]
- Schumann K. Engler HGA, editor. Zingiberaceae. Das Pflanzenreich. 1904;46:1–458. [Google Scholar]
- Sharma AK, Bhattacharya NK. Cytology of several members of Zingiberaceae. La Cellule. 1959;59:297–346. [Google Scholar]
- Sirirugsa P. The genus Curcuma of Thailand. In: Wu TL, Wu QG, Chen ZY, editors. Proceedings of the 2nd Symposium on the family Zingiberaceae.; Guangzhou: Zhongshan University Press; 1996. pp. 39–46. [Google Scholar]
- Sirisawad T, Sirirugsa P, Suwanthada C, Apavtjrut P. Investigation of chromosome numbers in 20 taxa of Curcuma. In: Chantaranothai P, Larsen K, Sirirugsa P, Simpson D, editors. Proceedings of the 3rd Symposium on the family Zingiberaceae; Khon Kaen: Applied Taxonomic Research Centre, Khon Kaen University; 2003. pp. 54–62. [Google Scholar]
- Škorničková J, Sabu M. The recircumscription of Curcuma L. to include the genus Paracautleya R.M.Sm. Gardens' Bulletin Singapore. 2005a;57:37–46. [Google Scholar]
- Škorničková J, Sabu M. Curcuma roscoeana Wall. (Zingiberaceae) in India. Gardens' Bulletin Singapore. 2005b;57:187–198. [Google Scholar]
- Škorničková J, Sabu M. The identity and distribution of Curcuma zanthorrhiza Roxb. (Zingiberaceae) Gardens' Bulletin Singapore. 2005c;57:199–210. [Google Scholar]
- Škorničková J, Sabu M, Prasanthkumar MG. A new species of Curcuma L. (Zingiberaceae) from Mizoram. Gardens' Bulletin Singapore. 2003a;55:89–95. [Google Scholar]
- Škorničková J, Sabu M, Prasanthkumar MG. Curcuma codonantha (Zingiberaceae) – a new species from the Andaman Islands, India. Gardens' Bulletin Singapore. 2003b;55:219–228. [Google Scholar]
- Škorničková J, Sabu M, Prasanthkumar MG. Curcuma mutabilis (Zingiberaceae): a new species from South India. Gardens' Bulletin Singapore. 2004;56:43–54. [Google Scholar]
- Škorničková J, Rehse T, Sabu M. Other economically important Curcuma species. In: Ravindran PN, Babu KN, Sivaraman K., editors. Turmeric: the genus Curcuma. Boca Raton, FL: CRC Press; 2007. pp. 451–467. [Google Scholar]
- Šmarda P, Bureš P. Intraspecific DNA content variability in Festuca pallens on different geographical scales and ploidy levels. Annals of Botany. 2006;98:665–678. doi: 10.1093/aob/mcl150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM. Synoptic keys to the genera of Zingiberaceae pro parte. Edinburgh: Royal Botanic Garden Edinburgh; 1981. [Google Scholar]
- Stace CA. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon. 2000;49:451–477. [Google Scholar]
- Suguira T. A list of chromosome numbers in angiospermous plants. Botanical Magazine Tokyo. 1931;45:353–355. [Google Scholar]
- Suguira T. Studies on the chromosome numbers in higher plants, with special reference to cytokenesis, I. Cytologia. 1936;7:544–595. [Google Scholar]
- Takano A. Cytological analyses of 19 taxa in Globba (Zingiberaceae) Acta Phytotaxonomica et Geobotanica. 2001;52:65–74. [Google Scholar]
- Takano A, Okada H. Multiple occurrence of triploid formation in Globba (Zingiberaceae) from molecular evidence. Plant Systematics and Evolution. 2002;230:143–159. [Google Scholar]
- Venkatasubban KR. A preliminary survey of chromosome numbers in Scitamineae of Bentham & Hooker. Proceedings of Indian Academy of Sciences Series B; 1946. pp. 281–300. [Google Scholar]
- Walker DJ, Monino I, Correal E. Genome size in Bituminaria bituminosa (L.) C. H. Stirton (Fabaceae) populations: separation of “true” differences from environmental effects on DNA determination. Environmental and Experimental Botany. 2006;55:258–265. [Google Scholar]
- Weerapakdee W, Krasaechai A. Collection and development studies of certain Curcuma spp. Journal of Agriculture. 1997;13:127–136. [Google Scholar]