Abstract
Thymidine glycol (Tg), which is also known as 5,6-dihydroxy-5,6-dihydrothymidine, and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) are two major types of DNA damage products induced by reactive oxygen species (ROS). Here we report the synthesis of oligodeoxyribonucleotides (ODNs) containing both Tg and 8-oxodG. The dual incorporation of the two single-base lesions was achieved by using a phosphoramidite building block of 8-oxodG with ultramild base protecting group and a building block of Tg whose nucleobase hydroxyl groups were protected with acetyl functionality. The availability of ODNs carrying neighboring 8-oxodG and Tg provided authentic substrates for assessing the formation and examining the replication and repair of this kind of tandem lesions. In addition, thermodynamic parameters derived from melting temperature data revealed that tandem lesions destabilized the double helix to a greater extent than either of the two single-base lesions alone. The thermodynamic results could offer a basis for understanding the repair of the tandem base lesions.
Introduction
Reactive oxygen species (ROS) can lead to a plethora of modifications to DNA, which yield nucleobase and deoxyribose lesions, strand breaks, and DNA-protein cross-links (1, 2). These oxidative DNA lesions may have implications in a variety of pathological conditions, including diseases associated with aging and cancer (1, 3). Thymidine glycol (or 5,6-dihydroxy-5,6-dihydrothymidine, Tg) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) are two common monomeric base lesions induced by ROS. Thymidine glycol lacks mutagenicity under most conditions (4-7). However, it effectively blocks DNA replication (8, 9). By contrast, 8-oxodG is a major mutagenic lesion and can result in G→T transversion mutation (10, 11).
In recent years, several types of tandem DNA lesions (12-20), where damage involves two adjacent nucleosides, have been isolated and characterized. A first example of tandem lesions, with a 8-oxodG and a formamido (dβF) residue being neighboring to each other, was reported by Box et al. (12, 21). Recently Bourdat et al. (15) showed that the amount of these tandem lesions, dβF-8-oxodG and its isomeric 8-oxodG-dβF, formed from γ radiation cannot account for the total amount of tandem lesions involving 8-oxodG. It was, therefore, postulated that other tandem lesions involving 8-oxodG may exist. Since thymidine glycol and 8-oxodG are major types of single-nucleobase lesions induced by ROS (2), we reason that tandem lesions with a 8-oxodG being adjacent to a Tg might be induced by ROS.
Clustered DNA damages, in which two or more lesions within one or two helical turns of the DNA helix, can be initiated from a single ionizing radiation track (22-24). It has been shown that such damaged sites were difficult to be repaired with purified repair enzymes and in mammalian cells (25-31). To understand the biological significances of tandem lesions with a Tg being neighboring to an 8-oxodG, it is crucial to assess their formation and to examine the replication and repair of this type of lesions. The lack of procedures for preparing pure and sufficient tandem lesion-bearing substrates, however, hampers such studies. This motivates us to design synthetic approaches for the incorporation of both types of lesions into oligodeoxyribonucleotides (ODNs).
Solid-phase synthesis of ODNs by using phosphoramidite building blocks is advantageous over the post-synthetic methods for the preparation of modified or damaged ODNs because the chain length and the sequence are not restricted and large-scale preparation is available (32). In this regard, Iwai (33, 34) described the synthesis of a building block of thymidine glycol where the nucleobase hydroxyl group(s) were protected with tert-butyldimethylsilyl (TBDMS) group. Very recently, Cadet et al. (35) reported the synthesis of a Tg building block where the nucleobase hydroxyl group(s) was protected with a base-labile levulinyl functionality. On the other hand, the deprotection of ODNs synthesized from the commercially available building block of 8-oxodG requires prolonged treatment with concentrated ammonium hydroxide; during such treatment, thymidine glycol, however, was shown to be significantly degraded (33). Therefore, dual insertion of 8-oxodG and thymidine glycol necessitates alternative synthetic strategies.
Here in this paper we reported the successful preparation of ODN substrates bearing both 8-oxodG and Tg, which provides substrates for examining the formation of the tandem lesions with neighboring 8-oxodG and Tg induced by ROS. The availability of this type of substrates also facilitates the investigation of the replication and repair of this type of lesions both in vitro and in vivo.
Experimental Section
Materials
Common reagents for solid-phase DNA synthesis were obtained from Glen Research Co. (Sterling, VA). Unmodified ODNs used in this study were purchased from Integrated DNA Technologies (Coraville, IA). Silica gel and TLC plates were obtained from EM Science (Gibbstown, NJ). All chemicals unless otherwise specified were from Sigma-Aldrich (St. Louis, MO).
Mass Spectrometry
Electrospray ionization-mass spectrometry (ESI-MS) and tandem MS (MS/MS) experiments were carried out on an LCQ Deca XP ion-trap mass spectrometer (ThermoFinnigan, San Jose, CA). An equal-volume solvent mixture of acetonitrile and water was used as solvent for electrospray, and a 2-μL aliquot of ∼5 μM sample solution was injected in each run. The spray voltage was 3.4 kV and the instrument was operated in the negative-ion mode.
HPLC
A 4.6×250 mm Apollo C18 column (5 μm in particle size, 300 Å in pore size, Alltech Associate Inc., Deerfield, IL) was used for the separation of synthetic ODNs. A solution of 50 mM triethylammonium acetate (TEAA, solution A) and a mixture of 50 mM TEAA and acetonitrile (70/30, v/v, solution B) were used as mobile phases. The flow rate was 0.8 mL/min, and a gradient of 5 min 0-20% B, 45 min 20-50% B and 5 min 50-100% B was employed.
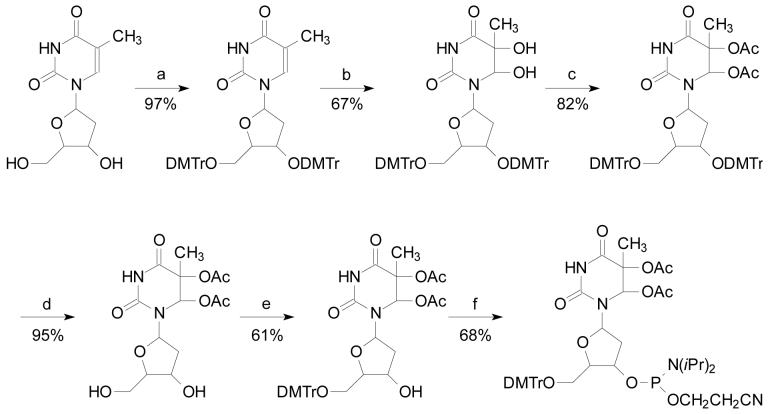
Synthesis and Characterization of Compounds (Scheme 1)
Scheme 1.
Synthesis of phosphoramidite building block of Tg.
Reagents: (a) DMTrCl/Pyridine/70°C; (b) OsO4/Pyridine; (c) Ac2O/DMAP/Pyridine; (d) 70% acetic acid; (e) DMTrCl/Pyridine; (f) NC(CH2)2OP(Cl)N(iPr)2/DIEA/CH2Cl2
3′,5′-O-(4,4′-dimethoxytrityl)thymidine (1)
The title compound was synthesized according to the procedures reported by Sekine et al. (36). In this respect, it is worth mentioning that the incorporation of the bulky 4,4′-dimethoxytrityl (DMTr) functionality to the 3′-hydroxyl group required a reaction temperature of 70 °C (36), though the traditional DMTr protection of the 5′-hydroxyl group is typically carried out at room temperature. Yield: 6.79 g (97%). Rf: 0.38 (EtOAc-hexane, 1:1). 1H NMR (400 MHz, DMSO-d6): δ 11.30 (br, 1H), 6.75-7.37 (m, 26H), 6.15 (m, 1H), 4.21 (m, 1H), 3.94 (m, 1H), 3.70 (s, 6H), 3.68 (d, J = 3.2 Hz, 6H), 3.00 (m, 1H), 2.92 (m, 1H), 1.77 (m, 1H), 1.61 (m, 1H), 1.36 (s, 3H). MALDI-MS: m/z 869.1 [M+Na]+.
(5R,6S)-3′,5′-O-(4,4′-dimethoxytrityl)thymidine glycol (2)
Compound 1 (1.67 g, 1.97 mmol) and OsO4 (0.5 g, 1.97 mmol) were dissolved in pyridine (7.5 mL), and the mixture was stirred at room temperature for 2 h. Sodium hydrogen sulfite (1.8 g), dissolved in a mixture of water (30 mL) and pyridine (20 mL), was then added to the reaction mixture. The mixture was stirred for another 2 h. The product was extracted twice with CHCl3 (150 mL) and the organic layer was dried with Na2SO4. The solvent was removed under reduced pressure. The reaction mixture was then loaded onto a silica gel column and eluted with a CH2Cl2 solution containing 0.5% MeOH and 0.1% Et3N. Yield: 1.17 g (67%). Rf: 0.24 (CH3OH-CH2Cl2, 1:19). 1H NMR (300 MHz, CDCl3): δ 6.77-7.50 (m, 26H), 6.29 (m, 1H), 5.09 (s, 1H), 4.44 (m, 1H), 3.92 (m, 1H), 3.79 (m, 12H), 3.02 (m, 2H), 2.05 (m, 1H), 1.77 (m, 1H), 1.28 (s, 3H). MALDI-MS: m/z 903.4 [M+Na]+.
(5R,6S)-3′,5′-O-(4,4′-dimethoxytrityl)-5,6-O-acetyl-thymidine glycol (3)
Compound 2 (281 mg, 0.32 mmol) was coevaporated twice with dry pyridine (10 mL) and the dried residue was dissolved again in dry pyridine (2 mL). DMAP (4-dimethylaminopyridine, 7.38 mg, 0.06 mmol) and acetic anhydride (0.36 mL, 3.84 mmol) were added into the solution under argon atmosphere. After 15 h, the solution was dried under reduced pressure, and the dried residue was redissolved in EtOAc (80 mL). The organic layer was washed with water (twice, 40 mL) and brine (40 mL), dried over Na2SO4, and concentrated. Glycol 3 (262 mg, 82%) was isolated with a silica gel column using a solvent gradient of 0-15% EtOAc in CH2Cl2. Rf: 0.58 (EtOAc-CH2Cl2, 15:85). 1H NMR (400 MHz, CDCl3): δ 8.64 (s, 1H), 7.15-7.40 (m, 18H), 6.69-6.81 (m, 8H), 6.35 (s, 1H), 6.03 (dd, 1H, J = 5.8, 8.7 Hz), 4.26 (m, 1H), 4.10 (m, 3H), 3.76 (s, 6H), 3.72 (d, J = 2.3 Hz, 6H), 3.22 (m, 2H), 2.02 (s, 3H), 2.00 (s, 3H), 1.90 (s, 3H). MALDI-MS: m/z 987.4 [M+Na]+.
(5R,6S)-5,6-O-acetyl-thymidine glycol (4)
Compound 3 (262 mg, 0.27 mmol) was treated with 70% acetic acid at room temperature for 6 h. The resulting aqueous solution was dried under reduced pressure. The dried residue was dispersed in ether (50 mL) and extracted twice with water (50 mL each). The aqueous solutions were combined and dried. Yield: 93 mg (95%). 1H NMR (400 MHz, CDCl3): δ 8.86 (s, 1H), 6.91 (s, 1H), 6.09 (t, 1H, J = 7.3 Hz), 4.48 (m, 1H), 3.96 (m, 1H), 3.81 (m, 2H), 2.142 (m, 2H), 2.10 (s, 3H), 2.08 (s, 3H), 1.86 (s, 3H). ESI-MS: m/z 383.1 [M+Na]+.
(5R,6S)-5′-O-(4,4′-dimethoxytrityl)-5,6-O-acetyl-thymidine glycol (5)
Compound 4 (90 mg, 0.26 mmol) was coevaporated twice with dry pyridine (5 mL). The dried residue was redissolved in dry pyridine (2 mL), and to the resulting solution was added 4,4′-dimethoxytrityl chloride (105 mg, 0.31 mmol) under argon atmosphere. After 4 h, the solvent was removed under reduced pressure. The product was loaded onto a silica gel column and eluted with 0-2% methanol in a solvent mixture of Et3N and CH2Cl2 (1:99, v/v). Yield: 102 mg (61%). Rf: 0.17 (CH2Cl2-CH3OH, 19:1). 1H NMR (400 MHz, CDCl3): δ 8.30 (s, 1H), 7.10-7.46 (m, 9H), 6.81-6.84 (m, 4H), 6.56 (s, 1H), 6.08 (t, 1H, J = 7.2 Hz), 5.27 (s, 1H), 4.35 (m, 1H), 3.42 (dd, 1H, J = 5.5, 9.8 Hz), 3.29 (dd, 1H, J = 5.5, 9.8 Hz), 2.32 (m, 1H), 2.06 (m, 1H), 2.05 (s, 3H), 2.03 (s, 3H), 1.78 (s, 3H). ESI-MS: m/z 685.1 [M+Na]+.
(5R,6S)-5′-O-(4,4′-dimethoxytrityl)-5,6-O-acetyl-thymidine glycol-3′-O-[(2-cyanoethyl)-N,N-diisopropyl-phosphoramidite] (6)
To a flask, which was suspended in an ice bath and contained a solution of compound 5 (150 mg, 226 μmol) in dry CH2Cl2 (1.8 mL), was added N,N-diisopropylethylamine (DIEA, 100 μL, 573 μmol) followed by dropwise addition of 2-cyanoethyl-N,N-diisopropylchlorophosphoramidite (78 μL, 348 μmol). The mixture was stirred at room temperature for 15 min. A second portion of DIEA (100 μL, 573 μmol) was added to the mixture and the solution was stirred for another 15 min. Workup was carried out by cooling down the mixture in an ice bath followed by addition of CH3OH (0.36 mL). The solution was quickly extracted with EtOAc (10 mL) and the organic layer was washed with NaHCO3 (4 mL) and brine (4 mL) and dried with anhydrous Na2SO4. The solvent was evaporated off to give 6 in white foam. The product was eluted with 0-5% methanol in a solvent mixture of Et3N and CH2Cl2 (0.1:99.9, v/v). Yield: 133 mg (68%). Rf: 0.64, 0.73 (CH2Cl2-CH3OH, 19:1); 31P-NMR (CDCl3): δ 149.5, 149.2. ESI-MS: m/z 863.1 [M+H]+.
5′-O-(4,4′-dimethoxytrityl)-2-N-(phenoxyacetyl)-8-oxo-7,8-dihydro-2′-deoxyguanosine-3′-O-[(2-cyanoethyl)-N,N-diisopropyl-phosphoramidite] (7)
Compound 7 was prepared following the previously published procedures (Details shown in the Supporting Information) (37-39).
ODN Synthesis
ODNs (sequences shown in Table 1) were synthesized on a Beckman Oligo 1000S DNA synthesizer (Fullerton, CA) at 1-μmol scale. The phosphoramidite building blocks 6 and 7 were dissolved in anhydrous acetonitrile at a concentration of 0.06 M, ultramild phosphoramidite building blocks (Glen Research Inc., Sterling, VA) of dG, dC and dA were employed, and the factory-installed ODN assembly protocol was used without any modification. After synthesis, the products were cleaved from the controlled-pore glass (CPG) support and deprotected with 30% NH4OH at room temperature for 1.5 h. The ODNs were then purified by reversed-phase HPLC.
Table 1.
Sequences of Synthesized Oligodeoxynucleotides
| ODNs | Sequence | Observed m/z | Theoretical m/z |
|---|---|---|---|
| ODN1 | 5′-ATG GCG* TGC TAT-3′ | 3691.2 | 3691.6 |
| ODN2 | 5′-ATG GCG TgGC TAT-3′ | 3709.2 | 3709.6 |
| ODN3 | 5′-ATG GCG* TgGC TAT-3′ | 3725.2 | 3725.6 |
| ODN4 | 5′-ATG GCTg G*GC TAT-3′ | 3724.9 | 3725.6 |
| ODN5 | 5′-ATG* GCG TgGC TAT-3′ | 3725.2 | 3725.6 |
8-oxodG
Thermodynamic Studies
UV absorbance-versus-temperature profiles were recorded on a Varian Cary 50 spectrophotometer (Varian Inc., Palo Alto, CA), and the ODNs were dissolved in a 1.2-mL solution containing 250 mM NaCl, 10 mM sodium cacodylate, and 0.1 mM EDTA (pH 7.0) at a total ODN concentration (Ct) of 2.0, 3.4, 5.6, 9.5, or 16 μM. The absorbance was recorded in the reverse and forward directions for a temperature range of 80-10°C at a rate of 1°C/min, and the melting temperature (Tm) value was obtained by the derivative method.
The thermodynamic parameters were obtained from the van’t Hoff plot (40), in which the reciprocal of Tm was plotted against :
and
where R is the ideal gas constant (= 1.987 cal mol-1 K-1). The error limits for ΔG°, ΔH° and ΔS° derived from fitted parameters were calculated by using previously described equations (41-43).
Results
Synthesis of Phosphoramidite Building Blocks for the Dual Incorporation of Tg and 8-oxodG into ODNs
Phosphoramidite building blocks of both Tg and 8-oxodG are commercially available (Glen Research). The labile nature of thymidine glycol, which was shown to be decomposed upon treatment with concentrated ammonia at room temperature for extended period of time (33), prevents the use of the commercially available building block of 8-oxodG, which necessitates deprotection with concentrated ammonia at room temperature for 24-48 h, for the dual insertion of the two oxidatively damaged products into ODNs. In addition, the 5- and 6-hydroxyl groups in the commercially available Tg building block were protected with TBDMS. The nucleobase deprotection of those ODNs that are synthesized with the Tg building block, therefore, requires treatments in two steps, one with ammonium hydroxide and the other with tetrabutyl ammonium fluoride (TBAF).
With the above analysis in mind, we decided to prepare the phosphoramidite building blocks of 8-oxo-dG, whose N2 is protected with a phenoxyacetyl group (37-39), and thymidine glycol, whose 5- and 6-dihydroxyl groups were protected with acetyl functionality. The latter protection of thymidine glycol obviates the needs for the deprotection with TBAF.
To synthesize the thymidine glycol phosphoramidite building block, we first protected the 3′ and 5′ hydroxyl groups of thymidine with DMTr. The DMTr protecting group was chosen for its easy removal upon acid treatment and its strong UV absorbance, which allows for the monitoring of the elution of the poorly UV-absorptive Tg during flash column chromatography separation. The DMTr-protected compound was then oxidized with OsO4 to offer the desired thymidine glycol (33). We then protected both hydroxyl groups in thymine glycol with acetyl groups, which was achieved by using DMAP as a catalyst (44). It is worth noting that the acetylation of the tertiary hydroxyl group was not complete without the addition of DMAP. This result is in line with what Gasparutto et al. (35) observed, where they showed that the protection of the 5-hydroxyl group of thymidine glycol with levulinyl functionality was incomplete. The resulting compound was treated with 70% acetic acid to give the 5,6-O-acetyl-thymidine glycol, which was converted to the phosphoramidite building block using published procedures (45).
With these two building blocks, we synthesized several ODNs (sequences listed in Table 1). We found that the acetyl protecting group of thymidine glycol can be readily removed upon treatment with 30% NH4OH at room temperature for 1.5 h and thymidine glycol remains intact after such treatment. The ODNs were then purified by reversed-phase HPLC (HPLC traces are shown in the Supporting Information). It is worth noting that the C6 position of the thymidine glycol functionality undergoes epimerization during the deprotection step (34, 46). Therefore, we expect that both the (5R,6S) and the (5R,6R) isomers are present in the ODN, though only the major isomer of the thymidine glycol, i.e., the (5R,6S) isomer, from the OsO4 oxidation was employed for phosphoramidite building block synthesis.
ESI-MS and MS/MS Characterization of ODNs Containing Both 8-oxodG and Tg
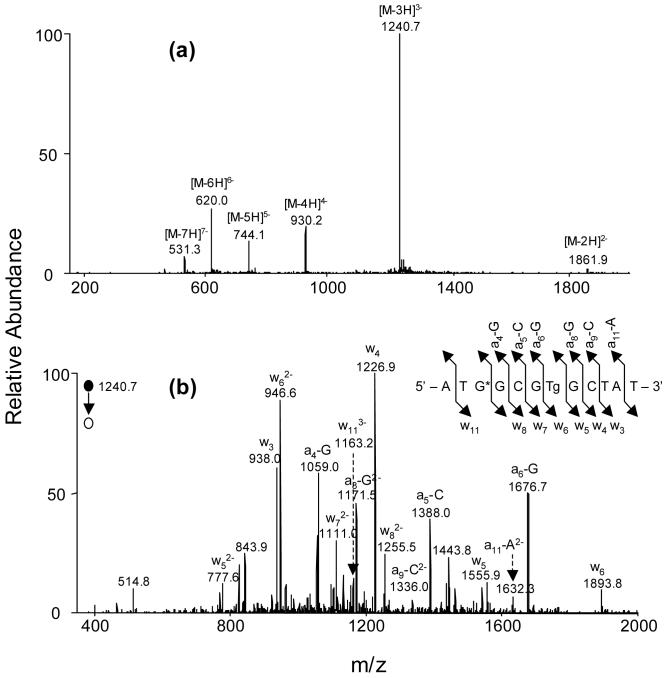
We next characterized the above ODNs by ESI-MS and MS/MS (Table 1, Figures 1-2 and Supporting Information). Here we began our discussion with the characterizations of d(ATG*GCGTgGCTAT) (ODN5, G* represents 8-oxodG). In this regard, ESI-MS shows that the deconvoluted mass of the ODN is 50 Da higher than the calculated mass of the unmodified d(ATGGCGTGCTAT) (Figure 1a), which is consistent with the presence of both Tg and 8-oxo-dG in this substrate.
Figure 1.
ESI-MS & MS/MS characterizations of d(ATG*GCGTgGCTAT): (a) Negative-ion ESI-MS; (b) product-ion spectrum of the [M-3H]3- ion (m/z 1241).
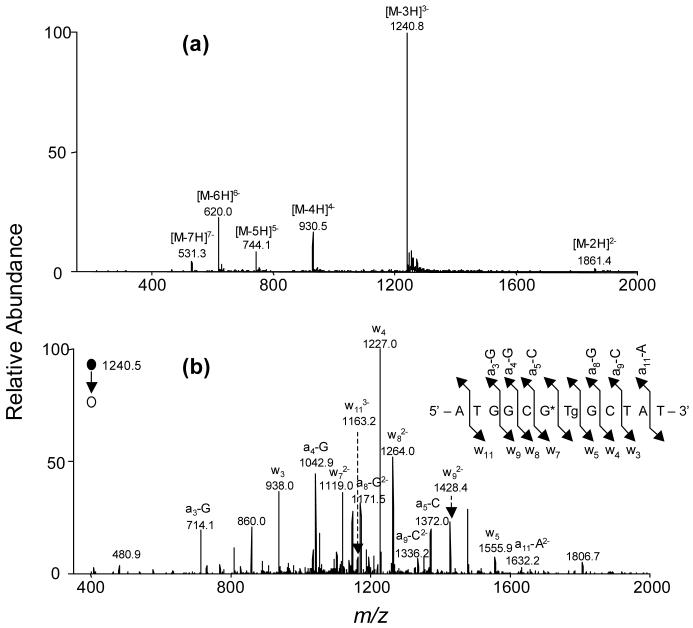
Figure 2.
ESI-MS & MS/MS characterizations of d(ATGGCG*TgGCTAT): (a) Negative-ion ESI-MS; (b) product-ion spectrum of [M-3H]3- ion (m/z 1241).
The product-ion spectrum of the [M - 3H]3- ion (m/z 1240.7, Figure 1b) of the ODN showed the formation of wn ions, i.e., w3, w4, w52-, w62-, w72-, w82-, and w113-, and [an - Base] ions, i.e., [a4 - Gua], [a5 - Cyt], [a6 - Gua], [a8 - Gua]2-, [a9 - Cyt]2- and [a11 - Ade]2- ions [nomenclature for fragment ions follows that reported by McLuckey et al. (47); “Ade”, “Cyt” and “Gua” represent adenine, cytosine and guanine, respectively]. Whereas the measured masses for the w3, w4, and w52- ions are the same as the calculated masses for the corresponding ions of the unmodified ODN, the w62-, w72- and w82- ions exhibited 34 Da higher, and the w113- ion showed 50 Da higher, in mass than the corresponding fragments formed from the unmodified d(ATGGCGTGCTAT). These results are consistent with the presence of 8-oxo-dG and Tg at the third and seventh positions in the ODN, respectively. The above conclusion is further substantiated by the observed masses for the [an - Base] ions. In this respect, the measured masses for the [a4 - Gua], [a5 - Cyt] and [a6 - Gua] ions are 16 Da higher, whereas the measured masses of the [a8 - Gua]2-, [a9 - Cyt]2- and [a11 - Ade]2- ions are 50 Da higher, than the calculated masses of the corresponding fragments from the unmodified d(ATGGCGTGCTAT). It is worth noting that we did not observe the [a3 - Base] and w9 ion (Figure 1b), which is consistent with previous findings about the fragmentations of ODNs containing a 8-oxodG (48).
The sequences of ODN3 and ODN4 were also confirmed by the similar ESI-MS and MS/MS analysis (Figure 2 shows the MS and MS/MS for ODN3, and the mass spectrometric results for ODN4 are shown in the Supporting Information). First of all, the measured masses of these two ODNs are consistent with their calculated masses (Table 1). In addition, the production spectrum of the [M - 3H]3- ion of ODN3 (Figure 2b) showed that the w72-, w82-, w93-, w113-, [a8 - Gua]2-, [a9 - Cyt]2- and [a11 - Ade]2- ions are 50 Da higher in mass than the calculated masses for the corresponding ions of the unmodified ODN. The measured masses of w3, w4, w5, [a3 - Gua], [a4 - Gua] and [a5 - Cyt] ions, however, are the same as the calculated masses for the respective ions of the unmodified ODN. The product-ion spectrum of the [M - 3H]3- ion of ODN4 is very similar to that of ODN4 except that the [a6 - 143], a characteristic ion for short ODNs carrying a Tg (49), and w62- ions are found in the product-ion spectrum of ODN4 (Figure S15b), but undetectable in that of ODN3 (Figure 2b). Furthermore, the w5 ion is observed for ODN3 (Figure 2b), but not for ODN4 (Figure S15b).
Thermodynamic Studies
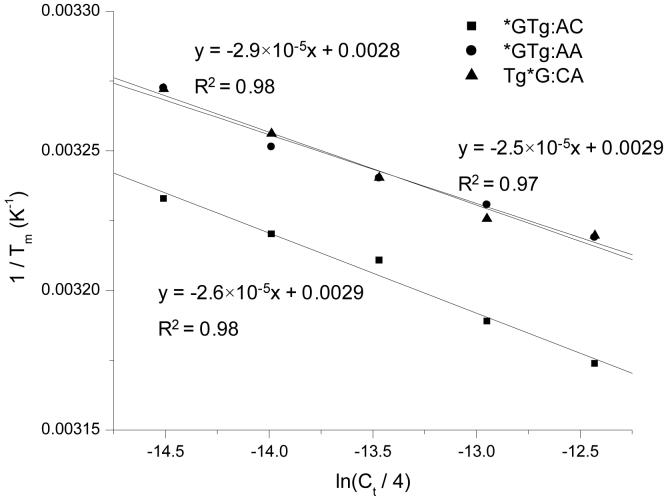
We next measured the thermodynamic parameters for duplex formation with the synthesized lesion-bearing substrates. The specific nucleotide sequences were: strand 1, 5′-ATGGCXYGCTAT-3′; strand 2, 5′-ATAGCMNGCCAT-3′, where XY/MN represents GT/AC, GT/AA, TG/CA, G*T/AC, G*T/AA, GTg/AC, G*Tg/AC, G*Tg/AA or TgG*/CA (G* represents 8-oxodG). We refer to the duplexes by abbreviations of the form of XY/MN (Table 2). We determined the ΔH and ΔS by fitting Tm-1 versus ln(Ct/4) as shown in Figure 3 (Data for unmodified and single base lesion-bearing ODNs are shown in the Supporting Information). The ΔH, ΔS, ΔG25°C, and the Tm values at Ct = 16.0 μM are listed in Table 2.
Table 2.
Thermodynamic Parameters for Duplex Formation
| Duplex | Tm[a] (°C) | ΔH0 (kcal/mol) | ΔS0 (cal mol-1 K-1) | ΔG25°C (kcal/mol) | ΔΔG25°C (kcal/mol) |
|---|---|---|---|---|---|
| 5′-ATGGCXYGCTAT-3′ 3′-TACCGNMCGATA-5′ |
|||||
| 1 (XY/MN: GT/AC) | 60.1 | -83±4.8 | -220±13 | -16.2±0.48 | |
| 2 (XY/MN: GT/AA) | 43.1 | -72±4.5 | -200±13 | -11.6±0.21 | 4.6 |
| 3 (XY/MN: TG/CA) | 60.1 | -77±1.3 | -207±4 | -15.6±0.13 | 0.6 |
| 4 (XY/MN: G*T/AC) | 56.4 | -83±2.4 | -230±7 | -15.3±0.21 | 0.9[b] |
| 5 (XY/MN: G*T/AA) | 52.4 | -90±7.2 | -250±20 | -15.1±0.58 | -3.5[b] |
| 6 (XY/MN: GTg/AC) | 44.7 | -78±1.5 | -222±4 | -12.2±0.08 | 4.0[b] |
| 7 (XY/MN: G*Tg/AC) | 40.9 | -69±2.0 | -195±6 | -11.1±0.08 | 5.1[b] |
| 8 (XY/MN: G*Tg/AA) | 37.5 | -81±5.7 | -240±17 | -10.7±0.21 | 0.9[b] |
| 9 (XY/MN: TgG*/CA) | 37.4 | -76±5.0 | -220±15 | -10.5±0.15 | 5.1[b] |
Ct = 16 μM
ΔG25°C (modified ODN) - ΔG25°C (corresponding unmodified ODN).
Figure 3.
Plots of 1/Tm vs. ln(Ct/4) for the duplexes containing thymidine glycol and 8-oxodG tandem lesions. The duplex is d(ATGGCXYGCTAT)/d(ATAGCMNGCCAT), where XY/MN represents G*Tg/AC(■), G*Tg/AA(●), TgG*/CA( ).
Our data on thermodynamic parameters (Table 2) showed that the free energy change associated with the formation of duplex at 25 °C (ΔG25°C) with 8-oxodG/dA base pair (-15.1 kcal/mol) is very similar to that for the formation of the duplex with 8-oxodG/dC base pair (-15.3 kcal/mol). These results are consistent with previous thermodynamic results as well as NMR and X-ray crystal structure studies (50-52), which showed that 8-oxodG can form base pairs with both dC and dA, depending on whether the damaged nucleoside adopts an anti or syn configuration about the glycosidic bond (50, 51).
Consistent with previous thermodynamic studies (34), our results showed that the presence of thymine glycol decreases markedly the duplex stability and it preferentially forms base pairing with adenine. In addition, duplex ODN with tandem lesion is less stable than the corresponding duplex with either of the two single-base lesions alone (Table 2). Moreover, there is no obvious difference in thermal stability of duplex ODNs with the two types of tandem lesions, i.e., 5′-G*Tg-3′ and 5′-TgG*-3′ (Duplexes 7 and 9, Table 2).
Discussion
In the present work, we successfully synthesized phosphoramidite building blocks of thymidine glycol and 8-oxodG, which facilitate the simultaneous insertion of the two lesions into ODNs. Additionally, with the use of acetyl group for the protection of the hydroxyl functionalities on the thymine glycol moiety, post-synthesis deprotection can be achieved in one step. The availability of authentic ODN substrates enables the assessment of the formation of this type of tandem lesions under various oxidative stress conditions. In this respect, the tandem lesion-carrying ODN substrates will facilitate the design and optimization of enzymatic digestion procedures for the successful release of this type of tandem lesion from duplex DNA.
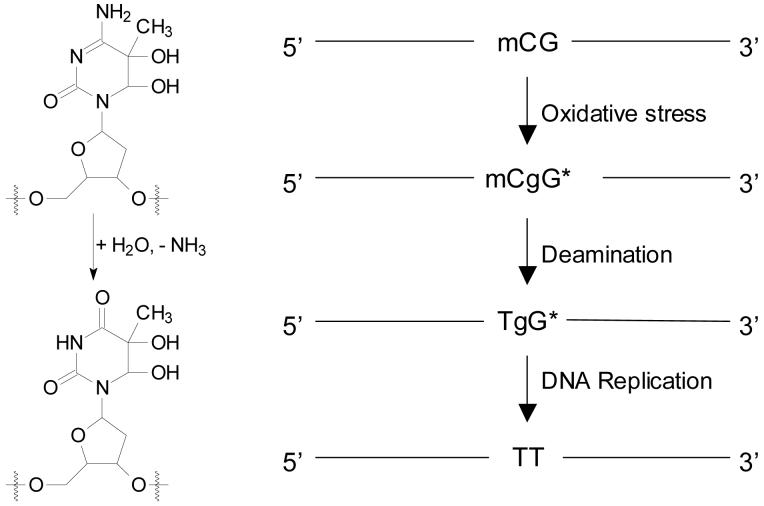
Methylation of cytosine at CpG sites plays an important role in the epigenetic silencing of genes in mammalian cells (53, 54). Damage occurring at CpG dinucleotide and the resulting mutation at these sites may perturb the binding of DNA to methyl-CpG binding proteins (55). We reason that the type of tandem lesion that we synthesized here may have implications in CpG mutagenesis. In this context, under oxidative stress, mC could be damaged to form 5-methylcytosine glycol (56), which is susceptible to deamination to yield thymine glycol. On the other hand, the dG at CpG site can be oxidized to give a 8-oxodG (Scheme 2). Thymidine glycol is mostly a blocking lesion. It, however, can be bypassed by translesion synthesis DNA polymerases including yeast DNA polymerases η and ζ as well as human DNA polymerase κ, and all three polymerases preferentially insert a dA opposite the lesion (5, 57, 58). On the other hand, 8-oxodG can be bypassed by various DNA polymerases and both dC and dA can be inserted opposite the lesion (59, 60). Therefore, the tandem lesions with a pyrimidine glycol being neighboring to 8-oxodG may contribute to the previously observed ROS-induced mCG→TT mutation (61). The availability of ODN substrates bearing structurely defined tandem lesions will allow for the replication studies of these lesions both in vitro and in vivo, which may offer important insights into the roles of this type of lesions in CpG mutagenesis.
Scheme 2.
Deamination of 5-methycytosine glycol and the implications of tandem lesions in mCG → TT mutation.
Recent repair studies on cluster lesions showed that the repair of one lesion can be hampered by the presence of another lesion nearby (25-28, 31). Our thermodynamic measurement results clearly showed that tandem lesions, where an 8-oxodG is adjacent to a Tg, can cause more destabilization to DNA duplexes than either of the two lesions alone. In this regard, the presence of Tg and 8-oxodG neighboring to each other destabilizes the duplex DNA by ∼5.1 kcal/mol (Table 2). Although either Tg or 8-oxodG, when present by itself, can be efficiently repaired by base excision repair (BER) enzymes (62), the elevated structural distortion caused by the vicinal single-base lesions may result in the compromised repair of these two lesions by BER machinery. In addition, both Tg and 8-oxodG could be excised from DNA by NER enzymes in vitro (63), the formation of these two lesions in tandem, due to the increased structural distortion, may also render the tandem lesions to be excised more efficiently by NER than while the two lesions are not proximal to each other. The availability of authentic ODN substrates will again enable us to examine such possibilities.
Taken together, we reported here for the first time the dual incorporation of Tg and 8-oxodG into ODNs. The availability of ODNs carrying neighboring 8-oxodG and Tg builds a solid foundation for examining the replication and repair of the tandem oxidative lesions.
Supplementary Material
Acknowledgement
The authors want to thank the National Institutes of Health (CA 96906 and CA101864) for supporting this research.
Abbreviations
- ROS
reactive oxygen species
- ODN
oligodeoxynucleotides
- BER
base excision repair
- NER
nucleotide excision repair
- ESI
electrospray ionization
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
References
- (1).Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- (2).Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- (3).Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- (4).Hayes RC, Petrullo LA, Huang H, Wallace SS, LeClerc JE. Oxidative damage in DNA. Lack of mutagenicity by thymine glycol lesions. J. Mol. Biol. 1988;201:239–246. doi: 10.1016/0022-2836(88)90135-0. [DOI] [PubMed] [Google Scholar]
- (5).Johnson R,E, Yu S-L, Prakash S, Prakash L. Yeast DNA polymerase zeta is essential for error-free replication past thymine glycol. Genes Dev. 2003;17:77–87. doi: 10.1101/gad.1048303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Seki M, Masutani C, Yang Lee W, Schuffert A, Iwai S, Bahar I, Wood Richard D. High-efficiency bypass of DNA damage by human DNA polymerase Q. EMBO J. 2004;23:4484–4494. doi: 10.1038/sj.emboj.7600424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Basu AK, Loechler EL, Leadon SA, Essigmann JM. Genetic effects of thymine glycol: site-specific mutagenesis and molecular modeling studies. Proc. Natl. Acad. Sci. USA. 1989;86:7677–7681. doi: 10.1073/pnas.86.20.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Clark JM, Beardsley GP. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986;14:737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ide H, Kow YW, Wallace SS. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985;13:8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wagner J, Kamiya H, Fuchs RPP. Leading versus lagging strand mutagenesis induced by 7,8-dihydro-8-oxo-2′-deoxyguanosine in Escherichia coli. J. Mol. Biol. 1997;265:302. doi: 10.1006/jmbi.1996.0740. [DOI] [PubMed] [Google Scholar]
- (11).Tan X, Grollman AP, Shibutani S. Comparison of the mutagenic properties of 8-oxo-7,8-dihydro-2′-deoxyadenosine and 8-oxo-7,8-dihydro-2′-deoxyguanosine DNA lesions in mammalian cells. Carcinogenesis. 1999;20:2287–2292. doi: 10.1093/carcin/20.12.2287. [DOI] [PubMed] [Google Scholar]
- (12).Box HC, Budzinski EE, Freund HG, Evans MS, Patrizyc HB, Wallace JC, Maccubbin AE. Vicinal lesions in x-irradiated DNA? Int. J. Radiat. Biol. 1993;64:261–263. doi: 10.1080/09553009314551411. [DOI] [PubMed] [Google Scholar]
- (13).Box HC, Budzinski EE, Dawidzik JD, Wallace JC, Evans MS, Gobey JS. Radiation-induced formation of a crosslink between base moieties of deoxyguanosine and thymidine in deoxygenated solutions of d(CpGpTpA) Radiat. Res. 1996;145:641–643. [PubMed] [Google Scholar]
- (14).Budzinski EE, Dawidzik JB, Rajecki MJ, Wallace JC, Schroder EA, Box HC. Isolation and characterization of the products of anoxic irradiation of d(CpGpTpA) Int. J. Radiat. Biol. 1997;71:327–336. doi: 10.1080/095530097144210. [DOI] [PubMed] [Google Scholar]
- (15).Bourdat AG, Douki T, Frelon S, Gasparutto D, Cadet J. Tandem base lesions are generated by hydroxyl radical within isolated DNA in aerated aqueous solution. J. Am. Chem. Soc. 2000;122:4549–4556. [Google Scholar]
- (16).Bellon S, Ravanat JL, Gasparutto D, Cadet J. Cross-linked thymine-purine base tandem lesions: Synthesis, characterization, and measurement in gamma-irradiated isolated DNA. Chem. Res. Toxicol. 2002;15:598–606. doi: 10.1021/tx015594d. [DOI] [PubMed] [Google Scholar]
- (17).Liu Z, Gao Y, Wang Y. Identification and characterization of a novel cross-link lesion in d(CpC) upon 365-nm irradiation in the presence of 2-methyl-1,4-naphthoquinone. Nucleic Acids Res. 2003;31:5413–5424. doi: 10.1093/nar/gkg736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhang Q, Wang Y. Independent generation of 5-(2′-deoxycytidinyl)methyl radical and the formation of a novel cross-link lesion between 5-methylcytosine and guanine. J. Am. Chem. Soc. 2003;125:12795–12802. doi: 10.1021/ja034866r. [DOI] [PubMed] [Google Scholar]
- (19).Zhang Q, Wang Y. Independent generation of the 5-hydroxy-5,6-dihydrothymidin-6-yl radical and its reactivity in dinucleoside monophosphates. J. Am. Chem. Soc. 2004;126:13287–13297. doi: 10.1021/ja048492t. [DOI] [PubMed] [Google Scholar]
- (20).Zhang Q, Wang Y. Generation of 5-(2′-deoxycytidyl)methyl radical and the formation of intrastrand cross-link lesions in oligodeoxyribonucleotides. Nucleic Acids Res. 2005;33:1593–1603. doi: 10.1093/nar/gki301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Budzinski EE, Dawidzik JD, Wallace JC, Freund HG, Box HC. The radiation chemistry of d(CpGpTpA) in the presence of oxygen. Radiat. Res. 1995;142:107–109. [PubMed] [Google Scholar]
- (22).Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int. J. Radiat. Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- (23).Jenner TJ, Fulford J, O’Neill P. Contribution of base lesions to radiation-induced clustered DNA damage: implication for models of radiation response. Radiat. Res. 2001;156:590–593. doi: 10.1667/0033-7587(2001)156[0590:cobltr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- (24).Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Budworth H, Dianova II, Podust VN, Dianov GL. Repair of clustered DNA lesions. Sequence-specific inhibition of long-patch base excision repair by 8-oxoguanine. J. Biol. Chem. 2002;277:21300–21305. doi: 10.1074/jbc.M201918200. [DOI] [PubMed] [Google Scholar]
- (26).Budworth H, Dianov GL. Mode of inhibition of short-patch base excision repair by thymine glycol within clustered DNA lesions. J. Biol. Chem. 2003;278:9378–9381. doi: 10.1074/jbc.M212068200. [DOI] [PubMed] [Google Scholar]
- (27).Lomax M,E, Cunniffe S, O’Neill P. Efficiency of repair of an abasic site within DNA clustered damage sites by mammalian cell nuclear extracts. Biochemistry. 2004;43:11017–11026. doi: 10.1021/bi049560r. [DOI] [PubMed] [Google Scholar]
- (28).Lomax ME, Cunniffe S, O’Neill P. 8-OxoG retards the activity of the ligase III/XRCC1 complex during the repair of a single-strand break, when present within a clustered DNA damage site. DNA Repair. 2004;3:289–299. doi: 10.1016/j.dnarep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- (29).Pearson CG, Shikazono N, Thacker J, O’Neill P. Enhanced mutagenic potential of 8-oxo-7,8-dihydroguanine when present within a clustered DNA damage site. Nucleic Acids Res. 2004;32:263–270. doi: 10.1093/nar/gkh150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kalam MA, Basu AK. Mutagenesis of 8-oxoguanine adjacent to an abasic site in simian kidney cells: tandem mutations and enhancement of G-->T transversions. Chem. Res. Toxicol. 2005;18:1187–1192. doi: 10.1021/tx050119r. [DOI] [PubMed] [Google Scholar]
- (31).Lomax ME, Salje H, Cunniffe S, O’Neill P. 8-OxoA inhibits the incision of an AP site by the DNA glycosylases Fpg, Nth and the AP endonuclease HAP1. Radiat. Res. 2005;163:79–84. doi: 10.1667/rr3284. [DOI] [PubMed] [Google Scholar]
- (32).Beaucage SL, Iyer RP. The synthesis of modified oligonucleotides by the phosphoramidite approach and their applications. Tetrahedron. 1993;49:6123–6194. [Google Scholar]
- (33).Iwai S. Synthesis of thymine glycol containing oligonucleotides from a building block with the oxidized base. Angew. Chem. Int. Ed. 2000;39:3874–3876. doi: 10.1002/1521-3773(20001103)39:21<3874::AID-ANIE3874>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- (34).Iwai S. Synthesis and thermodynamic studies of oligonucleotides containing the two isomers of thymine glycol. Chem.-Eur. J. 2001;7:4343–4351. doi: 10.1002/1521-3765(20011015)7:20<4343::aid-chem4343>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- (35).Gasparutto D, Cognet S, Roussel S, Cadet J. Synthesis of a convenient thymidine glycol phosphoramidite monomer and its site-specific incorporation into DNA fragments. Nucleosides, Nucleotides & Nucleic Acids. 2005;24:1831–1842. doi: 10.1080/15257770500267279. [DOI] [PubMed] [Google Scholar]
- (36).Sekine M, Hata T. 4,4′,4″-Tris(benzoyloxy)trityl as a new type of base-labile group for protection of primary hydroxyl groups. J. Org. Chem. 1983;48:3011–3014. [Google Scholar]
- (37).Bourdat A-G, Gasparutto D, Cadet J. Synthesis and enzymic processing of oligodeoxynucleotides containing tandem base damage. Nucleic Acids Res. 1999;27:1015–1024. doi: 10.1093/nar/27.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Nampalli S, Kumar S. Efficient synthesis of 8-oxo-dGTP: A mutagenic nucleotide. Bioorg. Med. Chem. Lett. 2000;10:1677–1679. doi: 10.1016/s0960-894x(00)00310-3. [DOI] [PubMed] [Google Scholar]
- (39).Gillet LCJ, Scharer OD. Preparation of C8-amine and acetylamine adducts of 2′-deoxyguanosine suitably protected for DNA synthesis. Org. Lett. 2002;4:4205–4208. doi: 10.1021/ol026474f. [DOI] [PubMed] [Google Scholar]
- (40).Breslauer KJ. Extracting thermodynamic data from equilibrium melting curves for oligonucleotide order-disorder transitions. Methods Enzymol. 1995;259:221–242. doi: 10.1016/0076-6879(95)59046-3. [DOI] [PubMed] [Google Scholar]
- (41).Meyer SL. Data Analysis for Scientists and Engineers. Wiley; New York: 1975. [Google Scholar]
- (42).SantaLucia J, Jr., Kierzek R, Turner DH. Functional group substitutions as probes of hydrogen bonding between GA mismatches in RNA internal loops. J. Am. Chem. Soc. 1991;113:4313–4322. [Google Scholar]
- (43).Persmark M, Guengerich FP. Spectroscopic and thermodynamic characterization of the interaction of N7-guanyl thioether derivatives of d(TGCTG*CAAG) with potential complements. Biochemistry. 1994;33:8662–8672. doi: 10.1021/bi00195a006. [DOI] [PubMed] [Google Scholar]
- (44).Hoefle G, Steglich W, Vorbrueggen H. New synthetic methods. 25. 4-Dialkylaminopyridines as acylation catalysts. 4 Puridine syntheses. 1. 4-Dialkylaminopuridines as highly active acylation catalysts. Angew. Chem. 1978;90:602–615. [Google Scholar]
- (45).Gait MJ. Oligonucleotide Synthesis: A Practical Approach. IRL Press Limited; Oxford, England: 1984. [Google Scholar]
- (46).Lustig MJ, Cadet J, Boorstein RJ, Teebor GW. Synthesis of the diastereomers of thymidine glycol, determination of concentrations and rates of interconversion of their cis-trans epimers at equilibrium and demonstration of differential alkali lability within DNA. Nucleic Acids Res. 1992;20:4839–4845. doi: 10.1093/nar/20.18.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).McLuckey SA, Van Berker GJ, Glish GL. Tandem mass spectrometry of small, multiply charged oligonucleotides. J. Am. Soc. Mass Spectrom. 1992;3:60–70. doi: 10.1016/1044-0305(92)85019-G. [DOI] [PubMed] [Google Scholar]
- (48).Luo H, Lipton MS, Smith RD. Charge effects for differentiation of oligodeoxynucleotide isomers containing 8-oxo-dG residues. J. Am. Soc. Mass Spectrom. 2002;13:195–199. doi: 10.1016/S1044-0305(01)00353-1. [DOI] [PubMed] [Google Scholar]
- (49).Wang Y. HPLC isolation and mass spectrometric characterization of two isomers of thymine glycols in oligodeoxynucleotides. Chem. Res. Toxicol. 2002;15:671–676. doi: 10.1021/tx0155855. [DOI] [PubMed] [Google Scholar]
- (50).Kouchakdjian M, Bodepudi V, Shibutani S, Eisenberg M, Johnson F, Grollman AP, Patel DJ. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991;30:1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- (51).McAuley-Hecht KE, Leonard GA, Gibson NJ, Thomson JB, Watson WP, Hunter WN, Brown T. Crystal structure of a DNA duplex containing 8-hydroxydeoxyguanine-adenine base pairs. Biochemistry. 1994;33:10266–10270. doi: 10.1021/bi00200a006. [DOI] [PubMed] [Google Scholar]
- (52).Plum GE, Grollman AP, Johnson F, Breslauer KJ. Influence of the oxidatively damaged adduct 8-xxodeoxyguanosine on the conformation, energetics, and thermodynamic stability of a DNA duplex. Biochemistry. 1995;34:16148–16160. doi: 10.1021/bi00049a030. [DOI] [PubMed] [Google Scholar]
- (53).Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- (54).Lorincz MC, Groudine M. CmC(a/t)GG methylation: A new epigenetic mark in mammalian DNA? Proc. Natl. Acad. Sci. USA. 2001;98:10034–10036. doi: 10.1073/pnas.201392598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Valinluck V, Tsai H-H, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Zuo S, Boorstein RJ, Teebor GW. Oxidative damage to 5-methylcytosine in DNA. Nucleic Acids Res. 1995;23:3239–3243. doi: 10.1093/nar/23.16.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Kusumoto R, Masutani C, Iwai S, Hanaoka F. Translesion synthesis by human DNA polymerase eta across thymine glycol lesions. Biochemistry. 2002;41:6090–6099. doi: 10.1021/bi025549k. [DOI] [PubMed] [Google Scholar]
- (58).Fischhaber PL, Gerlach VL, Feaver WJ, Hatahet Z, Wallace SS, Friedberg EC. Human DNA polymerase kappa bypasses and extends beyond thymine glycols during translesion synthesis in vitro, preferentially incorporating correct nucleotides. J. Biol. Chem. 2002;277:37604–37611. doi: 10.1074/jbc.M206027200. [DOI] [PubMed] [Google Scholar]
- (59).Zhang Y, Yuan F, Wu X, Wang M, Rechkoblit O, Taylor J-S, Geacintov NE, Wang Z. Error-free and error-prone lesion bypass by human DNA polymerase kappa in vitro. Nucleic Acids Res. 2000;28:4138–4146. doi: 10.1093/nar/28.21.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zhang Y, Yuan F, Wu X, Taylor J-S, Wang Z. Response of human DNA polymerase iota to DNA lesions. Nucleic Acids Res. 2001;29:928–935. doi: 10.1093/nar/29.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Lee D-H, O’Connor TR, Pfeifer GP. Oxidative DNA damage induced by copper and hydrogen peroxide promotes CG TT tandem mutations at methylated CpG dinucleotides in nucleotide excision repair-deficient cells. Nucleic Acids Res. 2002;30:3566–3573. doi: 10.1093/nar/gkf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Fromme JC, Verdine GL. Base excision repair. Adv. Protein Chem. 2004;69:1–41. doi: 10.1016/S0065-3233(04)69001-2. [DOI] [PubMed] [Google Scholar]
- (63).Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc. Natl. Acad. Sci. USA. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.