Abstract
The MAP kinase Fus3 regulates many different signal transduction outputs that govern the ability of Saccharomyces cerevisiae haploid cells to mate. Here we characterize Fus3 localization and association with other proteins. By indirect immunofluorescence, Fus3 localizes in punctate spots throughout the cytoplasm and nucleus, with slightly enhanced nuclear localization after pheromone stimulation. This broad distribution is consistent with the critical role Fus3 plays in mating and contrasts that of Kss1, which concentrates in the nucleus and is not required for mating. The majority of Fus3 is soluble and not bound to any one protein; however, a fraction is stably bound to two proteins of ∼60 and ∼70 kDa. Based on fractionation and gradient density centrifugation properties, Fus3 exists in a number of complexes, with its activity critically dependent upon association with other proteins. In the presence of α factor, nearly all of the active Fus3 localizes in complexes of varying size and specific activity, whereas monomeric Fus3 has little activity. Fus3 has highest specific activity within a 350- to 500-kDa complex previously shown to contain Ste5, Ste11, and Ste7. Ste5 is required for Fus3 to exist in this complex. Upon α factor withdrawal, a pool of Fus3 retains activity for more than one cell cycle. Collectively, these results support Ste5’s role as a tether and suggest that association of Fus3 in complexes in the presence of pheromone may prevent inactivation in addition to enhancing activation.
INTRODUCTION
The mating pathway of Saccharomyces cerevisiae is a simple model for negative growth control and differentiation by a MAP kinase cascade. In the presence of mating pheromone, haploid cells of opposite mating type stop dividing and mate. Mating is marked by the transcription of many genes, cell division arrest in G1 phase, and changes in cellular morphology that result in a uninuclear pear-shaped cell (or shmoo) that can fuse with a partner cell into a zygote (Sprague and Thorner, 1993).
Mating is regulated by one of the best characterized MAP kinase cascades to be described for any eukaryotic cell. A working model (Gustin et al., 1998; Leeuw et al., 1998) has emerged to explain how the pheromone signal is transmitted from the plasma membrane to Fus3 and Kss1, two MAP kinases (MAPKs) that provide unique and overlapping functions required for the many responses. Signaling begins when peptide pheromones secreted from each cell type bind to receptors on the opposite cell type. The receptor is then thought to interact with a heterotrimeric G protein to promote exchange of guanosine diphosphate for guanosine triphosphate on Gα and its dissociation from Gβγ. The Gβ subunit directly sends the signal through Ste20. The signal is then sent from Ste20 to the MAPKK kinase Ste11 by a mechanism that may involve direct phosphorylation of Ste11 by Ste20. The Ste50 protein may also regulate activation of Ste11, although it is not essential for signal transduction. Ste11 directly activates a MAPK kinase Ste7, which directly activates the two MAP kinases, Fus3 and Kss1.
Activation of Fus3 and Kss1 results in the phosphorylation of numerous substrates, many of which include pathway components (Elion et al., 1993; Errede et al., 1993; Peter et al., 1993; Choi et al., 1994; Kranz et al., 1994; Bardwell et al., 1996; Cook et al., 1996; Tedford et al., 1997). Genetic and biochemical evidence suggests that whereas Fus3 and Kss1 have both overlapping and nonoverlapping substrate specificity, Fus3 plays a significantly greater role in the control of mating. Fus3 and Kss1 both contribute to the activation of the transcription factor Ste12 (Elion et al., 1991; Gartner et al., 1992; Cherkasova et al., 1999; Farley et al., 1999), consistent with their mutual ability to phosphorylate Ste12 in vitro (Elion et al., 1993; Bardwell et al., 1996). However, catalytically inactive Fus3 nearly completely blocks the ability of Kss1 to activate Ste12-dependent genes whereas catalytically inactive Kss1 only partially blocks the ability of Fus3 to activate Ste12-dependent genes (Madhani et al., 1997; Cherkasova et al., 1999), suggesting that Kss1 may not play a significant role in the activation of Ste12 in the presence of Fus3 (Madhani et al., 1997). Fus3 also plays a larger role than Kss1 in the control of G1 arrest. fus3 null mutants are defective in G1 arrest, whereas kss1 null mutants are not (Elion et al., 1990, 1991; Cherkasova et al., 1999). This defect is due to a nearly complete dependency of the cyclin-dependent kinase inhibitor Far1 to be phosphorylated by Fus3 and a major requirement for Fus3 in the repression of G1/S cyclin genes (Peter et al., 1993; Tyers and Futcher, 1993; Cherkasova et al., 1999). Fus3 also has essential roles in projection formation, partner selection, and cell fusion that are not shared by Kss1 (Elion et al., 1990; Farley et al., 1999). That Fus3 is more critical than Kss1 for many of the responses to mating pheromone is consistent with the fact that the FUS3 gene is expressed predominantly in haploids and induced by mating pheromone (Elion et al., 1990) whereas the KSS1 gene is constitutively expressed in haploids and diploids (Ma et al., 1995).
The activity of Fus3 is tightly regulated, presumably because inappropriate activation would block vegetative growth. During vegetative growth, Fus3 is largely unphosphorylated and inactive (Gartner et al., 1992; Elion et al., 1993). Fus3 is prevented from being inappropriately activated by high osmolarity during vegetative growth through cross-regulation by the Hog1 MAP kinase (Hall et al., 1996; O’Rourke and Herskowitz, 1998). In the presence of mating pheromone, Fus3 is rapidly phosphorylated and activated by Ste7 (Gartner et al., 1992; Elion et al., 1993; Errede et al., 1993) and subsequently inactivated by at least three phosphatases (Msg5, Ptp2, and Ptp3), presumably to allow cells to recover and reenter the mitotic cycle in the absence of mating (Doi et al., 1994; Zhan et al., 1997).
The activation of Fus3 is strictly dependent upon Ste5, a scaffolding protein that associates with multiple components of the mating MAP kinase cascade (reviewed by Elion, 1995, 1998). Ste5 binds Gβ, Ste11, Ste7, and the MAP kinases through separate binding sites and tethers the kinases into a high-molecular-weight complex (Choi et al., 1994; Kranz et al., 1994; Whiteway et al., 1995; Lyons et al., 1996). Ste5 associates with free Gβ that is liberated by pheromone (Feng et al., 1998) through a RING-H2 metal-binding motif (Inouye et al., 1997; Feng et al., 1998). This association is essential for activation of Ste11 by Ste20 (Feng et al., 1998) and strongly argues that Ste5 directly channels the pheromone signal through the kinases. Ste5 oligomerizes both in the absence and presence of pheromone (Yablonski et al., 1996; Feng et al., 1998). Oligomerization is required for signal transduction (Yablonski et al., 1996), possibly for signal relay from Ste11 down to the MAP kinases (Feng et al., 1998). Ste5 could function in part to assure proper activation of Fus3 in the presence of pheromone, as well as to prevent Fus3 from being activated by the wrong signal. In addition, Ste5 might also regulate the access of Fus3 to substrates involved in mating and G1 arrest through interactions with Far1 and Bem1, a morphogenesis protein (Lyons et al., 1996).
To better understand how Fus3 functions, we characterized Fus3 localization and association with other proteins under a variety of conditions. By indirect immunofluorescence, Fus3 localizes throughout the cytoplasm and nucleus, with a greater fraction in cytoplasmic punctate spots in the presence of mating pheromone. Fus3 is more broadly localized than Kss1, providing one explanation for Fus3’s larger role in mating. The majority of Fus3 is soluble and not bound to any one protein; however, a fraction of Fus3 is stably bound to two proteins of ∼60 and ∼70 kDa. Fus3 exists in a number of complexes of widely varying size and specific activity, with ∼5.3% in a 350- to 500-kDa complex that is thought to contain Ste5, Ste11, and Ste7. Strikingly, Fus3 in this highest molecular-weight complex is most active, whereas the monomeric pool has little activity. The ability of Fus3 to localize in this complex requires Ste5. This result supports Ste5’s proposed role as a tether and suggests that complex formation may protect Fus3 from inactivation.
MATERIALS AND METHODS
Media and Microbiological Techniques
Standard methods (Sambrook et al., 1989) were used for manipulation of Escherichia coli and for all recombinant DNA manipulations. E. coli DH5α was the host for the construction and propagation of plasmids. Yeast transformations were performed as described previously (Ito et al., 1983). All yeast strains are isogenic to EY957, a bar1Δ derivative of W303a (Elion et al., 1993). Experiments were conducted with EY957, EY940 (a fus3–6::LEU2 derivative of EY957; Elion et al., 1993) containing either pYEE121 (FUS3-HA URA3 CEN) or pYEE128 (FUS3R42-HA URA3 CEN) (Elion et al., 1993), and with EY1881 (a ste5Δ :: TRP1 ste11Δ ::URA3 derivative of EY957), containing pKC11 (pGAL1-STE11M TRP1 ADE2 CEN) and pKCS5 (pGAL1-STE7M HIS3 CEN) or pKC11R444 (pGAL1-STE11R444M TRP1 ADE2 CEN) and pKCS5R220 (pGAL1-STE7R220M HIS3 CEN). Yeast cells were grown in YPD or selective SC media with either 2% dextrose, 2% raffinose, or 2% galactose as described (Sherman et al., 1986). Phosphate-free medium was made essentially as described previously (Rubin, 1975). MgSO4 (10 ml, 1 M) was added to 1 l of 2× SC-Ura-Cys-Met medium lacking carbon source. Concentrated NH4OH (10 ml) was slowly added with stirring, and phosphate salts were precipitated for 30 min at room temperature. The medium was filtered twice through 3-mm filters (Whatman, Clifton, NJ) with a Buchner funnel, the pH was adjusted to 4 with HCl, and then the medium was autoclaved.
Plasmids
pYEE1102 (FUS3-HA#5 CEN HIS3), pYEE1100 (FUS3 CEN HIS3), pYEE121 (FUS3-HA#5 CEN URA3), pYEE114 (FUS3 CEN URA3), pKC11 (GAL1-STE11M CEN TRP1 ADE2), PKCS5 (GAL1-STE7M CEN HIS3), pKC11R444 (pGAL1-STE11R444M TRP1 ADE2 CEN), and pKCS5R220 (pGAL1-STE7R220M HIS3 CEN) have all been described previously (Elion et al., 1993; Choi et al., 1994; Kranz et al., 1994).
Preparation of Antipeptide Antisera
Five milligrams of a 15-residue peptide (EE1), corresponding to amino acid residues 340–353 of Fus3 plus an N-terminal cysteine (synthesized by P. Kim, Whitehead Institute), was coupled to 10 mg of keyhole limpet hemocyanin (KLH, Pierce Chemical, Rockford, IL) that had been activated with 1.7 mg m-maleimidobenzoyl-H-hydroxysuccinimide ester (Pierce Chemical), according to manufacturers directions. The EE1–KLH conjugate was dialyzed against PBS (10 mM sodium phosphate, pH 7.4, 150 mM NaCl), yielding 7.2 ml of conjugate at ∼2 mg/ml protein (∼50% coupling efficiency); 2 mg of this conjugate was used for a series of four injections to each of three rabbits (by East Acres Biologicals, Southbridge, MA). An additional boost was made with 0.5 mg of EE1 conjugated to rabbit albumin (Sigma Chemical, St. Louis, MO), prepared as for the EE1–KLH conjugate. All sera were partially purified by ammonium sulfate precipitation (Ausubel et al., 1992), followed by dialysis against PBS, and stored in aliquots at −20°C. To affinity purify antiserum (from rabbit R2377, which provided the strongest immune response), the EE1–albumin conjugate was coupled to Affi-gel 10 (Bio-Rad, Richmond, CA) according to manufacturer’s instructions.
α Factor Time Course
Cells (900 ml) were grown at 30°C to an A600 of 0.4–0.5 in SC-Ura medium (SC medium for EY957), and 50 ml were removed as a zero time point. α Factor (synthesized by C. Dahl, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School) was added to a final concentration of 50 nM, and the culture was incubated at 30°C with shaking. Samples of 50 ml were collected at intervals over a 2-h period. The remaining 600 ml were then pelleted, washed once with 100 ml prewarmed sterile water, and then resuspended in fresh 30°C medium, and 50-ml samples were then removed at intervals over a 3-h period. All samples were immediately harvested, washed with ice-cold water, and frozen in dry ice/ethanol and stored at −80°C. In parallel, 1 ml of each sample was fixed with 0.1 ml 37% formaldehyde to monitor cell morphology. Fixed samples were stored overnight at 4°C, washed three times with 0.1 M potassium phosphate (pH 6.5), and then resuspended in 1 ml of this buffer and examined microscopically. Three separate tallies of 100 cells each were made for each sample.
In Vivo Labeling
Ten milliliters of a saturated culture grown in SC-Ura at 30°C was pelleted, resuspended in 300 ml of prewarmed SC-Ura-Cys-Met-phosphate–free medium and grown at 30°C for 8–10 h to an A600 of ∼0.4. To label with 35S, 10 ODs of cells were pelleted and resuspended in 5 ml of labeling medium containing 100 mCi 35S-trans-label (>1000 Ci/mmol; ICN, Costa Mesa, CA) per ml, and incubated for 30 min with shaking at 30°C. Cells were induced with 125 nM α factor (final concentration) by adding 5 ml more of labeling medium containing α factor (125 nM α factor was used because the cells are 2.5 times more concentrated than other experiments done with 50 nM α factor). Uniduced cells were treated identically, except that the labeling medium did not contain α factor. Cells were similarly labeled with 32PO4 using medium containing 0.5 mCi of 32PO4 (ICN) per 10 ODs of cells and a prelabeling incubation that was twice as long.
Indirect Immunofluorescence
Staining of fixed cells was carried out essentially as described previously (Elion et al., 1990). Yeast cells were grown in SC-Ura to an A600 of ∼0.5. Cells were found to form better shmoos if induced in YPD medium rather than SC medium; therefore, cultures were pelleted and resuspended in YPD medium containing 50 nM α factor and incubated for 2 h at 30°C. Cells were fixed by adding 0.1 volume of 37% formaldehyde and 0.1 volume of 0.1 M potassium phosphate (pH 6.5) and incubating samples on ice for 2 h. Samples were washed three times with sterile water, once with Buffer A (0.1 M potassium phosphate, pH 6.5, 1.2 M sorbitol). Spheroplasts were made by adding 10 μl β-mercaptoethanol, 100 μl of 5 μg/ml Zymolyase 20T to 1 ml cells, and incubating 15 min at 30°C until 70% spheroplasted (i.e., phase dark). Samples were then processed as described previously (Elion et al., 1995) using a 1:20 dilution of primary antibody in blocking buffer for 2 h at 30°C (12CA5 for hemagglutin [HA] epitope or 4A1 for tubulin) and then preadsorbed secondary antibody (Cy3-conjugated affinity-purified donkey anti-mouse IgG; Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature in the dark. To reduce background, the secondary antibody was preadsorbed against yeast proteins by diluting the antibody 1:1000 in blocking buffer containing 0.1% yeast whole-cell extract, incubating for 1–2 h on ice, and pelleting aggregates by centrifuging 10 min in a microfuge. Cells were viewed with a Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY). Ilford HP5 Plus ASA 400 (black and white) or Fuji Super HGII ASA 100 (color) film were used for photomicroscopy.
Cell Culture and Preparation of Yeast Extracts
Yeast strains grown in media containing 2% dextrose were induced with 50 nM α factor as described previously (Elion et al., 1990, 1993). Yeast strains containing GAL1-inducible plasmids were grown overnight at 30°C in SC selective media containing 2% glucose. The saturated culture was diluted into selective media containing 2% raffinose and grown overnight at 30°C to an A600 of 0.5–1.0. The raffinose culture was pelleted and resuspended in selective media containing 2% galactose at an A600 of 0.5 and grown at 30°C for 5–6 h. Cells were treated with 50 nM α factor for 60 min after 5 h in galactose. The cells were harvested, and extracts were prepared with modified H buffer containing 10% glycerol and 250 mM NaCl as described (Elion et al., 1993; Kranz et al., 1994).
Immunoprecipitation and Immune Complex Kinase Assays
All immune complex kinase assays were performed essentially as described (Elion et al., 1993). For glycerol gradient fractions, 400 μl of gradient fractions were diluted with modified H buffer containing 150 mM NaCl to 500 μl and incubated with 5 μg 12CA5 monoclonal antibody for 90 min on ice. Samples were then centrifuged for 10 min at 16,000 × g to remove insoluble aggregates, and supernatants were rocked with 30 μl Protein A–Sepharose CL-4B (Sigma Chemical) for 90 min at 4°C. Samples were pelleted and then washed five times with 1 ml of ice-cold modified H buffer, followed by two washes with kinase buffer (Elion et al., 1993). Samples were resuspended in 20 μl kinase buffer containing 1 μg casein, 20 μM cold ATP, 1 μCi [32P]ATP (6000 Ci/mmol, Amersham, Arlington Heights, IL) and incubated for 10 min at 30°C. Reactions were stopped by the addition of 30 μl 2× Laemmli buffer (Sambrook et al., 1989). Samples were boiled for 5 min before electrophoresis. Fus3 kinase activity was quantitated by determining the amount of radioactivity incorporated into the phospho-casein bands using a Beckman LS6500 Scintillation counter (Beckman, Fullerton, CA). The basal kinase activity present in a fus3Δ strain that lacked Fus3-HA (12% of 100% maximal activity) was subtracted from each fraction. For plot data, relative values of Fus3-HA kinase activity were normalized by the amount of Fus3-HA protein level in each fraction of the glycerol gradient by densitometric scanning of exposed films using a Bio-Rad Imager System (model GS-525) and Molecular Analyst version 1.5 software. Similar analysis was done for Fus3, Ste11M, and Ste7M. For each data point, the background was first calculated (based upon triplicate averaging of equivalent background areas of the films) and subtracted from each sample.
Immunoblot Analysis
The levels of Fus3, Fus3-HA, Ste11M, Ste7M, and tubulin were determined by immunoblot analysis according to standard procedures (Burnette, 1981) with the modifications noted by Elion et al. (1993). Proteins were detected with rabbit polyclonal Fus3 antibody (Elion et al., 1993), 12CA5 and 9E10 monoclonal antibodies (from the Harvard University Monclonal Antibody Facility; Upstate Biotechnology, Lake Placid, NY; and Santa Cruz Biotechnology, Santa Cruz, CA), 4A1 Drosophila tubulin monoclonal antibody (gift of C. Holm; University of California, San Diego), and a chemiluminescent detection kit (ECL, Amersham).
Glycerol Gradient Density Centrifugation
Glycerol gradient analysis was performed as described previously (Kranz, 1993) with the following modifications: 10 and 30% glycerol (wt/vol) stocks were prepared in modified H buffer containing 150 mM NaCl (Elion et al., 1993). A linear 10–30% glycerol gradient in a total volume of 11.4 ml was generated at 4°C in a SW41 polyallomer tube with a Hoefer SG13 gradient maker and peristaltic pump. One hundred fifty microliters of whole-cell extract (20 mg/ml) in modified H buffer containing 150 mM NaCl were precentrifuged for 10 min at 16,000 rpm in a microcentrifuge at 4°C, and the clarified supernatant was loaded onto the gradient. Gradients were centrifuged at 210,000 × g (35,000 rpm) at 4°C for 22 h in a SW41 swinging bucket rotor. Samples were collected with a 10-μl capillary pipet attached to narrow tubing, using a peristaltic pump to withdraw 580-μl aliquots from the bottom of the tube. Aliquots were collected at 4°C and stored at −70°C. Molecular weight standards were prepared in the same way as the samples, by mixing albumin, aldolase, catalase, and thyroglobulin to 1 mg/ml in H buffer with 150 mM NaCl, and loading 150 μl of the mixture on identically prepared gradients. Samples for immunoblot analysis and kinase assays were resolved on 8% polyacrylamide gels. Molecular weight standards were resolved on 7% polyacrylamide gels that were then stained with Coomassie Blue to localize the proteins.
RESULTS
Fus3 Is Rapidly Inactivated upon α Factor Withdrawal but Retains Residual Activity
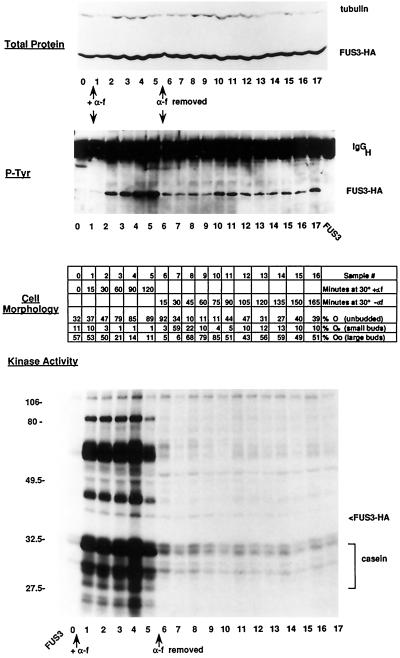
To determine the extent of α factor control of Fus3, we examined Fus3 activity during α factor addition and withdrawal, using phosphorylation of associated substrates as an assay for kinase activity (Elion et al., 1993). Cells were incubated with α factor until the majority were arrested in G1 phase, after which the α factor was washed out and the cells were allowed to recover. Samples were taken at various time points and monitored for cell morphology and Fus3 kinase activity using a strain containing a functional Fus3-HA (Elion et al., 1993) in place of native Fus3. Fus3-HA was activated rapidly in response to α factor, as measured by the phosphorylation of associated substrates in Fus3-HA–immune complexes (Figure 1). The increase in Fus3 activity correlated with increased tyrosine phosphorylation and protein abundance (Figure 1; note that the relatively low level of tyrosine phosphorylation at the 15-min time point may be caused by sample loss in this particular experiment). Fus3-HA began to lose activity at the 2-h time point, possibly from induced levels of the Msg5 phosphatase (Doi et al., 1994). α Factor withdrawal led to a dramatic inhibition of Fus3-HA kinase at the earliest time point taken, consistent with the resumption of budding. Surprisingly, however, the inhibition of Fus3 was not complete. Some Fus3 activity still persisted for at least 180 min after α factor withdrawal, as evidenced by both tyrosine phosphorylation of Fus3 and residual phosphorylation of Fus3-associated substrates. The bulk of Fus3-HA did not change in activity through the subsequent first cell cycle, suggesting that this basal activity of Fus3 is not cell cycle regulated (although this does not rule out the possibility of cell cycle-dependent regulation of a subset of Fus3 molecules). These results suggest that α factor induces a form of Fus3 that is resistant to inactivation by phosphatases.
Figure 1.
Effect of α factor addition and withdrawal on Fus3-HA kinase activity. Strain EY960 (EY940 + pYEE121, FUS3-HA CEN URA3) was induced with α factor for 2 h, and then the α factor was washed out and the G1-arrested cells were allowed to recover for 3 h. Duplicate samples were taken at the indicated time intervals during the α factor addition and withdrawal; one sample was pelleted and frozen for cell extracts, and the other was fixed with formaldehyde for microscopic analysis. A total of 18 time points (0–17, 120 min in the presence of α factor, 180 min after α factor washout) were analyzed for protein levels, tyrosine phosphorylation, and kinase activity, and a total of 17 time points were analyzed for cell morphology (0–16, 120 min in the presence of α factor, 165 min after α factor washout). Fus3-HA was detected with 12CA5 antibody, and Fus3-HA tyrosine phosphorylation was detected with an anti-phosphotyrosine antibody as previously described (Elion et al., 1993). Kinase assays were performed as described (see MATERIALS AND METHODS). Top panel, Abundance of Fus3-HA by immunoblot analysis of 25 μg of whole-cell extract. Second panel, Tyrosine phosphorylation of Fus3-HA immunoprecipitated from 200 μg of whole-cell extract. Third panel, Cell morphology. Fourth panel, Fus3-HA kinase assay of associated substrates immunoprecipitated from 200 μg of whole-cell extract. Samples were separated on a 10% (38:2) acrylamide:bis-acrylamide SDS gel. Cell percentages are averages of three fields of 100 cells each.
The Majority of Fus3 Is Not Stably Associated with Any One Protein
Previous work argues that the coimmunoprecipitating substrates in the Fus3 kinase assay are physiologically relevant (Elion et al., 1993; Choi et al., 1994; Kranz et al., 1994; Lyons et al., 1996; Tedford et al., 1997). The appearance of a highly reproducible profile of numerous associated substrates in the Fus3-HA kinase assays during α factor addition and withdrawal (Figure 1; substrates during withdrawal visualized by prolonged exposure of the autoradiogram; our unpublished data), suggests that a fraction of Fus3 is associated with a fixed set of substrates that may be present at stoichiometric levels. We immunoprecipitated Fus3-HA from in vivo labeled cells to determine whether Fus3 stably associates with a subset of proteins. Because potential substrates are likely to be dissociated once phosphorylated, we compared extracts from cells labeled with 35S and 32PO4.
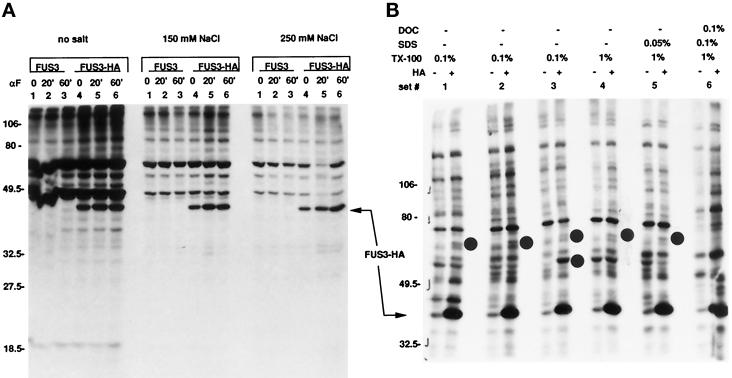
Immunoprecipitation of Fus3-HA from 35S-labeled extracts under a variety of nondenaturing conditions (including those used in the Fus3 kinase assay) revealed two specifically associated proteins of ∼70 and 60 kDa (Figure 2, sets 1–5, molecular mass ∼70 kDa; sets 1 and 3, molecular mass, ∼60 kDa). The two proteins associate nonidentically with Fus3. The 70-kDa protein associated with Fus3 under all immunoprecipitation conditions tested except for those of set 6 (0.1% deoxycholate, 0.1% SDS, 1% Triton-X 100), whereas the 60-kDa protein was less stably associated and only detected under the conditions of set 3 (0.1% Triton X-100 without added ovalbumin or bovine serum albumin (sets 1 and 2, respectively). If we assume that these proteins are not underrepresented for methionine and cysteine, then we can conclude that they are present in the immune complex at levels considerably less than Fus3. We did not detect any of the other proteins predicted to associate with Fus3 on the basis of the kinase assays. Thus, the majority of Fus3 is not stably associated with any one protein under these conditions, and the substrates detected in the Fus3 kinase assay are represented as a minority.
Figure 2.
Immunoprecipitation of Fus3-HA from 35S-labeled cells. Strains EY960 and EY1124 (EY940 with FUS3 [pYEE114] or FUS3-HA [pYEE121]) was labeled with 35S (see MATERIALS AND METHODS), and cells were harvested either without inducing with α factor, or after 20 and 60 min of α factor induction. Whole-cell extracts were prepared as described (see MATERIALS AND METHODS) and 200 μg of total protein was immunoprecipitated with 12CA5 for analysis. (A) Effect of salt in the immunoprecipitation. Samples were separated on a 10% (38:3 acrylamide:bis-acrylamide) SDS gel. Shown is an 18-h exposure of the autoradiogram. (B) Effect of detergents in the immunoprecipitation. Samples were separated on a 7.5% (30:0.8 acrylamide:bis-acrylamide) SDS gel. Shown is a 90-h exposure of the autoradiogram. For both panels A and B, extracts were prepared as described in MATERIALS AND METHODS and were then immunoprecipitated under the conditions described in the figure (note that in panel B, Set 1 also contained 1% bovine serum albumin and Set 2 also contained 1% ovalbumin). After immunoprecipitation, samples were washed with modified H-buffer (Elion et al., 1993). Fus3-HA is indicated by the arrow. ±HA indicates whether the strain contains Fus3-HA (+HA) or Fus3 (−HA). The dots indicate the positions of the 60- and 70-kDa proteins in panel B. DOC is deoxycholate. Extracts in panels A and B are from separate experiments.
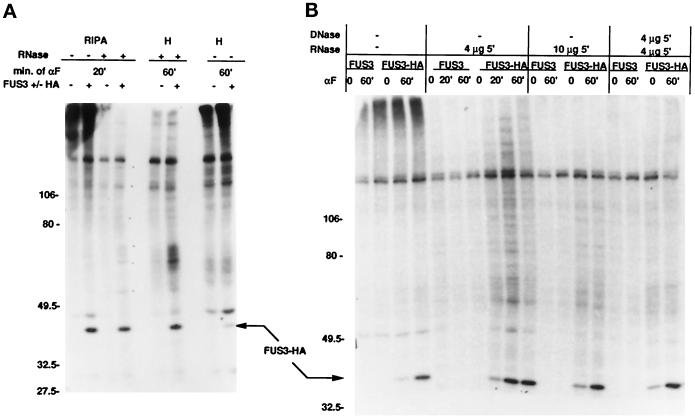
Immunoprecipitation of Fus3-HA from 32PO4-labeled extracts (Figure 3) showed that Fus3 is the only major phosphoprotein, although other minor phosphoproteins barely above background can be detected (Figure 3A). Similar results were found after varying the conditions of immunoprecipitation and after ribonuclease (RNase) and deoxyribonuclease (DNase) digestion, even though these variations improved the Fus3 signal. Thus, the vast majority of Fus3 is not stably associated with a phosphoprotein and must dissociate from its substrates once they are phosphorylated.
Figure 3.
Immunoprecipitation of Fus3-HA from 32P04-labeled cells. Strain EY940 containing FUS3 or FUS3-HA on a CEN plasmid (pYEE114 or pYEE121) was labeled with 32P-orthophosphate (see MATERIALS AND METHODS), and cells were harvested either in the absence of α factor induction or after 20 and 60 min of induction. Whole-cell extracts were prepared as described (see MATERIALS AND METHODS), and 200 μg of total protein were immunoprecipitated with 12CA5 antibody. Samples were separated on 7.5% (30:0.8 acrylamide:bis-acrylamide) SDS gels. (A) Immunoprecipitation of Fus3-HA in RIPA or modified H buffer, with or without final incubation with 4 μg RNAse for 5 min on ice. Shown is a 4.5-d exposure of the autoradiogram. ±HA indicates whether the strain contains Fus3-HA (+HA) or Fus3 (−HA). (B) Samples were immunopreciptated in modified H buffer and then treated with RNAse and DNAse as indicated. Shown is a 6.5-d exposure of the autoradiogram. Arrows indicate the position of Fus3-HA.
Fus3 Localizes throughout Dividing and α Factor-Arrested Cells
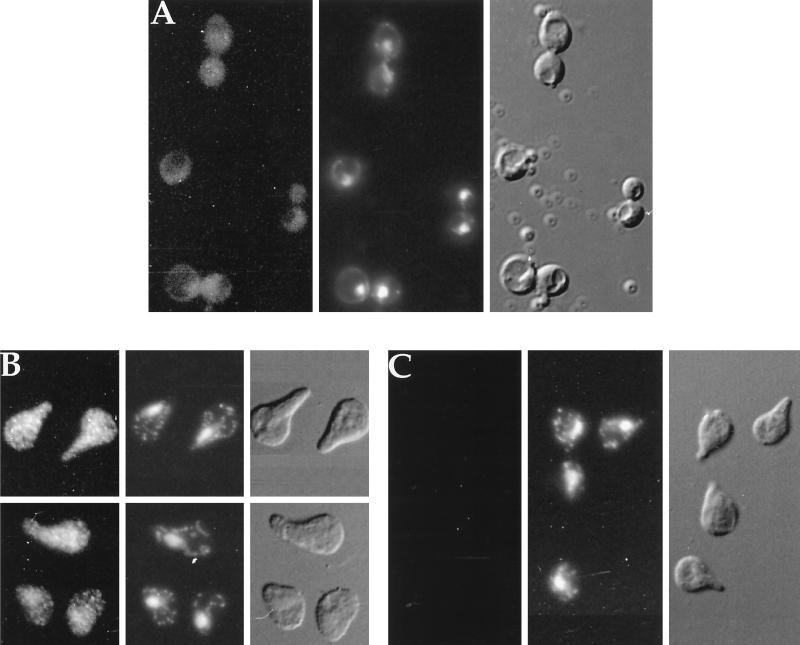
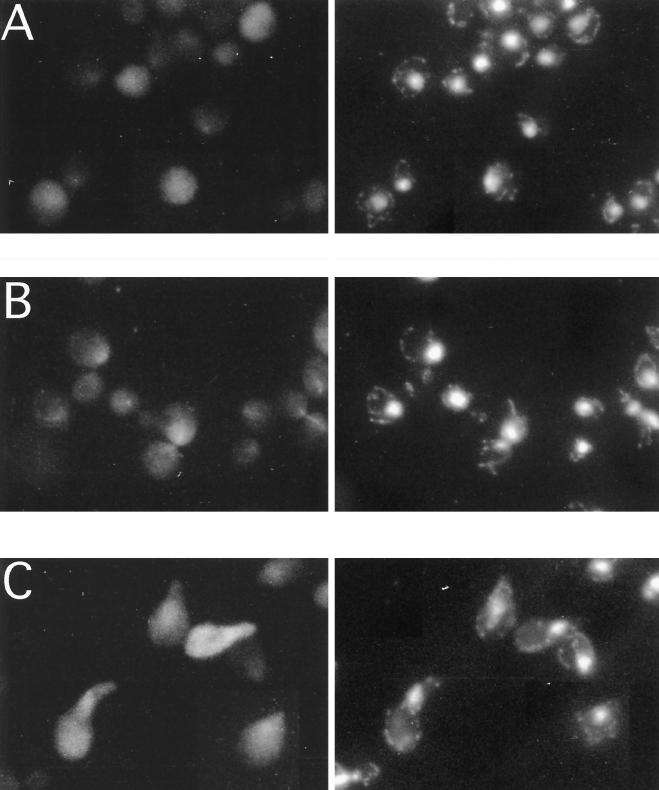
Fus3 was localized by indirect immunofluorescence. In dividing cells, the Fus3-HA pattern (Figure 4A) consisted of tiny spots throughout the cell with possible exclusion from the vacuole and slightly enhanced localization in the nucleus. This staining was specific to Fus3-HA, based upon the absence of staining from cells that lack the HA epitope (Figure 4C). The distribution of Fus3-HA changed in three respects upon stimulation with α factor. First, after a 10-min exposure to α factor, a greater percentage of Fus3 appeared to be in the nucleus (compare Figure 5, A and B). With longer exposure to α factor (Figures 4C and 5C), the staining pattern was significantly more intense and more punctate and appeared to be distributed throughout the cell (seen best in Figure 4C). In addition, Fus3 no longer appeared to be excluded from the vacuole, possibly because of its increased abundance in the cytoplasm. Thus, Fus3 localization involves both the nucleus and cytoplasm.
Figure 4.
Localization of Fus3-HA by indirect immunofluorescence. (A) FUS3-HA, vegetative growth; (B) FUS3-HA, 60-min α factor induction; (C) FUS3, 90-min α factor induction; (EY940 containing either FUS3 or FUS3-HA on a CEN plasmid [pYEE121]) grown either in the absence or presence of α factor, was prepared as described in MATERIALS AND METHODS and stained with 12CA5 antibody and affinity-purified donkey-anti-mouse IgG antibody conjugated to rhodamine-like Cy3. Micrographs shown are Cy3 fluorescence, DAPI fluorescence, and Nomarski differential interference contrast, as labeled. Cells were photographed with Fuji Super HGII-100 film using comparable exposure times.
Figure 5.
Comparison of short- and long-term exposure to α factor on Fus3-HA localization. (A) FUS3-HA, vegetative growth; (B) FUS3-HA, 10-min α factor induction; (C) FUS3-HA; 3-h α factor induction. Cells were prepared as in Figure 4 and photographed with comparable exposure times. Micrographs shown are Cy3 fluorescence and DAPI fluorescence.
The punctate pattern of Fus3 localization that was most apparant after longer exposure to α factor is clearly distinguished from other specifically localized proteins tagged with the HA epitope (Kranz, 1993), such as Pmr1-HA, which localizes in the Golgi (Antebi and Fink, 1992), Ssl2-HA, which localizes in the nucleus (Gulyas and Donahue, 1992), and β-galactosidase, which localizes nonspecifically in the cytoplasm (Elion et al., 1995). Thus, the spots may represent the association of Fus3 with a novel macromolecular structure or subcellular organelle. Mating pheromone may enhance the ability of Fus3 to associate in the structures represented by the punctate spots since they are more apparant after α factor treatment of cells. Alternatively, it is possible that Fus3 associates with the same structures in the absence of α factor but that we cannot detect them due to lower levels of Fus3 and the limits of resolution.
A Fraction of Fus3 Is in a Complex That Sediments at 100,000 × g
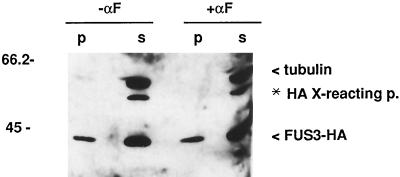
To determine whether Fus3 is in a large macromolecular structure, as suggested by the punctate staining pattern of Fus3 by indirect immunofluorescence (Figure 4), we subjected whole-cell extracts to high-speed centrifugation at 100,000 × g and determined the proportion of Fus3 associated with soluble and insoluble cell fractions. As a control, we assayed the amount of tubulin in the same samples. Under the lysis conditions used, microtubules are depolymerized and exist as soluble monomers (Barnes et al., 1992). Consistent with this, all detectable tubulin protein was present in the supernatant (Figure 6). The majority of Fus3 was also in the supernatant, indicating that it is, for the most part, a soluble enzyme. However, in contrast to tubulin, 10% of Fus3-HA was consistently found in the pellet of extracts made from both uninduced and pheromone-induced cells. Thus, a proportion of Fus3 is associated with very large macromolecular structures that are stable to these extraction methods, and α factor does not affect the amount of Fus3 that is associated.
Figure 6.
High-speed fractionation of Fus3-HA. Strain EY960 was either uninduced (lanes 1 and 2) or induced with α factor for 1 h (lanes 3 and 4) and whole-cell extracts prepared as described in MATERIALS AND METHODS, including the clarification step of 10 min at 13,000 rpm. Total protein (1 mg) in 500 μl of modified H buffer was centrifuged at 100,000 × g (61,000 rpm in a microcentrifuge) for 30 min at 4°C. The 500-μl supernatant (s) was removed, diluted with 500 μl of 2× SDS sample buffer, and boiled. The pellet (p) was resuspended by boiling in 1 ml of 1× SDS sample buffer. Equal amounts (30 μl) of each sample were separated on a SDS polyacrylamide gel and immunoblotted with 12CA5 and tubulin (4A1) antibodies. Alternate lanes were used to ensure that signals are not from spillover from an adjacent lane.
Fus3-Specific Activity Varies Greatly across a Glycerol Gradient
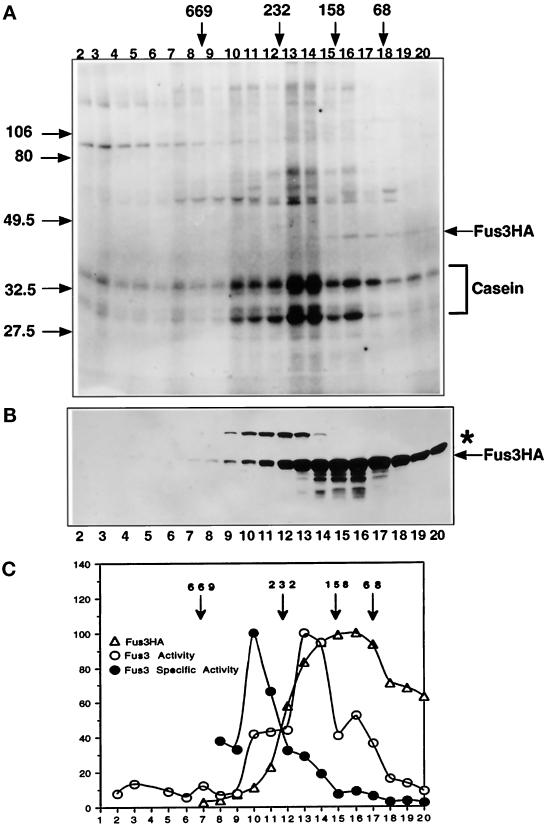
Gradient density centrifugation was used in parallel to high-speed fractionation to determine the relative specific activity of Fus3 as a monomer or when present in higher-molecular-weight forms that may not be stable to the rigors of immunoprecipitation. Whole-cell extracts were prepared from a Fus3-HA strain that was induced with α factor. Samples were precentrifuged to pellet aggregates, and the supernatant was then separated on a linear 10–30% glycerol gradient. Individual fractions were assayed for the amount of Fus3-HA by immunoblot analysis and for kinase activity using casein as a substrate (Figure 7, A and B). Several phosphoproteins were detected across the gradient, in addition to phosphocasein; they may be the same associated substrates detected in Fus3-HA kinase assays (Figure 1). The ∼42-kDa phosphoprotein is phosphorylated Fus3-HA (Elion et al., 1993).
Figure 7.
Fus3 abundance and kinase activity across a 10–30% glycerol density gradient. (A) Fus3-HA kinase activity across the gradient. Fus3-HA from each fraction was immunoprecipitated with 12CA5 antibody and assayed for Fus3 activity using exogenous casein as described (see MATERIALS AND METHODS). Shown is an 8-h exposure of the autoradiogram. (B) Distribution of Fus3-HA protein across a 10–30% glycerol density gradient, performed as in Figure 8. A portion of each fraction (50 μl) was used for immunoblot analysis to detect Fus3-HA with 12CA5 antibody. (C) Graphic representation of Fus3-HA kinase activity, abundance, and specific activity across the glycerol gradient (see MATERIALS AND METHODS). Open circle, Fus3-HA kinase activity; triangles, Fus3-HA protein; solid circles, Fus3-HA-specific activity. Strain EY940 containing FUS3-HA on a CEN plasmid (pYEE121) was used for extract preparation. Cells were grown and induced with 50 nM α factor for 1 h. The asterisk indicates a ∼55 kDa protein that cross-reacts with 12CA5.
Fus3-HA sedimented broadly across the gradient (Figure 7B), in contrast to more localized sedimentation profiles for the 55-kDa protein that cross-reacts with 12CA5 and tubulin, which sediments as a monomer at 55 kDa under the same conditions (Kranz, 1993). On the basis of qualitative analysis using a chemiluminescent detection system (see MATERIALS AND METHODS), the vast majority of Fus3-HA was associated with other proteins (∼81% of Fus3-HA in fractions 10–17). The specific activity of Fus3 varied greatly across the gradient and did not correlate with the abundance of the various sedimenting species (Figure 7C). For example, although a significant fraction of Fus3 sedimented at or close to its monomeric size (e.g., the pool with a lower apparent molecular mass than the 68-kDa standard), this pool had lowest specific activity. The fraction of Fus3-HA with the highest specific activity was the least represented (∼5.3%) and sedimented within a molecular weight range of 350–500 KDa (fractions 9–12). The majority of active Fus3-HA (∼35%) sedimented at a molecular mass of ∼170–220 kDa (fractions 13–14), indicating it was associated in a distinct complex(es). Thus, Fus3-HA exists in several distinct complexes and has significantly higher specific activity when associated with other proteins. Moreover, the position of Fus3-HA of highest specific activity closely overlaps the position where Ste5, Ste11, and Ste7 also cosediment (Choi et al., 1994), strongly suggesting that the Ste5–multikinase complex plays a key role either in activating Fus3 or maintaining high activity.
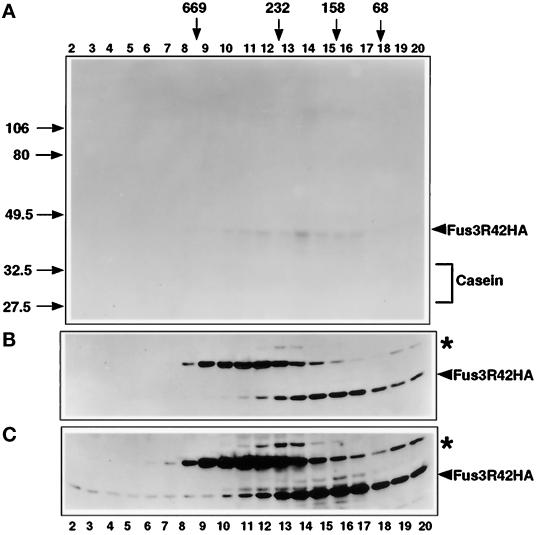
A parallel glycerol gradient was done on extracts prepared from a strain harboring catalytically inactive Fus3 (Fus3R42-HA). No phosphorylation of casein was detected across this glycerol gradient (Figure 8A) even in the fractions with the highest levels of Fus3R42-HA protein (Figure 8B), indicating that all of the phosphorylation of casein we detected for Fus3-HA (Figure 7A) was due to Fus3 activity. Phosphorylated Fus3R42-HA was weakly detected in fractions 10–17 upon prolonged exposure of the gel (Figure 8A). This phosphorylation may be caused by residual autophosphorylation activity of Fus3R42-HA (Elion et al., 1993; Brill et al., 1994) or by phosphorylation by Ste7, which tightly associates with Fus3 and cosediments with Fus3 in a glycerol gradient (Choi et al., 1993; Bardwell et al., 1996). Longer exposure of the Fus3R42-HA immunoblot (Figure 8C) indicated that a greater fraction sediments at the bottom of the gradient compared with Fus3-HA. Thus, Fus3R42-HA may associate more stably in macromolecular structures than does Fus3-HA.
Figure 8.
Fus3R42 abundance and kinase activity across a 10–30% glycerol density gradient. (A) Fus3R42-HA kinase activity across the gradient assayed as described in Figure 6. (B) Distribution of Fus3R42-HA protein across the 10–30% glycerol density gradient, performed as in Figure 8. A portion of each fraction (50 μl) was used for immunoblot analysis to detect Fus3R42-HA with 12CA5 antibody. Strain EY940 containing FUS3R42-HA on a CEN plasmid was used for extract preparation. Cells were grown as in Figure 6. A similar exposure time is shown as in Figure 7B. (C) Longer exposure of the immunoblot in panel B showing Fus3R42-HA at the bottom of the gradient. The asterisk indicates a ∼55 kDa protein that cross-reacts with 12CA5.
Fus3, Ste11, and Ste7 Do Not Cosediment in a High-Molecular-Weight Complex in the Absence of Ste5
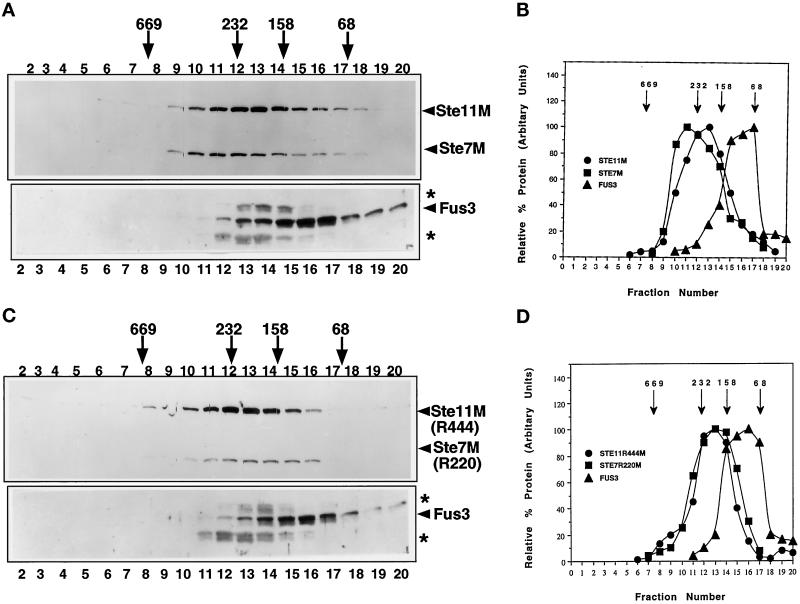
To further support a role for the Ste5–multikinase complex in the generation of a species of Fus3 of high specific activity, we localized Fus3, Ste7, and Ste11 kinases on a 10–30% glycerol gradient using extracts prepared from a ste5Δ strain. Because it is not possible to detect native levels of Ste7 and Ste11 using available polyclonal antibodies or monoclonal antibodies to tagged derivatives, Ste7 and Ste11 were each overexpressed. Wild-type levels of native Fus3 were monitored. We compared the pattern of Fus3 sedimentation in strains expressing both functional and nonfunctional forms of Ste11 and Ste7 (i.e., Ste11R444 and Ste7R220), because previous work has shown that a larger percentage of Fus3 associates with the inactive forms of Ste11 and Ste7 (Choi et al., 1994).
In the absence of Ste5, little or no Fus3 was found to sediment at the position of 350–500 kDa (Figure 9, lanes 9–11) compared with what has been observed in the STE5 strain in Figure 7 or in a STE5 strain harboring GST-Ste5 (Choi et al., 1994). A greater percentage of Fus3 was found to sediment in a tighter peak between the 158- and 68-KDa molecular mass standards. Similar results were found with functional and nonfunctional Ste11M and Ste7M. Ste11M and Ste7M (as well as the inactive forms) were found to sediment in novel peaks in the range of ∼170–300 kDa. Ste11M and Ste7M each shift in opposite directions compared with their sedimentation pattern in the presence of Ste5 (Choi et al., 1994), arguing that the pattern of sedimentation of the three kinases is not due to an artifactual shifting of the entire gradient. This result strongly argues that the sedimentation of Fus3 at the 350–500 kDa position in the gradient is directly due to a tethering function provided by Ste5.
Figure 9.
Distribution of Fus3, Ste11M, and Ste7M across a 10–30% glycerol density gradient in the absence of Ste5. Whole-cell extracts were prepared from a ste5Δ ste11Δ strain (EY1881) harboring either pKC11 (pGAL1-STE11M) and pKCS5 (pGAL1-STE7M) or pKC11R444 (pGAL1-STE11R444M) and pKCS5R220 (pGAL1-STE7R220M) and separated across a 10–30% glycerol gradient as described (see MATERIALS AND METHODS). (A and C) Immunoblots of the distribution of Fus3, Ste11M and Ste7M, Ste11R444M, and Ste7R220M across the gradients. A portion of each fraction (50 μl) was used for immunoblot analysis to detect Ste11M and Ste7M with the 9E10 antibody, and 120 μl of each fraction was used to detect Fus3 with a rabbit polyclonal antibody to Fus3 (see MATERIALS AND METHODS; Choi et al., 1994). (B and D) Plots of the relative amount of each protein. Protein levels were quantitated by densitometric scans of exposures of the immunoblots as in Figure 7C. The highest value for each protein was standardized as 100%. Arrows above the figure represent the positions of native-state-size markers separated in a separate glycerol gradient (albumin, 68 kDa; aldolase, 158 kDa; catalase, 232 kDa; throglobulin, 669 kDa). The asterisks indicate proteins that cross-react with the anti-Fus3 antiserum.
DISCUSSION
Fus3 Localization Is Consistent with Its Critical Role in Mating
The indirect immunolocalization results suggest that Fus3 is broadly distributed in mating cells, in accordance with functions in the cytoplasm and nucleus. After brief exposure of cells to α factor, Fus3 appears to concentrate somewhat at or within the nucleus, consistent with the requirement for Fus3 in the activation of the Ste12 transcription factor (Elion et al., 1991). After longer exposure to α factor, Fus3 appears to localize throughout the cell, with increased appearance in the cytoplasm. This cytoplasmic distribution agrees with the requirement for Fus3 in mating responses that may involve the cytoplasm, such as projection formation, partner selection, and cell fusion (Elion et al., 1990; Farley et al., 1999). However, we do not detect an obvious pool of Fus3 at the shmoo tip, as might be expected for functions in partner selection and cell fusion. Localization of Fus3 at the shmoo tip could involve a small population of molecules and not be detectable, or it could involve a significant population of molecules that associate transiently or in a manner that is not stable to the fixation procedures used for indirect immunofluorescence.
Pheromone may regulate the ability of Fus3 to either enter or exit from the nucleus. For example, the enhanced nuclear localization of Fus3 after short-term exposure to α factor may be due to enhanced nuclear import. Growth factor-induced nuclear import has been noted for mammalian MAP kinases (Chen et al., 1992; Gonzalez et al., 1993; Lenormand et al., 1993; Fukuda et al., 1997) that phosphorylate nuclear targets such as transcription factors (Treisman, 1996). While it is possible that α factor induces a selective turnover of subcellular pools of Fus3, this seems unlikely because Fus3 abundance increases in response to α factor (Elion et al., 1993). The enhanced cytoplasmic localization observed after long exposure to α factor could be caused by the synthesis of more Fus3 protein in the cytoplasm as well as changes in the ability of Fus3 to either enter or exit the nucleus.
Fus3 is more broadly distributed in the cell than is Kss1, which is localized almost exclusively in the nucleus (Ma et al., 1995), thus providing one explanation for functional differences between the two kinases. Fus3 is more important than Kss1 for a number of pheromone responses that may take place at or near the shmoo tip (Elion et al., 1990; Lyons et al., 1996; Farley et al., 1999) where the concentration of Kss1 is very low (Ma et al., 1995). Further work is needed to determine the cellular location of phosphorylation events and the relative contribution of the two kinases to substrate phosphorylation when both kinases are present. For example, Fus3 makes a greater contribution to the up-regulation of the transcription factor Ste12 in response to pheromone than does Kss1 (Elion et al., 1991; Madhani et al., 1997), although both kinases are nuclear. Thus, additional levels of specificity may mediate the different functions of Fus3 and Kss1 during mating.
Fus3 Exists in a Variety of Complexes throughout the Cell
Our study suggests that in vivo, most of Fus3 (∼81%) exists in association with a variety of other proteins, rather than as a simple monomer. First, by indirect immunofluorescence, Fus3 localizes throughout the cell in discrete entities that appear as punctate staining. This pattern of localization may be due to the concentration of Fus3 in large structures of some kind, for it contrasts the smooth staining pattern seen for soluble proteins such as β-galactosidase that localize nonspecifically throughout the cytoplasm (Elion et al., 1995). The punctate pattern is not reminiscent of a particular organelle such as the Golgi apparatus or secretory vesicles, nor does it ressemble the pattern of actin or tubulin and therefore may represent a novel subcellular superstructure or organelle. Second, ∼10% of total Fus3 is pelleted at 100,000 × g, indicating that a significant pool of Fus3 is in very large macromolecular structures, consistent with the punctate pattern of localization. Third, glycerol gradient density centrifugation of preclarified concentrated extracts show that a significant fraction of soluble Fus3 (∼74%) is in complexes ranging in apparant size from ∼70 kDa to > 500 kDa. Collectively, our analysis argues that Fus3 is complexed to a variety of proteins, consistent with the fact that it coprecipitates with numerous proteins (Elion et al., 1993; Choi et al., 1994; Kranz et al., 1994; Bardwell et al., 1996; Lyons et al., 1996; Tedford et al., 1997). The fact that it is difficult to detect any of these proteins in coimmunoprecipitation experiments of 35S-labeled extracts suggests either that these associations involve a small pool of Fus3 or that they are not stable to the conditions of the immunoprecipitation.
Active Fus3 Is Complexed with Other Proteins
A striking finding from our analysis is that the active Fus3 is complexed to other proteins whereas monomeric Fus3 is largely inactive. All of the most active Fus3 was found in at least three pools of higher molecular mass (in the ranges of 68–158, 158–232, and 232–500 kDa), suggesting that nearly all of the active Fus3 in the cell is bound to other proteins. The majority of active Fus3 is found in a discrete peak in the molecular mass range of ∼158–200 kDa, suggesting that it exists in a distinct complex of unknown identity. One of these pools may contain Ste7/Fus3 complexes, which are known to exist in the absence of Ste5 (Choi et al., 1994; Bardwell et al., 1996). A second pool may involve Fus3 dimers, by analogy to ERK2 (Khokhlatchev et al., 1998). Although Fus3 may be unusual in its propensity to exist in complexes, the notion that association with other proteins is required to maintain kinase activity may apply to other MAP kinases.
We find that Fus3 forms particularly stable complexes with two proteins of ∼60 and ∼70 kDa (p60 and p70) both in the absence and presence of mating pheromone. p60 and p70 could be novel or previously identified in Fus3-immune complexes; their apparant size is relatively similar to several Fus3 substrates, including Dig1/Rsr1 and Dig2/Rsr2 (∼45 and ∼65 kDa, respectively; Tedford et al., 1997) and Ste7 (∼55 kDa; Bardwell et al., 1996). Stable complexes involving MAP kinases have been reported elsewhere and include dimers between Ste7 and Fus3/Kss1 (Bardwell et al., 1996), Xenopus MAP kinase and Rsk (Hsiao et al., 1994), and c-Jun-NH2-terminal kinases and c-Jun (Kallunki et al., 1994).
Ste5 Is Responsible for Maintaining Ste11, Ste7, and Fus3 in a High-Molecular-Weight Complex and Maintaining Fus3 Activity
We previously suggested that Ste5 serves as a tether for Ste11, Ste7, and Fus3 on the basis of the fact that Ste5 provides separable binding sites for the three kinases and that the four proteins cosediment at a high molecular mass (∼350–500 kDa) on a glycerol-density gradient. Here we demonstrate that the ability of Ste11, Ste7, and Fus3 to cosediment within this molecular mass range requires Ste5. In the absence of Ste5, all three proteins shift dramatically in their sedimentation profile, with no cosedimentation within the 350–500 kDa range. This simple result provides important evidence that Ste5 holds the kinases together in a complex.
Previous studies implicated Ste5 as serving an important role in the activation of Fus3 (Choi et al., 1994; Kranz et al., 1994). Fus3 is not activated by mating pheromone in the absence of Ste5 (Choi et al., 1994; Kranz et al., 1994), indicating that Ste5 is essential for its activity. In addition, Fus3 associates with Ste5 in complexes that also contain Ste11 and Ste7 (Choi et al., 1994), and overexpression of Ste5 stimulates Fus3 activity and suppresses a variety of loss-of-function fus3 mutations (Kranz et al., 1994). However, the dependence of Fus3 activity on Ste5 is not necessarily due to Ste5’s putative tethering function. For example, Ste5 is required for the activation of Ste11 (Choi et al., 1994) and plays a direct role in the activation of Ste11 by Gβ (Feng et al., 1998) and therefore could enhance Fus3 activity indirectly. We present two findings that argue strongly that Ste5 enhances the activity of Fus3 through its function as a tether. First, we show that the pool of Fus3 in a wild-type cell that has highest specific activity sediments to a position in the gradient (∼350–500 kDa) that has previously been shown to contain Ste5, Ste11, and Ste7 (Choi et al., 1994). Second, we show that Ste5 is responsible for holding Fus3 in this complex.
Association of Fus3 in Complexes May Protect Fus3 from Inactivation
Why does active Fus3 exist almost exclusively in complexes? It is possible that complex formation via Ste5 and other proteins is required to prevent inhibition of Fus3 in addition to mediating its activation. Fus3 is largely inactive in the presence of α factor and immediately inactivated upon α factor withdrawal (Figure 1), consistent with the existence of multiple inhibitory phosphatases (Doi et al., 1994; Zhan et al., 1997). The observation that a fraction of Fus3 becomes resistant to inactivation by phosphatases after α factor treatment (Figure 1) supports the notion that α factor induces a form of Fus3 that is protected from inactivation. One possibility is that Fus3 is constitutively inactivated and that pheromone induction overrides constitutive inhibitory forces. This notion is consistent with the fact that all of the phosphatases that are known to inactivate Fus3 are constitutively expressed in vivo and are constitutively active in vitro (Msg5, Doi et al., 1994; Ptp2 and Ptp3, Zhan et al., 1997). In addition, Fus3 tyrosine phosphorylation has been shown to be constitutively inhibited by the high-osmolarity response pathway during vegetative growth (Hall et al., 1996). Consitutive negative control of Fus3 might protect dividing cells from inappropriate arrest and entry into the mating pathway. It is interesting to speculate that Ste5 may prevent constitutive negative regulation of Fus3.
Does Catalytically Inactive Fus3 Regulate Signal Transduction during Mating?
A signifcant pool of monomeric Fus3 (∼19% of total Fus3, if we assume that it is the pool that is of lower apparant molecular mass than the 68-kDa standard) is nearly completely inactive at a 1-h α factor induction time point (Figure 7). This pool could arise through the action of contaminating phosphatases during our extract preparation, or it could simply be the fraction of Fus3 that has not been activated or has already been inactivated. The existence of an inactive pool of Fus3 during the course of a pheromone response raises the question of whether inactive Fus3 might have a function during signal transduction. For example, catalytically inactive Fus3 could attenuate signal transduction during mating through the sequestration of activators or substrates. That the inactive pool of Fus3 could serve an attenuating function is consistent with the ability of catalytically inactive Fus3R42 to exist in variety of complexes (Figure 8) and the precedence of inhibitory functions for catalytically inactive forms of Fus3 and Kss1 during invasive growth (Cooke et al., 1997; Madhani et al., 1997). Recent genetic evidence suggests that in a wild-type cell, catalytically inactive Fus3 inhibits active Fus3 and promotes recovery (Farley et al., 1999), consistent with this possibility.
ACKNOWLEDGMENTS
We thank Bill Braell for advice on glycerol-gradient density centrifugation, Tamar Enoch for advice on preadsorption of antibodies with yeast extracts, and Yvonne DeCelis for generating the prints for Figure 4. We also thank B.N. Lee, A. Leza, and V. Cherkasova for their comments on the manuscript and Mark Rose for helpful discussions. This research was supported by the following grants to E.A.E.: Harcourt Charitable Foundation Junior Investigator Award, American Cancer Society Junior Investigator Award, and National Institutes of Health grant RO1 GM-46962. K.-Y.C. was also supported by a grant from a basic research medical fund, Ministry of Education, Korea.
REFERENCES
- Antebi A, Fink GR. The yeast calcium-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent K, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K. Short Protocols in Molecular Biology. New York, NY: John Wiley and Sons; 1992. [Google Scholar]
- Bardwell L, Cook JG, Chang EC, Cairns BR, Thorner J. Signaling in the yeast pheromone response pathway: Specific and high-affinity interaction of the mitogen-activated protein (MAP) kinases Kss1 and Fus3 with the upstream MAP kinase kinase Ste7. Mol Cell Biol. 1996;16:3637–3650. doi: 10.1128/mcb.16.7.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G, Louie KA, Botstein D. Yeast proteins associated with microtubules in vitro and in vivo. Mol Biol Cell. 1992;3:29–47. doi: 10.1091/mbc.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill JA, Elion EA, Fink GR. A role for autophosphorylation revealed by activated alleles of FUS3, the yeast MAP kinase homolog. Mol Biol Cell. 1994;5:297–312. doi: 10.1091/mbc.5.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette WN. Western blotting: electrophoretic transfer of proteins from SDS polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radio-iodinated protein. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova V, Lyons DM, Elion EA. Fus3 and Kss1 control G1 arrest through a balance of distinct arrest and proliferative functions that operate in parallel with Far1. Genetics. 1999;151:989–1004. doi: 10.1093/genetics/151.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K-Y, Satterberg B, Lyons DM, Elion EA. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Cook JG, Bardwell L, Kron SJ, Thorner J. Two novel targets of the MAP kinase Kss1 are negative regulators of invasive growth in the yeast Saccharomyces cerevisiae. Genes & Dev. 1996;10:2831–2848. doi: 10.1101/gad.10.22.2831. [DOI] [PubMed] [Google Scholar]
- Doi K, Gartner A, Ammerer G, Errede B, Shinkawa H, Sugimoto K, Matsumoto K. MSG5, a novel protein phosphatase promotes adaptation to the pheromone response in S. cerevisiae. EMBO J. 1994;13:61–70. doi: 10.1002/j.1460-2075.1994.tb06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA. Ste5: a meeting place for MAP kinases and their associates. Trends Cell Biol. 1995;5:322–327. doi: 10.1016/s0962-8924(00)89055-8. [DOI] [PubMed] [Google Scholar]
- Elion EA. Routing MAP kinase cascades. Science. 1998;281:1615–1626. doi: 10.1126/science.281.5383.1625. [DOI] [PubMed] [Google Scholar]
- Elion EA, Brill JA, Fink GR. FUS3 represses CLN1 and CLN2 and in concert with KSS1 promotes signal transduction. Proc Natl Acad Sci USA. 1991;88:9392–9396. doi: 10.1073/pnas.88.21.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA, Grisafi PL, Fink GR. FUS3 encodes a cdc2/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990;60:649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Elion EA, Satterberg B, Kranz JE. FUS3 phosphorylates multiple components of the mating signal transduction cascade: evidence for STE12 and FAR1. Mol Biol Cell. 1993;4:495–510. doi: 10.1091/mbc.4.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA, Trueheart J, Fink GR. Fus2 localizes near the site of cell fusion and is required for both cell fusion and nuclear alignment during zygote formation. J Cell Biol. 1995;130:1283–1296. doi: 10.1083/jcb.130.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B, Gartner A, Zhou Z, Nasmyth K, Ammerer G. MAP kinase-related FUS3 from S. cerevisiae is activated by STE7 in vitro. Nature. 1993;362:261–264. doi: 10.1038/362261a0. [DOI] [PubMed] [Google Scholar]
- Farley, F.W., Satterberg, B., Goldsmith, E.A., and Elion, E.A. (1999). Relative dependence of different outputs of the pheromone response pathway on Fus3 function. Genetics (in press). [DOI] [PMC free article] [PubMed]
- Feng Y, Song L-Y, Kincaid E, Mahanty SM, Elion EA. Functional binding between Gβ and the LIM domain of Ste5 is required to activate the MEKK Ste11. Curr Biol. 1998;8:267–278. doi: 10.1016/s0960-9822(98)70108-3. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Nasmyth K, Ammerer G. Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes & Dev. 1992;6:1280–1292. doi: 10.1101/gad.6.7.1280. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin MC, Albertyn J, Alexander MR, Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PJ, Cherkasova V, Elion EA, Gustin MC, Winter EC. The osmo-regulatory pathway represses mating pathway activity in S. cerevisiae: Isolation of a fus3 mutant that is immune to repression. Mol Cell Biol. 1996;16:6715–6723. doi: 10.1128/mcb.16.12.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KM, Chou SY, Shih SJ, Ferrell JE., Jr Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc Natl Acad Sci USA. 1994;91:5480–5484. doi: 10.1073/pnas.91.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye C, Dhillon N, Thorner J. Ste5 Ring-H2 domain: role in Ste4-promoted oligomerization for yeast pheromone signaling. Science. 1997;278:103–106. doi: 10.1126/science.278.5335.103. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukada Y, Murata K, Kimura A. Transformation of intact yeast cells with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki T, Su B, Tsigelny I, Sluss HK, Derijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes & Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Dimerization of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kranz JA. Signal Transduction in S. cerevisiae. Ph.D. Thesis. Cambridge MA: Harvard University; 1993. [Google Scholar]
- Kranz JA, Satterberg B, Elion EA. The MAP kinase Fus3 associates with and phosphorylates the upstream signaling component Ste5. Genes & Dev. 1994;8:313–327. doi: 10.1101/gad.8.3.313. [DOI] [PubMed] [Google Scholar]
- Leeuw T, Fourest-Lieuvin A, Wu C, Chenevert J, Clark K, Whiteway M, Thomas DY, Leberer E. Pheromone response in yeast: association of Bem1p with proteins of the MAP kinase cascade and actin. Science. 1995;270:1210–1213. doi: 10.1126/science.270.5239.1210. [DOI] [PubMed] [Google Scholar]
- Leeuw T, Wu C, Schrag JD, Thomas DY, Leberer E. Interaction of a G-protein beta-subunit with a conserved sequence in/PAK family protein kinases. Nature. 1998;39:191–195. doi: 10.1038/34448. [DOI] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Mahanty SK, Choi K-Y, Manandhar M, Elion EA. The SH3-domain protein Bem1 coordinates mitogen-activated protein kinase cascade activation with cell cycle control. Mol Cell Biol. 1996;16:4095–4106. doi: 10.1128/mcb.16.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Cook JG, Thorner J. Phosphorylation and localization of Kss1, a MAP kinase of the Saccharomyces cerevisiae pheromone response pathway. Mol Biol Cell. 1995;6:889–909. doi: 10.1091/mbc.6.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani HD, Styles CA, Fink GR. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;9:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- O’Rourke SM, Herskowitz I. The Hog1 MAPK prevents cross talk between the HOG and pheromone response pathways in Saccharomyces cerevisiae. Genes & Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Gartner A, Horecka J, Ammerer G, Herskowitz I. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell. 1993;73:747–760. doi: 10.1016/0092-8674(93)90254-n. [DOI] [PubMed] [Google Scholar]
- Rubin GM. Prparation of RNA and ribosomes from yeast. Methods Cell Biol. 1975;12:45–64. doi: 10.1016/s0091-679x(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- Sprague GF, Jr, Thorner JW. Pheromone response and signal transduction during the matig process of Saccharomyces cerevisiae. In: Jones E, Pringle J, Broach J, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 657–744. [Google Scholar]
- Tedford K, Kim S, Sa D, Stevens K, Tyers M. Regulation of the mating pheromone and invasive growth responses in yeast by two MAP kinase substrates. Curr Biol. 1997;7:228–238. doi: 10.1016/s0960-9822(06)00118-7. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–216. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Tyers M, Futcher B. Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol Cell Biol. 1993;13:5659–5669. doi: 10.1128/mcb.13.9.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway MS, Wu C, Leeuw T, Clark K, Fourest-Lieuvin A, Thomas DY, Leberer E. Association of the yeast pheromone response G protein βγ subunits with the MAP kinase scaffold Ste5p. Science. 1995;269:1572–1575. doi: 10.1126/science.7667635. [DOI] [PubMed] [Google Scholar]
- Yablonski D, Marbach I, Levitzki A. Dimerization of Ste5, a mitogen-activated protein kinase cascade scaffold protein, is required for signal transduction. Proc Natl Acad Sci USA. 1996;93:13864–13869. doi: 10.1073/pnas.93.24.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan XL, Deschenes RJ, Guan KL. Differential regulation of FUS3 MAP kinase by tyrosine-specific phosphatases PTP2/PTP3 and dual-specificity phosphatase MSG5 in Saccharomyces cerevisiae. Genes & Dev. 1997;11:1690–1702. doi: 10.1101/gad.11.13.1690. [DOI] [PubMed] [Google Scholar]