Abstract
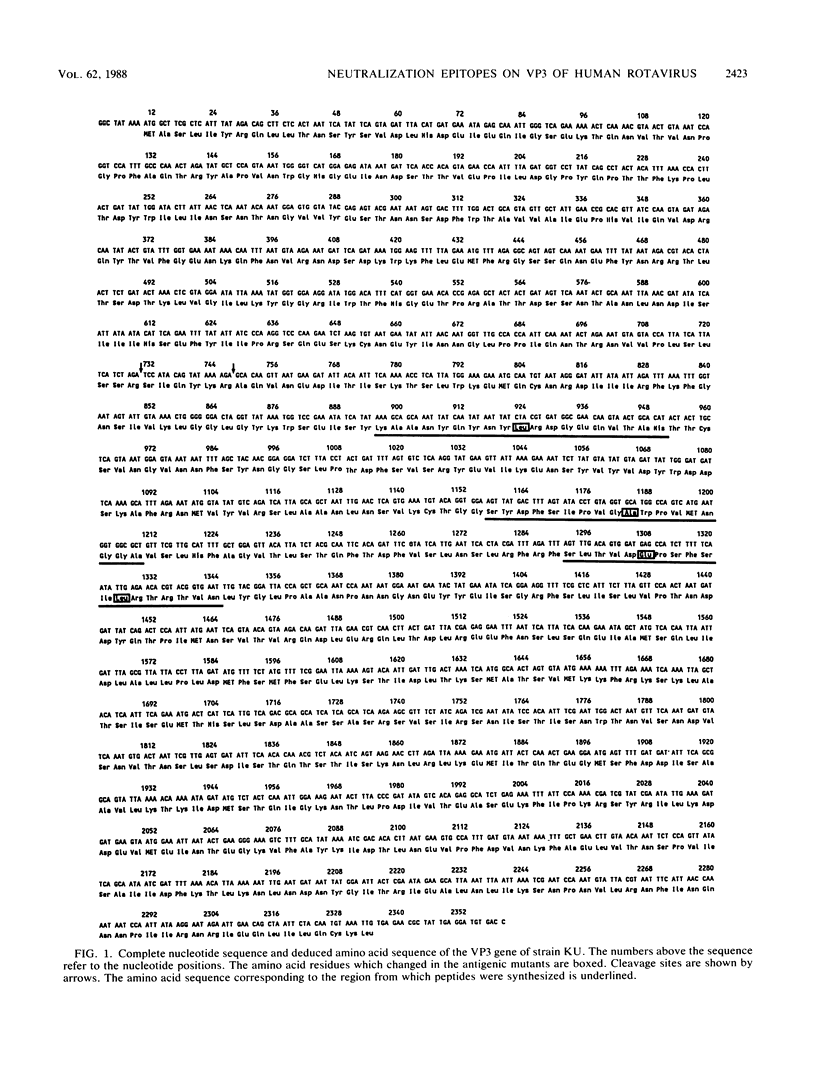
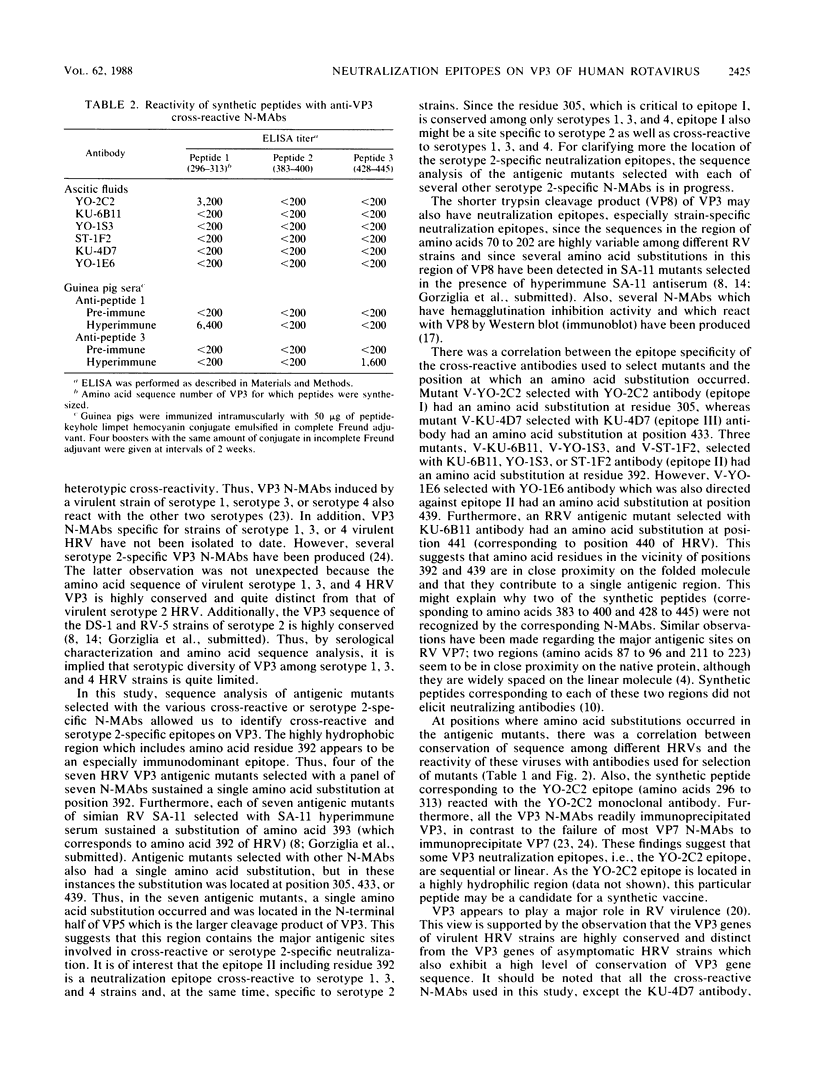
The group A rotaviruses are composed of at least seven serotypes. Serotype specificity is defined mainly by an outer capsid protein, VP7. In contrast, the other surface protein, VP3 (775 amino acids), appears to be associated with both serotype-specific and heterotypic immunity. To identify the cross-reactive and serotype-specific neutralization epitopes on VP3 of human rotavirus, we sequenced the VP3 gene of antigenic mutants resistant to each of seven anti-VP3 neutralizing monoclonal antibodies (N-MAbs) which exhibited heterotypic or serotype 2-specific reactivity, and we defined three distinct neutralization epitopes on VP3. The mutants sustained single amino acid substitutions at position 305, 392, 433, or 439. Amino acid position 305 was critical to epitope I, whereas amino acid position 433 was critical to epitope III. In contrast, epitope II appeared to be more dependent upon conformation and protein folding because both amino acid positions 392 and 439 appeared to be critical. These four positions clustered in a relatively limited area of VP5, the larger of the two cleavage products of VP3. At the positions where amino acid substitutions occurred, there was a correlation between amino acid sequence homology among different serotypes and the reactivity patterns of various viruses with the N-MAbs used for selection of mutants. A synthetic peptide (amino acids 296 to 313) which included the sequence of epitope I reacted with its corresponding N-MAb, suggesting that the region contains a sequential antigenic determinant. These data may prove useful in current efforts to develop vaccines against human rotavirus infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Rahman A. S., Yunus M., Alim A. R., Huq I., Yolken R. H., Curlin G. T. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J Infect Dis. 1980 Nov;142(5):660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn P. R., Sato F., Powell K. F., Bellamy A. R., Napier J. R., Harding D. R., Hancock W. S., Siegman L. J., Both G. W. Rotavirus neutralizing protein VP7: antigenic determinants investigated by sequence analysis and peptide synthesis. J Virol. 1985 Jun;54(3):791–797. doi: 10.1128/jvi.54.3.791-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Marked sequence variation between segment 4 genes of human RV-5 and simian SA 11 rotaviruses. Arch Virol. 1987;93(1-2):111–121. doi: 10.1007/BF01313897. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Fukuhara N., Tazawa F., Suzuki H., Sato T., Konno T., Ebina T., Ishida N. Characterization of monoclonal antibodies against human rotavirus hemagglutinin. J Med Virol. 1986 Aug;19(4):313–323. doi: 10.1002/jmv.1890190404. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E., Barra Y., Jay G. Detection of a secreted form of the murine H-2 class I antigen with an antibody against its predicted carboxyl terminus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1216–1220. doi: 10.1073/pnas.81.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Lazdins I., Holmes I. H. Coding assignments of double-stranded RNA segments of SA 11 rotavirus established by in vitro translation. J Virol. 1980 Mar;33(3):976–982. doi: 10.1128/jvi.33.3.976-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Three human rotavirus serotypes demonstrated by plaque neutralization of isolated strains. Infect Immun. 1982 Nov;38(2):781–784. doi: 10.1128/iai.38.2.781-784.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa T., Urasawa S., Taniguchi K. Sequential passages of human rotavirus in MA-104 cells. Microbiol Immunol. 1981;25(10):1025–1035. doi: 10.1111/j.1348-0421.1981.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P., Marbehant P., Marissens D., Lobmann M., Charlier P., Delem A., Zygraich N. Protection studies in colostrum-deprived piglets of a bovine rotavirus vaccine candidate using human rotavirus strains for challenge. J Infect Dis. 1983 Dec;148(6):1061–1068. doi: 10.1093/infdis/148.6.1061. [DOI] [PubMed] [Google Scholar]


