Abstract
Deflagellation of Chlamydomonas reinhardtii, and other flagellated and ciliated cells, is a highly specific process that involves signal-induced severing of the outer doublet microtubules at a precise site in the transition region between the axoneme and basal body. Although the machinery of deflagellation is activated by Ca2+, the mechanism of microtubule severing is unknown. Severing of singlet microtubules has been observed in vitro to be catalyzed by katanin, a heterodimeric adenosine triphosphatase that can remove tubulin subunits from the walls of stable microtubules. We found that purified katanin induced an ATP-dependent severing of the Chlamydomonas axoneme. Using Western blot analysis and indirect immunofluorescence, we demonstrate that Chlamydomonas expresses a protein that is recognized by an anti-human katanin antibody and that this protein is localized, at least in part, to the basal body complex. Using an in vitro severing assay, we show that the protein(s) responsible for Ca2+-activated outer doublet severing purify with the flagellar-basal body complex. Furthermore, deflagellation of purified flagellar-basal body complexes is significantly blocked by the anti-katanin antibody. Taken together, these data suggest that a katanin-like mechanism may mediate the severing of the outer doublet microtubules during Chlamydomonas deflagellation.
INTRODUCTION
Severing has been proposed as a mechanism of microtubule disassembly distinct from the depolymerization of microtubules undergoing dynamic instability (Vale, 1991; Karsenti, 1993; Shiina et al., 1995; McNally, 1996). Vale (1991) reported that Xenopus oocyte extracts could sever in vitro polymerized microtubules. Taxol-stabilized individual microtubules were fragmented into numerous short segments, presumably by disruption of tubulin–tubulin bonds at indiscriminate points along the length of the microtubule, eventually leading to complete disassembly. In contrast, depolymerization via dynamic instability is restricted to loss of tubulin subunits from the ends of microtubules (Mitchison and Kirschner, 1984). The microtubule-severing activity was shown to be regulated by the cell cycle; activity was low in interphase extracts and stimulated in extracts prepared from M phase oocytes (Vale, 1991) or interphase extracts activated by p34cdc2 kinase (Verde et al., 1992). Subsequently, biochemical purification identified several proteins with microtubule-severing activity from oocyte extracts; p56 (Shiina et al., 1992) and EF-1α (Shiina et al., 1994) were purified from Xenopus, while the heterodimeric severing protein katanin was purified from sea urchin (Strongylocentrotus purpuratus; McNally and Vale, 1993). Similar to the activity observed with mitotic Xenopus extracts, katanin requires the hydrolysis of ATP to disassemble microtubules. Neither p56, which has low severing activity compared with katanin, nor EF-1α requires ATP for severing activity (reviewed by Shiina et al., 1995). Although biochemical analysis has identified proteins with severing activity, we know little about the regulation and in vivo function of microtubule severing.
The severing of axonemal microtubules during flagellar excision of the biflagellate alga Chlamydomonas reinhardtii may provide a useful system for the study of microtubule severing. Chlamydomonas, like other ciliated or flagellated cells, sheds its flagella in response to a variety of stimuli (Mintz and Lewin, 1954; Blum, 1971; Thompson et al., 1974; Lewin et al., 1980; Witman, 1986; Quarmby et al., 1992). This cellular behavior, known as deflagellation or flagellar autotomy, is a highly specific process that involves the severing of the nine outer doublet axonemal microtubules. Electron microscopy reveals that the microtubules are severed at a precise site distal to the transition zone between the axoneme and the basal bodies (Lewin and Lee, 1985; Sanders and Salisbury, 1989, 1994; Jarvik and Suhan, 1991; Taillon et al., 1992). Previously, we have shown that cytosolic acidification triggers deflagellation via the activation of a Ca2+ influx pathway (Hartzell et al., 1993; Quarmby and Hartzell, 1994; Quarmby, 1996). In addition, Ca2+ is both necessary and sufficient to induce deflagellation in cells permeabilized with low concentrations of the nonionic detergent NP-40 (Sanders and Salisbury, 1989, 1994).
The molecular mechanism responsible for outer doublet severing is not known. However, it has been proposed that calcium-induced contraction of centrin-containing fibers within the transition zone between the axoneme and the basal bodies provides a shear force critical for the breakage of the outer doublet microtubules (Sanders and Salisbury, 1994). Investigation into the role that centrin plays in Chlamydomonas deflagellation has focused mainly on analysis of the variable flagella number mutant vfl-2. The vfl-2 mutation is a point mutation in the centrin gene resulting in gross abnormalities in all centrin-containing structures including complete loss of the contractile stellate fibers within the transition zone (Jarvik and Suhan, 1991; Taillon et al., 1992; Sanders and Salisbury, 1994). Although Sanders and Salisbury (1994) reported that vfl-2 cells fail to deflagellate under certain experimental conditions, we (Lohret and Quarmby, unpublished observations) and others (Jarvik and Suhan, 1991) have found that vfl-2 cells deflagellate normally. We conclude that centrin is not necessary for the flagellar excision process. In the absence of a centrin-induced microtubule severing, Jarvik and Suhan (1991) speculated that a transition zone-localized microtubule-severing activity, similar to that reported by Vale (1991), may be responsible for outer doublet severing.
In this study, we investigate the mechanism responsible for outer doublet severing during deflagellation. We find that micromolar free calcium induces axonemal severing in preparations of purified flagellar-basal body complexes (FBBCs) demonstrating that both the calcium sensor and microtubule-severing activity isolate with this cytoskeletal complex of axonemes plus basal bodies. The severing of axonemal doublet microtubules may proceed by a mechanism analogous to that of single microtubules (Vale, 1991; McNally and Vale, 1993; McNally et al., 1996). To test this possibility, we asked whether the stable doublet microtubules of the axoneme are susceptible to cleavage by the microtubule-severing protein katanin. We found that purified sea urchin katanin severed both cell-attached and isolated Chlamydomonas axonemes. Like the severing of in vitro polymerized microtubules, the activity required ATP hydrolysis. This is a critical finding because it is the first demonstration of a microtubule-severing protein breaking the complex doublet microtubules of an axoneme and raises the exciting possibility that the severing of Chlamydomonas outer doublet microtubules during deflagellation may involve the specific action of a katanin-like severing activity. In support of this model, we show that affinity-purified antibodies raised against the 60-kDa subunit of human katanin recognize a single predominant Chlamydomonas protein at ≈55 kDa on Western blots of both whole-cell and purified FBBCs. In addition, the antibody produced an intense staining of the basal body/flagellar transition region using indirect immunofluorescence in both whole cells and purified FBBCs. Importantly, the human p60 antibody significantly blocked Ca2+-stimulated axonemal severing in preparations of FBBCs. Taken together, these data provide evidence that an endogenous Chlamydomonas katanin may be involved in outer doublet severing during deflagellation.
MATERIALS AND METHODS
Chlamydomonas Strains and Culture Conditions
Chlamydomonas reinhardtii wild-type strains 137c, cc620, and cc621 were obtained from Dr. E. Harris (Chlamydomonas Genetics Center, Botany Department, Duke University, Durham, NC). Cells were grown on 1.5% agar TAP plates (Harris, 1989) at 21°C under constant illumination for 4–5 d. Cells were transferred from Tris acetate phosphate (TAP) plates into 4 ml of M-N media (M media of Sagar and Granick, 1953 [Harris, 1989] excluding NH4N03) and incubated for 3–5 h under constant light and agitation. Cells were collected by brief centrifugation, washed in a Ca2+-free deflagellation buffer ([DB] 10 mM PIPES, pH 7.0, 5 mM EGTA, 0.5 mM MgCl2), and resuspended in DB to an approximate final density of 1 × 107 cells/ml.
Detergent Permeabilization and Deflagellation Assay
Cells (1 × 107 cells/ml) were permeabilized by addition of 10 volumes of DB containing 0.05% Nonidet P-40 (Sigma Chemical, St. Louis, MO). For a typical experiment, 5 ml of 0.05% NP-40 in DB was added to 500 μl cells. Deflagellation was induced by addition of CaCl2 to the desired calculated free Ca2+ concentration (computer program of Fabiato, 1988). Cells were assayed for the loss of flagella using phase contrast microscopy. Percent deflagellation was measured by the following formula: [(Cf − Ef)/Cf ] × 100% where Cf and Ef equal the total number of flagella per 100 cells (detergent permeabilized) for control cells (no Ca2+) and experimental cells (Ca2+-treated), respectively. Cf was typically in the range of 180–200. Except where noted otherwise, all samples were viewed with a Zeiss Axioskop microscope equipped with a Achroplan 100×, 1.25 NA oil immersion objective and Zeiss MC-80 photographic unit (Zeiss, Göttingen, Germany).
Isolation and Assay of FBBCs
Our protocol for the preparation of FBBCs was adapted from Dutcher (1995). Cells from 4- to 5-d-old TAP plates were transferred to liquid M-N media (see above) and incubated for 2–3 h under constant light and gentle shaking. Cells were harvested by centrifugation for 5 min at 800 × g, and cell walls were removed by incubation in gametic lytic enzyme (GLE) for 30 min. GLE was prepared from the supernatant of agglutinating cc620 and cc621 cells as described by Harris (1989). GLE-treated cells were pelleted at 800 × g for 5 min and resuspended in MT buffer (30 mM Tris-acetate, pH 7.3, 5 mM MgSO4, 5 mM EDTA, 25 mM KCl, 1 mM DTT) and placed on ice. All subsequent steps were performed at 4°C. An equal volume of cells and lysis solution (MT buffer plus 2% NP-40, 0.01% aprotinin, 0.005% PMSF) were mixed rapidly and stirred for 1–5 min on ice. A small aliquot representing whole-cell protein was removed after detergent lysis and stored at −80°C. An equal volume of 50% Percoll was added and the mixture was centrifuged in 40-ml aliquots for 30 min at 14,500 × g. The FBBCs were removed from the top of the gradient at the interface of the Percoll and aqueous phases. The fraction was diluted 25-fold in MT buffer, and the FBBCs were pelleted by centrifugation for 15 min at 14,500 × g. The FBBC pellet was resuspendend in MT buffer and repelleted as above. The purified FBBCs were resuspendend in 1 ml MT. For experiments in which calcium-induced deflagellation was assayed, the complexes were washed and resuspended in DB. Deflagellation was induced by addition of CaCl2 to the desired calculated free Ca2+ concentration (as above). Flagella were determined to be severed if they had been released from their basal body attachment. Percent deflagellation was assayed by the following formula: (CBB − EBB)/CBB × 100% where CBB and EBB equal the total number of basal body-associated flagella (nonsevered) for control FBBCs (no Ca2+) and experimental FBBCs (Ca2+-treated), respectively. Typically, 50–100 flagella were analyzed for each experiment.
Katanin Experiments
Purification of sea urchin katanin has been previously described (McNally and Vale, 1993). Aliquots of purified katanin ([p81-p60 katanin] = 200 μg/ml in 30 mM PIPES, pH = 6.8, 0.1 mM EGTA, 4 mM MgCl2, 10% glycerol, 0.5 mg/ml soy bean trypsin inhibitor) were added to approximately 1 × 105 cells/ml permeabilized with 0.05% NP-40 (DB containing no free Ca2+) to obtain the desired final katanin concentration. ATP, ADP, and/or ATPγS were added from 100 mM stocks with the detergent treatment to obtain the desired final concentration of nucleotides. Cells were incubated for the indicated time, and katanin activity was stopped by addition of 10 mM ADP or fixation with 0.8% glutaraldehyde. Cells were viewed for changes in flagellar morphology under phase contrast as described above. Individual flagella were quantitatively scored based on appearance in one of four categories: no effect (NE), kinked (K), severed (S), and deflagellated (D) as defined in RESULTS. For each experiment, a minimum of 50 flagella were analyzed, and the percentages of flagella scored in each category were determined. For experiments in which purified katanin was added to isolated axonemes, flagella were obtained by treating wild-type cells with DB plus 1 μM free Ca2+. Cells were removed by brief centrifugation (16,000 × g for 10 s), and the supernatant was used as a crude axonemal preparation.
Antibodies
To produce human p60 katanin-specific antibodies, rabbits were immunized and antibodies were affinity purified with six histidine-tagged human p60 katanin purified from Escherichia coli. The human p60 sequence will be described in another paper. The control antibody was isolated from serum from the same rabbits by protein A chromatography after affinity depletion of p60-specific antibodies.
SDS-PAGE and Western Blotting
Whole-cell and purified FBBC protein samples were prepared as described above. Protein samples were diluted 1:1 with 8% SDS sample buffer, separated on a 4–15% polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to Super HYBOND-C nitrocellulose membranes (Amersham, Arlington Heights, IL) using the method of Towbin et al. (1979). Transfer was for 75 min at a constant voltage of 200 V in a Bio-Rad Mini Trans-Blot cell. Membranes were probed with anti-human p60 katanin antibodies at a 1:750 dilution, and detection was with either ECL of a HRP-conjugated anti-rabbit antibody (Amersham) or alkaline phosphatase-conjugated anti-rabbit antibody (Bio-Rad). All protein concentrations were determined by the standard Bio-Rad Protein Assay (catalog no. 500-0002) using BSA as a standard.
Indirect Immunofluorescence
Indirect immunofluorescence of GLE-treated WT137c cells and purified FBBCs was performed essentially as described by Sanders and Salisbury (1995). Samples were allowed to adhere to permanox-coated eight-well slide chambers (Nalge Nunc, Naperville, IL) for 10 min. The samples were fixed with ice-cold methanol for 10 min at −20°C. The methanol was removed and the slides allowed to air dry. The samples were rehydrated with three changes of PBS followed by blocking with 3% BSA in PBS for 30 min at room temperature. Blocking agent was removed and the samples incubated with a 1:100 or 1:200 dilution of the anti-human p60 antibody in 1% BSA in PBS overnight at 4°C. The samples were washed 2 times in PBS and incubated with a FITC-conjugated goat anti-rabbit antibody (ICN, Costa Mesa, CA) at a 1:400 dilution for 2 h at room temperature. Samples were washed 3 times in PBS and mounted in Citifluor mountant media (Ted Pella, Redding, CA). Coverslips were sealed with nail polish to prevent dessication. Whole-cell preparations were viewed with a Zeiss Axiovert 135 microscope equipped with a 100 W mercury lamp and standard FITC and UV filter sets. Whole-cell and FBBC preparations were viewed with 40× and 100× oil immersion Neofluar objectives, respectively. Photographs were recorded on TMAX 100 film (Eastman Kodak, Rochester, NY) at exposure times of 15–30 sec. All washes were for 5 min.
RESULTS
The Machinery of Doublet-severing Isolates with the FBBC
The ability of permeabilized cells to deflagellate in response to an increase in free Ca2+ suggests that the protein(s) responsible for outer doublet severing during deflagellation are not only localized to the transition zone between the basal body and the axoneme but are also tightly associated with the axoneme. To investigate whether the severing mechanism could isolate in a structural complex with the axoneme, we purified complexes that contained the two basal body-anchored flagella with associated proteins after detergent lysis of autolysin-treated cells (called FBBCs; Hyams and Borisy, 1978; Dutcher, 1995). The FBBCs were assayed for the ability to deflagellate in response to an increase in free Ca2+ before and after purification on a Percoll gradient (Figure 1 and Table 1). Both the crude and purified complexes displayed calcium-dependent microtubule severing, as evidenced by the fact that the flagella were severed from their basal body attachment (Figure 1). Similar to deflagellation induced by calcium in permeabilized cells, axonemal severing in both FBBC preparations was strictly dependent on micromolar or greater free calcium (Table 1). These results demonstrate conclusively that both the calcium sensor and machinery of microtubule severing isolate with the FBBC and, therefore, must be tightly associated with the flagellar axoneme.
Figure 1.
The machinery of microtubule-severing isolates with the FBBC. Representative phase-contrast micrographs of purified FBBCs immediately after purification (A) and after calcium treatment (B). Each flagella is connected to a basal body and the two basal bodies are linked by connecting fibers. FBBCs were purified on a Percoll gradient after detergent lysis of GLE-treated wild-type cells and resuspended in calcium-free DB (see MATERIALS AND METHODS). Deflagellation of the FBBCs was induced by addition of CaCl2 to a final free Ca2+ concentration of 1 μM. As shown in panel B, the addition of micromolar free Ca2+ resulted in the complete severing of the axonemes from the basal bodies. The flagella and basal bodies are labeled F and B, respectively.
Table 1.
The machinery of axonemal severing isolates with the flagellar-basal body complex
| Sample | %Deflagellationa
|
|
|---|---|---|
| pCa6 | pCa7 | |
| Permeabilized cell models | 96 ± 3b | 14 ± 8 |
| Flagellar-basal body complexes | ||
| Step 1 (crude)c | 100 | 11 |
| Step 2 (purified)d | 94 ± 3 | 10 ± 1 |
Calculated using the formulae described in MATERIALS AND METHODS.
Average±SE (n ≥ 4).
After detergent lysis of GLE-treated cells.
After Percoll gradient purification.
Katanin, a Microtubule-severing Adenosine Triphosphatase (ATPase), Induces ATP-dependent Kinking and Severing of Chlamydomonas Flagellar Axonemes
As described above, the key event in the deflagellation response of Chlamydomonas involves the precise severing of the outer doublet microtubules just distal to the transition zone between the basal bodies and the axoneme. The identity of the protein(s) that mediate this severing are unknown. Severing of taxol-stabilized singlet microtubules, however, has been previously observed in vitro to be catalyzed by katanin, a heterodimeric ATPase purified from sea urchin oocytes (McNally and Vale, 1993). Katanin is likely the protein responsible for the severing activity observed in M phase Xenopus oocytes (Vale, 1991) and thus may be the predominant severing protein in vivo (McNally et al., 1996). An analogous mechanism may play a central role in the severing of outer doublet microtubules during deflagellation.
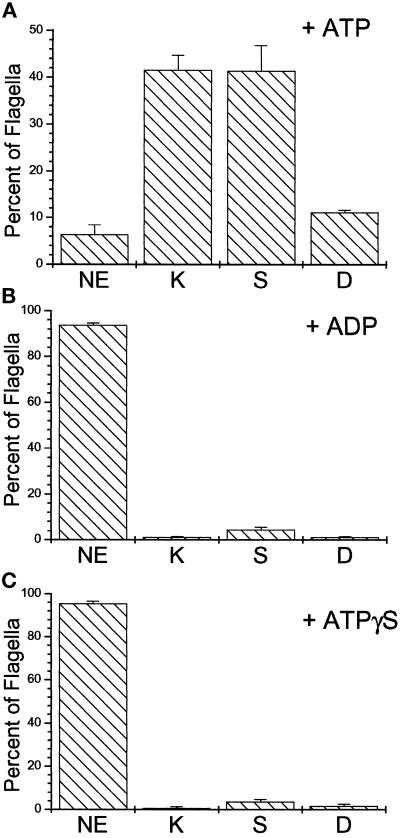
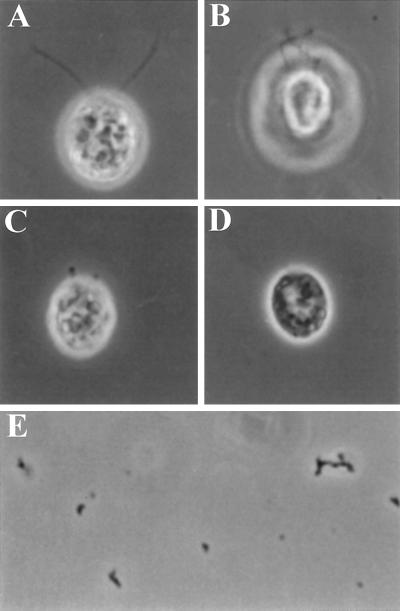
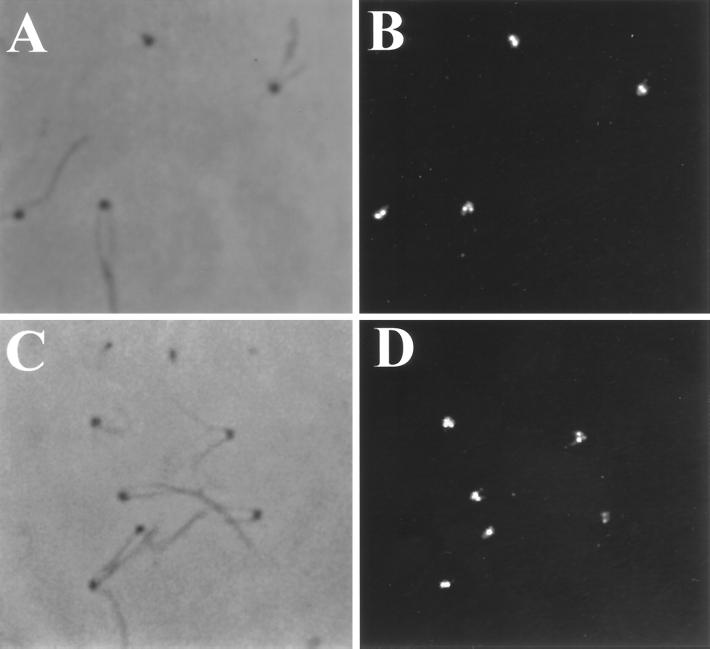
To test whether katanin can sever the in situ stabilized axonemal microtubules of Chlamydomonas, we incubated either NP-40 permeabilized Chlamydomonas cells or isolated axonemes with purified sea urchin katanin. As shown in Figure 2, A–D, katanin caused a dramatic change in Chlamydomonas flagellar morphology. Cell-attached flagella appeared kinked and, in many cases, were severed at various sites along the axoneme. In isolated flagellar preparations (Figure 2, E and F), the flagella were fragmented into numerous short and kinked segments in the presence of katanin and ATP. The effects produced by katanin on Chlamydomonas flagella were completely ATP-dependent; kinking and severing of the flagellar axonemes occurred only in the presence of hydrolyzable ATP (Figure 3). Neither ADP nor ATPγS could support katanin-mediated disruption of flagellar microtubules. In addition, both ADP and ATPγS inhibited the ATP-dependent katanin activity. The presence of a fourfold excess of either ADP or ATPγS (10 mM) inhibited katanin activity in the presence of ATP (2.5 mM). The percentage of kinked or severed flagella was reduced from a control level of 94% (ATP alone) to 26% and 22% in the presence of either ADP or ATPγS, respectively. The nucleotide dependence of the katanin-mediated breakage of Chlamydomonas flagellar microtubules is, therefore, the same as that described by McNally and Vale (1993) for the katanin-mediated severing of in vitro polymerized, taxol-stabilized microtubules.
Figure 2.
Katanin severs Chlamydomonas axonemal microtubules. NP-40–permeabilized cells (≈1 × 105 cells/ml) were incubated with purified katanin (100 μg/ml) plus 2.5 mM ADP (A) or 2.5 mM ATP (B–D) for 10 min. In the presence of ATP, katanin causes kinking (B), severing (C), and complete deflagellation (D) of individual flagella. (E and F) Katanin induces kinking and breakage of isolated flagellar axonemes. Flagella were excised from permeabilized wild-type cells by 1 × 10−6 M free Ca2+ and treated with either 5 mM ATP (E) or 5 mM ATP plus katanin (F) (100 μg/ml). As shown, the axonemes are fragmented only in the presence of both katanin and ATP.
Figure 3.
Katanin-mediated flagellar microtubule severing is supported by ATP but not by either ADP or ATPγS. The percentage of flagella scored in each of the categories illustrated in Figure 3 was determined in the presence of katanin (100 μg/ml) plus the indicated nucleotide (2.5 mM). As shown, katanin-induced flagellar axonemal microtubule kinking and breakage was supported by ATP (A) but not by ADP or ATPγS (B and C). Bars represent the average ± SE, n = 4; 50 cells counted for each experiment. All other conditions as in Figure 2.
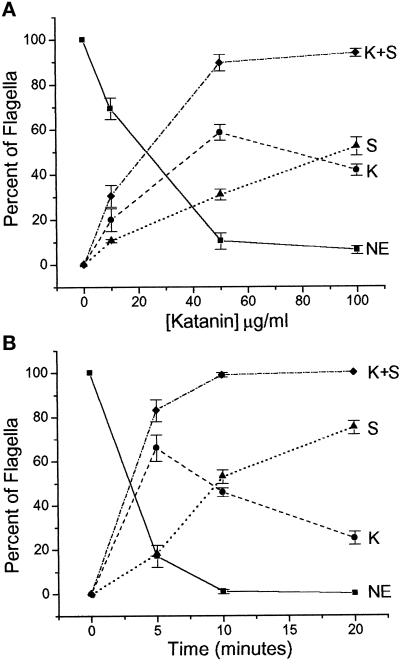
Katanin-mediated severing of flagellar axonemes was both dose and time dependent (Figure 4). At katanin concentrations between 50 and 100 μg/ml, nearly all of the flagella analyzed were either kinked or severed when assayed after 10 min (Figure 4A). The cell density during these experiments (and all other experiments in which katanin was used) was ≈5 × 105 cells/ml, thereby making the katanin concentration approximately 0.7–1.4 fmol/cell. The percentage of kinked flagella rose with increasing katanin concentrations and then declined (compare levels at 50 and 100 μg/ml katanin). This decrease in kinked flagella was accompanied by a continual increase in severed flagella, suggesting that axonemal kinking may be an early step in the progression to complete severing of the doublet microtubules and breakage of the axoneme. This idea is also supported by the time dependence of katanin activity (Figure 4B). At brief incubation intervals (5–10 min), many of the flagella were kinked but did not appear to have severed completely. At later time points (15–20 min), however, the majority of the flagella were severed at indiscriminate sites along their length, similar to that shown in Figure 2.
Figure 4.
Katanin-mediated breakage of Chlamydomonas flagella is both dose and time dependent. (A) NP-40–permeabilized wild-type cells (≈1 × 105 cells/ml) were incubated with purified katanin plus ATP for 10 min at various katanin concentrations (μg/ml). The percent of kinked and/or severed flagella (scored and quantified as in Figure 3) after 10 min of incubation was determined at various katanin concentrations (μg/ml). Note that flagella scored as deflagellated were added to those scored as severed. (B) Wild-type cells were permeabilized with 0.05% NP-40, 5 mM ATP, 100 μg/ml katanin for various amounts of time. At the indicated time points, katanin activity was stopped by addition of 0.8% glutaraldehyde and the flagella were assayed as in panel A. For assays at 20 min, 5 mM ATP was added after 10 min of incubation. For parts A and B, data points represent the average ± SE, n = 3–5; minimum of 50 cells counted for each experiment.
Lewin and Burrascano (1983) isolated a Chlamydomonas reinhardtii mutant that was defective in deflagellation (fa1–1, formerly fa–1 for flagellar autotomy mutant; see Finst et al., 1998). One possibility for the inability of the fa1–1 mutant to deflagellate is that the mutant axonemes are resistant to severing. To test this possibility, we treated permeabilized fa1–1 cells with katanin. The effects observed on detergent-permeabilized fa1–1 cells with purified katanin are indistinguishable from wild-type cells (Figure 5). Flagella on fa1–1 cells are kinked and severed by katanin. In addition, flagellar preparations made from katanin-treated fa1–1 cells (Figure 5E) consisted of small, kinked flagellar fragments resembling isolated wild-type flagella treated with katanin (Figure 2E). The katanin-mediated effects on the fa1–1 flagella showed a similar nucleotide, dose, and time dependence as that of the wild-type (Lohret and Quarmby, unpublished results). This is the first time that we have observed any type of flagellar microtubule severing in the fa1–1 mutant and demonstrates that the flagellar microtubules are not structurally resistant to severing.
Figure 5.
Katanin induces kinking and severing of fa1–1 flagella. NP-40 (0.05%)-permeabilized fa1 mutant cells (≈1 × 105 cells/ml) were incubated with purified katanin (100 μg/ml) plus 5 mM ADP (A) or 5 mM ATP (B–D) for 10 min. In the presence of ATP, katanin causes kinking (B), severing (C), and deflagellation (D) of fa1–1 flagella. (E) Flagellar preparations made from the katanin-treated fa1 cells consisted of small, kinked flagellar fragments resembling isolated wild-type flagella treated with katanin (Figure 2F).
A Katanin-related Protein Is Present in the Chlamydomonas FBBC
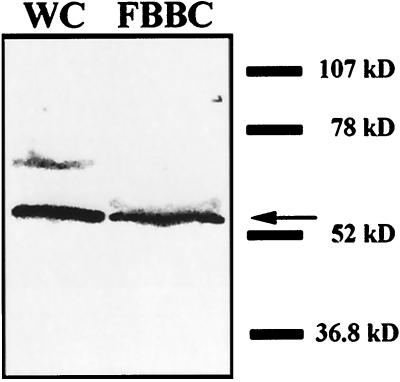
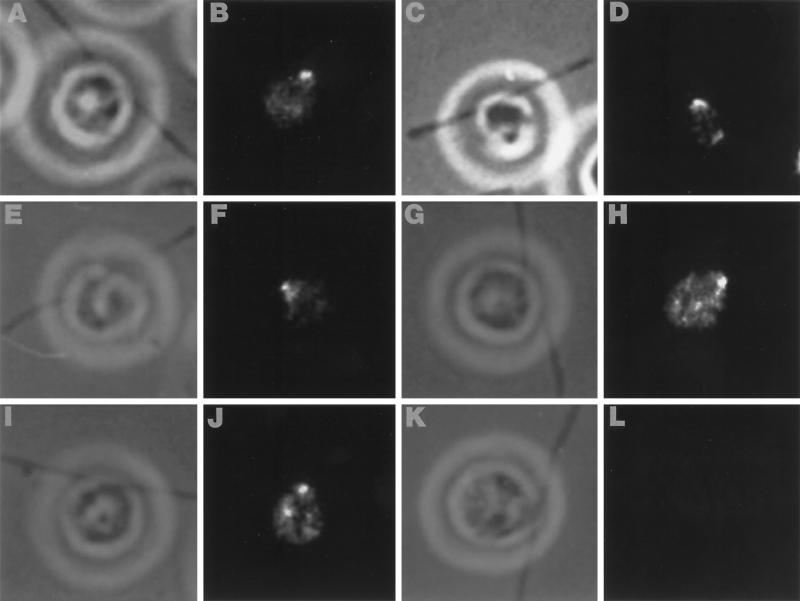
The above results demonstrate that sea urchin katanin can break the complex doublet microtubules of the axoneme. However, it remains unclear whether an endogenous Chlamydomonas katanin mediates in vivo axonemal severing. To determine whether Chlamydomonas expresses a katanin-related protein, affinity-purified antibodies, raised against the 60-kDa subunit of human katanin, were used in Western blot analysis. As shown in Figure 6, the human p60 antibody recognized a Chlamydomonas protein of ≈55 kDa in whole-cell extracts and in purified FBBCs. These results are critical because they not only demonstrate that Chlamydomonas expresses a katanin-related protein but, importantly, that this protein is present in the purified FBBCs. Additionally, we used indirect immunofluorescence to localize the protein within the cell and in the purified FBBCs. In whole cells, the human p60 antibody produced an intense fluorescent staining in an area at the base of the flagella, in the proximity of the area in which severing occurs (Figure 7). A similar fluorescence pattern was obtained in experiments with purified FBBCs. As shown in Figure 8, the anti-human katanin antibody produced a bright fluorescence that was restricted to the basal bodies and the extreme proximal part of the axoneme. The results presented above demonstrate that Chlamydomonas expresses a katanin-related protein that is localized, in part, to the site of deflagellation.
Figure 6.
Anti-human p60 antibodies strongly recognize a ≈55-kDa Chlamydomonas protein in both whole-cell and flagellar–basal body protein preparations. Whole-cell and FBBC proteins (10 μg) were separated on a 4–15% SDS-PAGE gel and analyzed by Western blot with an affinity-purified antibody to the 60-kDa subunit of human katanin. Detection was with an alkaline phosphatase-conjugated secondary antibody.
Figure 7.
Indirect immunofluorescence of whole cells demonstrates immunoreactivity near the basal bodies, but not along the length of the axoneme. Alternating phase contrast (A, C, E, G, and I) and anti-human p60 katanin fluorescence images (B, D, F, H, and J) of several different cells illustrating strong immunoreactivity at the base of the FBBC. Immune detection is with a FITC-conjugated secondary antibody. (K and L) Representative phase contrast and fluorecence images of a control cell that was treated with secondary antibody.
Figure 8.
Anti-human p60 antibodies specifically recognize basal body-localized epitopes in preparations of purified FBBCs. Phase contrast (A and C) and anti-human p60 katanin fluorescence images (B and D) of FBBCs illustrating intense staining of the basal bodies. No staining was observed along the length of the axonemes. Controls were performed with secondary antibody alone and showed no fluorescent staining of the basal bodies.
Anti-human p60 Antibodies Inhibit the Deflagellation of Isolated FBBCs
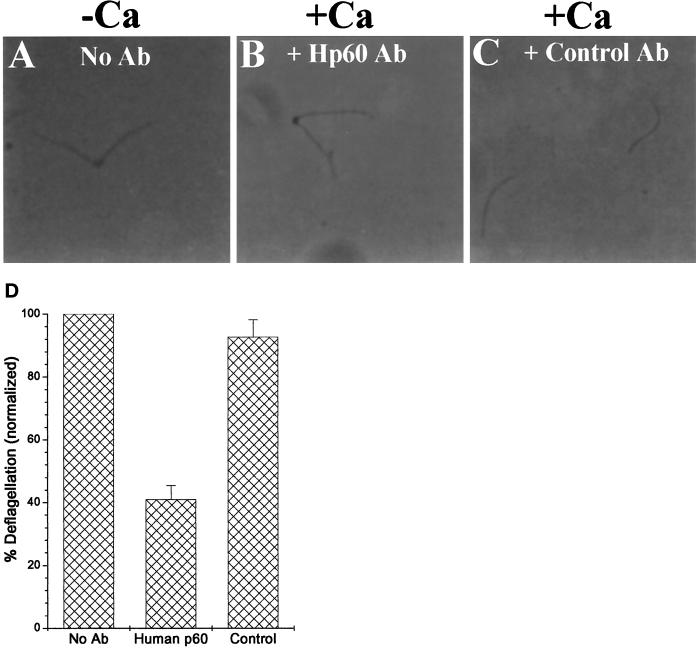
To test the involvement of an endogenous katanin activity in deflagellation, we asked whether anti-p60 antibodies inhibit Chlamydomonas axonemal severing. We incubated purified FBBCs with the p60 antibodies and then assayed calcium-stimulated deflagellation. Previously, we showed that untreated FBBCs deflagellate (quantified as release of the axoneme from its basal body attachment) after 1 μM Ca2+ treatment (Figure 1). Incubation of the complexes with the human p60 antibody at a concentration of 60 μg/ml inhibited severing nearly 60% (Figure 9). Similar concentrations of this antibody inhibit severing activity in Xenopus extracts (McNally, unpublished results). As a control, we used protein A-purified antibodies from the IgG fraction that did not bind to the human p60 affinity column. In contrast to the p60 antibody, the presence of an equivalent amount of control antibody had virtually no inhibitory effect. These results directly implicate a role for a katanin-related protein in Chlamydomonas deflagellation.
Figure 9.
Anti-human p60 antibodies inhibit deflagellation of isolated FBBCs. Purified FBBCs were treated with either no antibody (A), 60 μg/ml anti-human p60 antibody (B), or an equivalent amount of control antibodies (C) for 15 min and then assayed for Ca-induced deflagellation by addition of 1 μM free Ca2+. (D) The percent deflagellation of each sample was calculated as described (see MATERIALS AND METHODS) and normalized to the untreated sample.
DISCUSSION
Microtubule severing is the key event in the deflagellation response of C. reinhardtii, but little is known about the in vivo mechanism that mediates this process. In this report, we present evidence that the machinery of calcium-induced axonemal microtubule severing is tightly associated with the FBBC. An ≈55-kDa protein in this complex was recognized by a katanin antibody, and the same antibody blocked deflagellation. These observations suggest a possible role for katanin in axonemal microtubule severing. We also report that purified sea urchin katanin severed flagellar axonemes in an ATP-dependent manner, demonstrating that the complex doublet microtubules of the axoneme can be severed by the same ATPase previously shown to sever single microtubules.
Katanin has been purified from sea urchin oocytes on the basis of microtubule-severing activity in an in vitro assay (McNally and Vale, 1993), but the in vivo function of katanin and microtubule severing remains enigmatic. Microtubule severing may play a role in varied cellular events, including regulating poleward flux of tubulin in the metaphase spindle (McNally et al., 1996), degradation of sperm axonemal microtubules after fertilization of sea urchin oocytes (Fechter et al., 1996), microtubule reorganization during the transition from interphase to mitosis in dividing cells (Vale, 1991; Karsenti, 1993; McNally and Vale, 1993), and the release of centrosome-nucleated microtubules (Rodionov and Borisy, 1997; Keating et al., 1997). The deflagellation system of Chlamydomonas provides a unique experimental system to study Ca2+-regulated microtubule severing.
We find that both the Ca2+-sensor and the microtubule-severing activity of deflagellation purify with the FBBC. We isolated complexes consisting of the two flagellar axonemes, basal bodies, and associated proteins after detergent lysis of cells treated with GLE to remove the cell wall. An array of fibers, called the distal and proximal striated fibers, connects the basal bodies to each other (Ringo, 1967; Goodenough and Weiss, 1978) and allows the FBBC to be isolated as a single cytoskeletal complex. When treated with Ca2+, the axonemes were severed from the basal bodies and the complex dissociated into its component parts (see Figure 1). To our knowledge, we are the first to report that purified FBBCs deflagellate in response to Ca2+. The strict requirement for micromolar levels of free Ca2+ to induce axonemal severing in the complexes (Table 1) indicates that both the calcium sensor and the severing proteins purify with the FBBCs. Thus, the protein(s) responsible for outer doublet severing are tightly associated with the axoneme.
To determine whether katanin could sever the doublet microtubules of the axoneme, detergent-permeabilized cells were incubated in solution with purified katanin in the presence of ATP. The flagella were unambiguously fractured at various sites along the length of the axoneme (Figures 2 and 5). This is evidenced by the fact that small flagellar fragments were broken off or severed from the flagellum proper. A similar effect is seen when axonemes, previously isolated from detergent-permeabilized cells with Ca2+ treatment, are incubated with purified katanin plus ATP. The size of these fragments was not uniform, suggesting that the entire length of the axoneme can be affected by katanin activity. Microtubule severing by katanin has been characterized previously for in vitro polymerized microtubules in solution (McNally and Vale, 1993); therefore, we conclude that the severing of the axoneme was a direct result of the complete disassembly of the outer doublet microtubules at an arbitrary site in the axoneme.
In some instances, the flagella appeared normal in length but contained one or more prominent kinks. We hypothesize, based on the concentration and time dependence (Figure 4), that the kinked nature of both cell-attached and isolated flagella may indicate disruption of some, but not all, of the axonemal outer doublet microtubules. Interestingly, kinking of single microtubules is observed before severing by both extracts and katanin (Vale, 1991; McNally and Vale, 1993). In addition, loss or disassociation of tubulin subunits from the microtubule wall has been shown to result in bending or kinking of stable individual microtubules (Dye et al., 1992). The structural arrangement of the nine outer doublet microtubules in the axoneme means that axonemal kinking could result from either complete severing of one or more doublets, partial severing of a doublet, or some other mechanism.
The effects of katanin on Chlamydomonas flagella described above are absolutely dependent on ATP (Figure 3); kinking and severing of flagella are not seen with katanin alone or with katanin plus ADP or ATPγS. Under these conditions, flagella appear long and unaffected. Similarly, katanin did not sever isolated flagella in the absence of ATP. In addition, both ADP and ATPγS inhibited the ATP-dependent activity of katanin. The nucleotide dependence that we describe for the activity of katanin on axonemes is identical to that described previously for the severing of taxol-stabilized microtubules in vitro (McNally and Vale, 1993). We conclude, therefore, that katanin severs the double-walled axonemal microtubules of Chlamydomonas using a similar ATP-dependent mechanism.
In light of this result, it is important to note that the “deflagellation” of our isolated FBBCs does not require the addition of ATP. Furthermore, the presence of 5 mM ADP during isolation and assay did not inhibit Ca2+-induced severing (Lohret and Quarmby, unpublished results). If axonemal microtubule severing is ATP-dependent, then we infer that the endogenous katanin is purified in its ATP-bound state. This would imply that a single round of ATP hydrolysis is sufficient for axonemal severing. Given that purified sea urchin katanin has a turnover rate of one ATP per molecule of katanin per second (McNally and Vale, 1993), and that deflagellation occurs in less than 1 sec (Quarmby et al., 1992), it is feasible that one cycle of hydrolysis by a localized population of katanin molecules could sever the axoneme.
The results described above establish that katanin could be responsible for deflagellation. To determine whether a katanin-like protein is expressed in Chlamydomonas, we performed Western analysis using a polyclonal, affinity-purified antibody raised against the 60-kDa subunit of human katanin. The antibody recognized a Chlamydomonas protein of ≈55 kDa that was present in both whole-cell protein extracts and purified FBBCs (Figure 6). Because this protein localizes, in part, to the FBBC, the ≈55-kDa protein is a good candidate for an endogenous katanin in Chlamydomonas. Using immunofluorescence, McNally et al. (1996) showed that sea urchin katanin is highly concentrated at centrosomes. In most eukaryotic cells, the centrosome serves as the microtubule organizing center for both interphase and mitotic spindle microtubules (Kellogg et al., 1994; Stearns and Winey, 1997). In Chlamydomonas, the basal bodies serve similar functions including organizing both cellular and axonemal doublet microtubules. Our indirect immunoflourescence experiments with the anti-human p60 antibody produced an intense staining of the basal body region in both whole cells and in purified FBBCs (Figures 7 and 8). We observed no staining along the length of the axoneme, suggesting that the protein is highly localized to the basal bodies in the FBBC. We conclude that an endogenous katanin-like protein is correctly localized to mediate Ca2+-induced axonemal severing.
To test the hypothesis that the Chlamydomonas katanin-like protein is involved in Ca2+-activated severing of the axoneme, we incubated FBBCs with the antibody before treatment with Ca2+. The finding that affinity-purified antibodies against human p60 katanin block Ca2+-induced deflagellation in purified FBBCs implicates the involvement of a katanin-related protein in deflagellation (Figure 9). The possible involvement of katanin in deflagellation means that a genetic system is now available to study katanin regulation.
Axonemes of the fa1-1 deflagellation-defective mutant were severed by katanin as readily as axonemes from wild-type cells (Figure 5). One interpretation of this observation is that the fa defect affects the severing activity, as opposed to the susceptibility of the axoneme to severing. We have recently completed a genetic analysis of this pathway and identified several alleles of each of two genes, FA1 and FA2, involved in axonemal microtubule severing (Finst et al., 1998). Mutant strains with defects in either of these genes fail to sever their axonemes in response to Ca2+ (Finst et al., 1998). One prediction was that defects in these genes would affect expression or localization of the katanin-like protein. Western blot analysis of FBBC proteins isolated from fa1 and fa2 strains revealed no defects in the expression of the 55-kDa protein (Lohret and Quarmby, unpublished results). Further analysis using both immunofluorescence and electron microscopy will determine whether the fa mutations affect the precise localization of the katanin-like protein within the flagellar–basal body structure.
It is likely that FA1 and FA2 are the only genes in this pathway that can be identified via loss-of-function alleles, because multiple alleles of each gene were isolated using two independent forms of mutagenesis. As discussed above, strains that are putative knock-out alleles of FA1 or FA2 show no obvious defects in the expression levels or localization of the katanin-related protein. There are two interpretations of this result. Either katanin is not involved in deflagellation or katanin is essential to the life of the cell and would not be isolated in a screen for loss-of-function mutations. The data presented in this report strongly implicate a role for katanin in deflagellation; therefore, we favor the latter explanation. In this case, the FA1 and FA2 gene products are likely to be specific regulators of katanin activity. In particular, Fa1p and Fa2p likely mediate the activation of katanin by Ca2+.
One form of mutagenesis that we used was the nonhomologous insertion of exogenous DNA (Tam and Lefebvre, 1993). It is noteworthy that we have isolated molecularly tagged alleles of both FA1 and FA2 (Finst et al., 1998). We have recently cloned the FA1 gene, and the molecular tag is being used to clone the FA2 gene (Kim, Finst, and Quarmby, unpublished observations). Characterization of the genes and proteins controlling the deflagellation response of Chlamydomonas will provide valuable knowledge concerning the mechanism and regulation of microtubule severing.
ACKNOWLEDGMENTS
We are grateful to Dr. Criss Hartzell and members of the Quarmby and Hartzell laboratories for stimulating discussions. We thank Drs. Win Sale and Harish Joshi for helpful comments and for the use of their microscopes. We also appreciate the constructive criticism of two anonymous reviewers of an earlier verison of this work. The work was supported by National Science Foundation grant MCB-9603716 (L.M.Q.) and, in part, by National Institutes of Health grant GM-53060 (F.J.M.). T.A.L. is supported by a postdoctoral fellowship from the National Institutes of Health (GM-19138-01).
REFERENCES
- Blum JJ. Existence of a breaking point in cilia and flagella. J Theor Biol. 1971;33:257–263. doi: 10.1016/0022-5193(71)90065-8. [DOI] [PubMed] [Google Scholar]
- Dutcher SK. Purification of basal bodies and basal body complexes from Chlamydomonas reinhardtii. In: Dentler W, Witman G, editors. Cilia and Flagella. San Diego, CA: Academic Press; 1995. . Methods in Cell Biology 47, 323–334. [DOI] [PubMed] [Google Scholar]
- Dye RB, Flicker PF, Lien DY, Williams RC. End-stabilized microtubules observed in vitro: stability, subunit interchange, and breakage. Cell Motil Cytoskeleton. 1992;21:171–186. doi: 10.1002/cm.970210302. [DOI] [PubMed] [Google Scholar]
- Fabiato. A. Computer programs for calculating total from free or free from specified total ionic concentrations in aqueous solution containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fechter J, Schoneberg A, Schatten G. Excision and disassembly of sperm tail microtubules during sea urchin fertilization: Requirements for microtubule dynamics. Cell Motil Cytoskeleton. 1996;35:281–288. doi: 10.1002/(SICI)1097-0169(1996)35:4<281::AID-CM1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Finst, R.J., Kim, P.J., and Quarmby, L.M. (1998). Genetics of the deflagellation pathway in Chlamydomonas. Genetics (in press). [DOI] [PMC free article] [PubMed]
- Goodenough UW, Weiss RL. Interrelationships between microtubules, a striated fiber, and the gametic mating structure of Chlamydomonas reinhardtii. J Cell Biol. 1978;76:430–438. doi: 10.1083/jcb.76.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook. 1st ed. Vol. 1. Berkeley, CA: Academic Press; 1989. [Google Scholar]
- Hartzell LB, Hartzell HC, Quarmby LM. Mechanisms of flagellar excision: I. The role of intracellular acidification. Exp Cell Res. 1993;208:148–153. doi: 10.1006/excr.1993.1232. [DOI] [PubMed] [Google Scholar]
- Hyams JS, Borisy GG. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J Cell Sci. 1978;33:235–253. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- Jarvik JW, Suhan JP. The role of the flagellar transition region: inferences from the analysis of a Chlamydomonas mutant with defective transition region structures. J Cell Sci. 1991;99:731–740. [Google Scholar]
- Karsenti E. Severing microtubules in mitosis. Curr Biol. 1993;3:208–210. doi: 10.1016/0960-9822(93)90334-k. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D, Moritz M, Alberts B. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Lewin RA, Lee T-H, Fang L-S. Effects of various agents on flagellar activity, flagellar autotomy and cell viability in four species of Chlamydomonas (Chlorophyta: Volvocales) Symp Soc Exp Biol. 1980;35:421–437. [PubMed] [Google Scholar]
- Lewin RA, Burrascano C. Another new kind of Chlamydomonas mutant with impaired flagellar autotomy. Experientia. 1983;39:1397–1398. [Google Scholar]
- Lewin RA, Lee KW. Autotomy of algal flagella: electron microscope studies of Chlamydomonas and Tetraselmis. Phycologia. 1985;24:311–316. [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- McNally FJ. Modulation of microtubule dynamics during the cell cycle. Curr Opin Cell Biol. 1996;8:23–29. doi: 10.1016/s0955-0674(96)80044-5. [DOI] [PubMed] [Google Scholar]
- McNally FJ, Okawa K, Iwamatsu A, Vale RD. Katanin, the microtubule severing ATPase, is concentrated at centrosomes. J Cell Sci. 1996;109:561–567. doi: 10.1242/jcs.109.3.561. [DOI] [PubMed] [Google Scholar]
- Mintz RH, Lewin RA. Studies on the flagella of algae. V. Serology paralyzed mutants of Chlamydomonas. Can J Microbiol. 1954;1:65–67. doi: 10.1139/m55-009. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Quarmby LM, Yueh YG, Cheshire JL, Keller LR, Snell WJ, Crain RC. Inositol phospholipid metabolism may trigger flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1992;116:737–744. doi: 10.1083/jcb.116.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM, Hartzell HC. Two distinct, calcium-mediated, signal transduction pathways can trigger deflagellation in Chlamydomonas reinhardtii. J Cell Biol. 1994;124:807–815. doi: 10.1083/jcb.124.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM. Ca2+ influx activated by cytosolic acidification in Chlamydomonas. J Gen Physiol. 1996;108:351–361. doi: 10.1085/jgp.108.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol. 1967;33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Borisy GG. Microtubule treadmilling in vivo. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- Sagar R, Granick S. Nutritional studies with Chlamydomonas reinhardti. Ann NY Acad Sci. 1953;56:831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1989;108:1751–1760. doi: 10.1083/jcb.108.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1994;124:795–806. doi: 10.1083/jcb.124.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Immunofluorescence microscopy of cilia and flagella. In: Dentler W, Witman G, editors. Cilia and Flagella. San Diego, CA: Academic Press; 1995. , Methods in Cell Biology 47, 163–169. [DOI] [PubMed] [Google Scholar]
- Shiina N, Gotoh Y, Kubomura N, Iwamatsu A, Nishida E. Microtubule severing by elongation factor-1α. Science. 1994;262:282–285. doi: 10.1126/science.7939665. [DOI] [PubMed] [Google Scholar]
- Shiina N, Gotoh Y, Nishida E. A novel homo-oligomeric protein responsible for an MPF-dependent microtubule severing activity. EMBO J. 1992;11:4723–4731. doi: 10.1002/j.1460-2075.1992.tb05577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N, Gotoh Y, Nishida E. Microtubule-severing activity in M-phase. Trends Cell Biol. 1995;5:283–286. doi: 10.1016/s0962-8924(00)89040-6. [DOI] [PubMed] [Google Scholar]
- Stearns T, Winey M. The cell center at 100. Cell. 1997;91:303–309. doi: 10.1016/s0092-8674(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Taillon BE, Adler SA, Suhan JP, Jarvik JW. Mutational analysis of centrin: An EF-hand protein associated with three distinct contractile fibers in the basal body apparatus of Chlamydomonas. J Cell Biol. 1992;119:1613–1624. doi: 10.1083/jcb.119.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LW, Lefebvre PA. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993;135:375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GA, Baugh LC, Walker LF. Nonlethal deciliation of Tetrahymena by a local anaesthetic and its utility as a tool for studying cilia regeneration. J Cell Biol. 1974;61:253–257. doi: 10.1083/jcb.61.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991;64:827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- Verde F, Labbe JC, Doree M, Karsenti E. Control of microtubule dynamics and length by cyclin A and cyclin B dependent kinases in Xenopus egg extracts. J Cell Biol. 1992;118:1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134:280–290. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]