Abstract
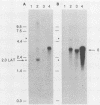
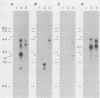
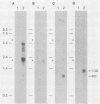
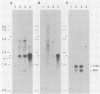
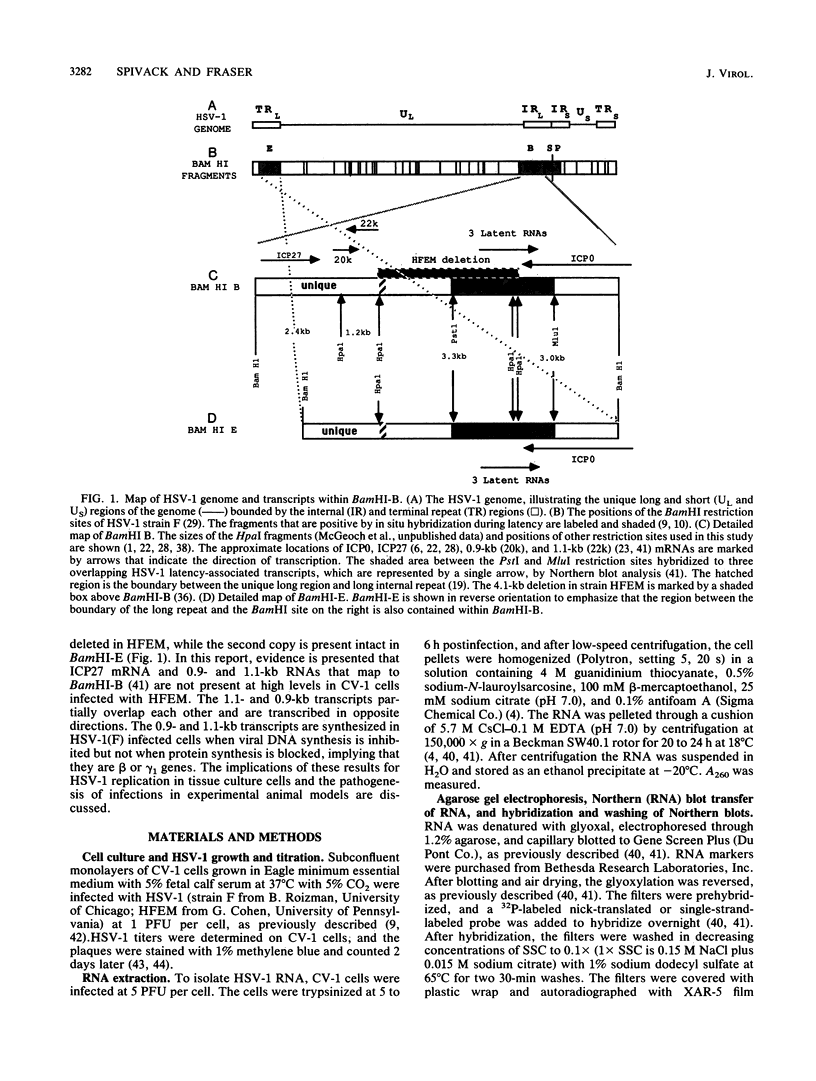
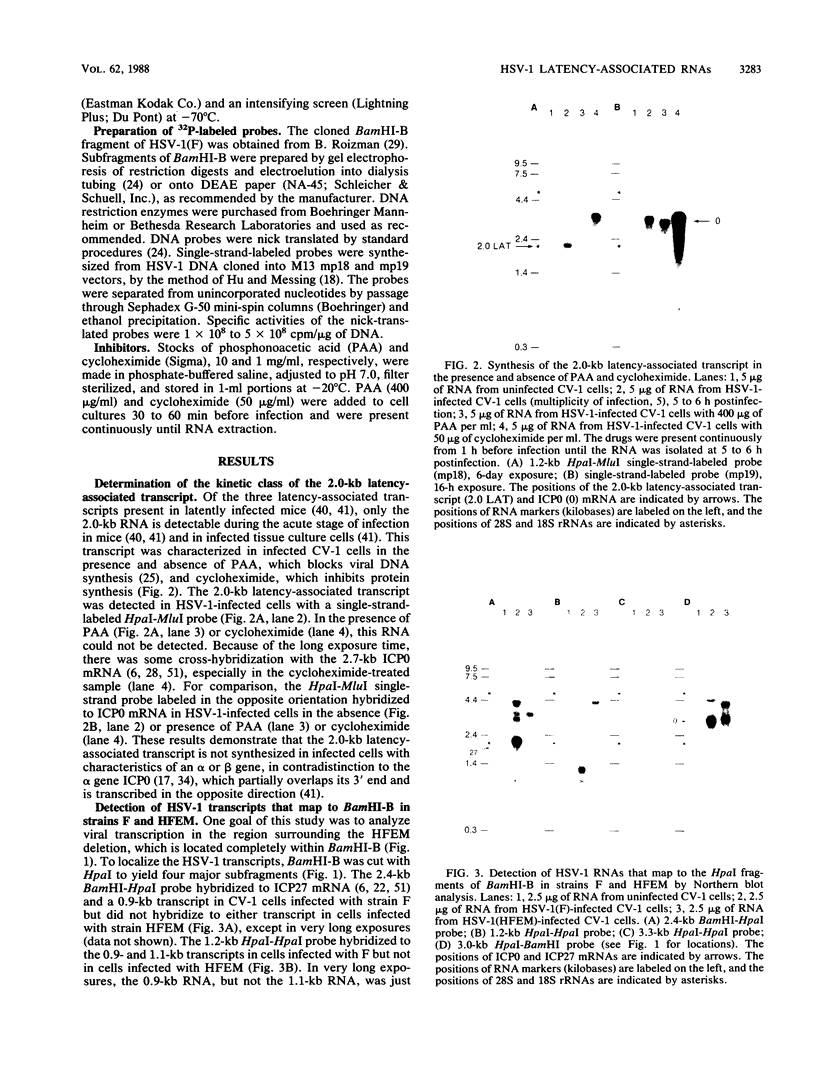
During latent herpes simplex virus type 1 (HSV-1) infection in the trigeminal ganglia of mice, three virus-specific transcripts, 2.0, 1.5, and 1.45 kilobases (kb), are detectable by Northern (RNA) blot analysis, but only the 2.0-kb transcript can be detected in HSV-1-infected tissue culture cells (J.G. Spivack and N. W. Fraser, J. Virol. 61:3842-3847, 1987). Since these latency-associated genes map to a diploid region of the genome, transcription from the deletion mutant HFEM, which contains only one complete copy of these genes, was investigated to determine the effect of gene dosage. The 4.1-kb HFEM deletion is located between the alpha genes ICP0 and ICP27. ICP0 mRNA and the 2.0-kb latency-associated transcript were present at normal levels during HFEM infection, but ICP27 mRNA and 0.9- and 1.1-kb transcripts that map near the deletion were not readily detectable. The levels of expression of one or more of these genes might be an important determinant of HSV-1 virulence in animal hosts. ICP27 mRNA accumulated when protein synthesis was inhibited before HFEM infection, implying that the deletion may affect ICP27 regulatory rather than coding elements. Expression of the 2.0-kb latency-associated transcript was characterized in infected CV-1 cells with metabolic inhibitors and strand-specific probes. On the basis of metabolic inhibitor studies, the gene encoding the 2.0-kb latency-associated transcript is not an alpha gene. During HSV-1 replication in infected tissue culture cells, the beta and gamma genes require the prior expression of alpha gene products. However, the latency-associated RNAs are expressed in the absence of detectable levels of alpha transcripts in latently infected mice. Thus, this latency-associated gene family appear to be regulated quite differently than alpha, beta, or gamma genes. For these reasons, and because the latency-associated genes may perform latent rather than replicative functions, we propose that they should be considered members of a new HSV-1 gene class, the lambda genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y., Hadar J., Tabor E., Ben-Hur T., Raibstein I., Rösen A., Darai G. A sequence in HpaI-P fragment of herpes simplex virus-1 DNA determines intraperitoneal virulence in mice. Virology. 1986 Mar;149(2):255–259. doi: 10.1016/0042-6822(86)90128-5. [DOI] [PubMed] [Google Scholar]
- Centifanto-Fitzgerald Y. M., Yamaguchi T., Kaufman H. E., Tognon M., Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982 Feb 1;155(2):475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C. M., McDougall J. K. Limited transcription of the herpes simplex virus genome when latent in human sensory ganglia. J Virol. 1982 Feb;41(2):686–691. doi: 10.1128/jvi.41.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Nickol J. M., Lieber M. R., Felsenfeld G. Regulated gene expression in transfected primary chicken erythrocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4312–4316. doi: 10.1073/pnas.83.12.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Koch H. G., Rösen A., Ernst F., Becker Y., Darai G. Determination of the nucleotide sequence flanking the deletion (0.762 to 0.789 map units) in the genome of an intraperitoneally avirulent HSV-1 strain HFEM. Virus Res. 1987 Apr;7(2):105–115. doi: 10.1016/0168-1702(87)90073-6. [DOI] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. C., Spandidos D. A., Wilkie N. M. Transcriptional regulation of a herpes simplex virus immediate early gene is mediated through an enhancer-type sequence. EMBO J. 1984 Feb;3(2):389–395. doi: 10.1002/j.1460-2075.1984.tb01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean A. R., Brown S. M. A herpes simplex virus type 1 variant which fails to synthesize immediate early polypeptide VmwIE63. J Gen Virol. 1987 May;68(Pt 5):1339–1350. doi: 10.1099/0022-1317-68-5-1339. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of alpha genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan J. L., Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980 Dec;51(Pt 2):233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Mavromara-Nazos P., Sears A. E., Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988 Jan;162(1):251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. Characterization of the IE110 gene of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2365–2380. doi: 10.1099/0022-1317-67-11-2365. [DOI] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Rosenthal J. D., Openshaw H., Notkins A. L. Herpes simplex virus DNA and mRNA sequences in acutely and chronically infected trigeminal ganglia of mice. Virology. 1978 Aug;89(1):102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Campbell M. E., Clements J. B. A tandemly reiterated DNA sequence in the long repeat region of herpes simplex virus type 1 found in close proximity to immediate-early mRNA 1. J Virol. 1984 Nov;52(2):715–718. doi: 10.1128/jvi.52.2.715-718.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Rösen A., Darai G. Mapping of the deletion in the genome of HSV-1 strain HFEM responsible for its avirulent phenotype. Med Microbiol Immunol. 1985;173(6):329–343. doi: 10.1007/BF02125037. [DOI] [PubMed] [Google Scholar]
- Rösen A., Ernst F., Koch H. G., Gelderblom H., Darai G., Hadar J., Tabor E., Ben-Hur T., Becker Y. Replacement of the deletion in the genome (0.762-0.789 mu) of avirulent HSV-1 HFEM using cloned MluI DNA fragment (0.7615-0.796 mu) of virulent HSV-1 F leads to generation of virulent intratypic recombinant. Virus Res. 1986 Aug;5(2-3):157–175. doi: 10.1016/0168-1702(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedarati F., Stevens J. G. Biological basis for virulence of three strains of herpes simplex virus type 1. J Gen Virol. 1987 Sep;68(Pt 9):2389–2395. doi: 10.1099/0022-1317-68-9-2389. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988 May;62(5):1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., O'Boyle D. R., 2nd, Fraser N. W. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J Virol. 1987 Oct;61(10):3288–3291. doi: 10.1128/jvi.61.10.3288-3291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Prusoff W. H., Tritton T. R. Dissociation of the inhibitory effects of 2-deoxy-D-glucose on Vero cell growth and the replication of herpes simplex virus. Antimicrob Agents Chemother. 1982 Aug;22(2):284–288. doi: 10.1128/aac.22.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Prusoff W. H., Tritton T. R. Inhibition of herpes simplex virus replication by methyl daunosamine. Antimicrob Agents Chemother. 1982 Jul;22(1):176–179. doi: 10.1128/aac.22.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., O'Boyle D. R., 2nd, Lavi E., Fraser N. W. Latent herpes simplex virus type 1 transcription in human trigeminal ganglia. J Virol. 1988 Sep;62(9):3493–3496. doi: 10.1128/jvi.62.9.3493-3496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Tenser R. B., Dawson M., Ressel S. J., Dunstan M. E. Detection of herpes simplex virus mRNA in latently infected trigeminal ganglion neurons by in situ hybridization. Ann Neurol. 1982 Mar;11(3):285–291. doi: 10.1002/ana.410110309. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Shedden W. I., Elliot A., Tetsuka T., Wildy P., Bourgaux-Ramoisy D., Gold E. Virus specific antigens in mammalian cells infected with herpes simplex virus. Immunology. 1966 Oct;11(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Preston C. M., Clements J. B. Separation and characterization of herpes simplex virus type 1 immediate-early mRNA's. J Virol. 1979 Jul;31(1):42–52. doi: 10.1128/jvi.31.1.42-52.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988 Feb;162(2):406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Rixon F. J., Easton A. J., Clements J. B. Immediate-early mRNA-2 of herpes simplex viruses types 1 and 2 is unspliced: conserved sequences around the 5' and 3' termini correspond to transcription regulatory signals. Nucleic Acids Res. 1983 Sep 24;11(18):6271–6287. doi: 10.1093/nar/11.18.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]