Abstract
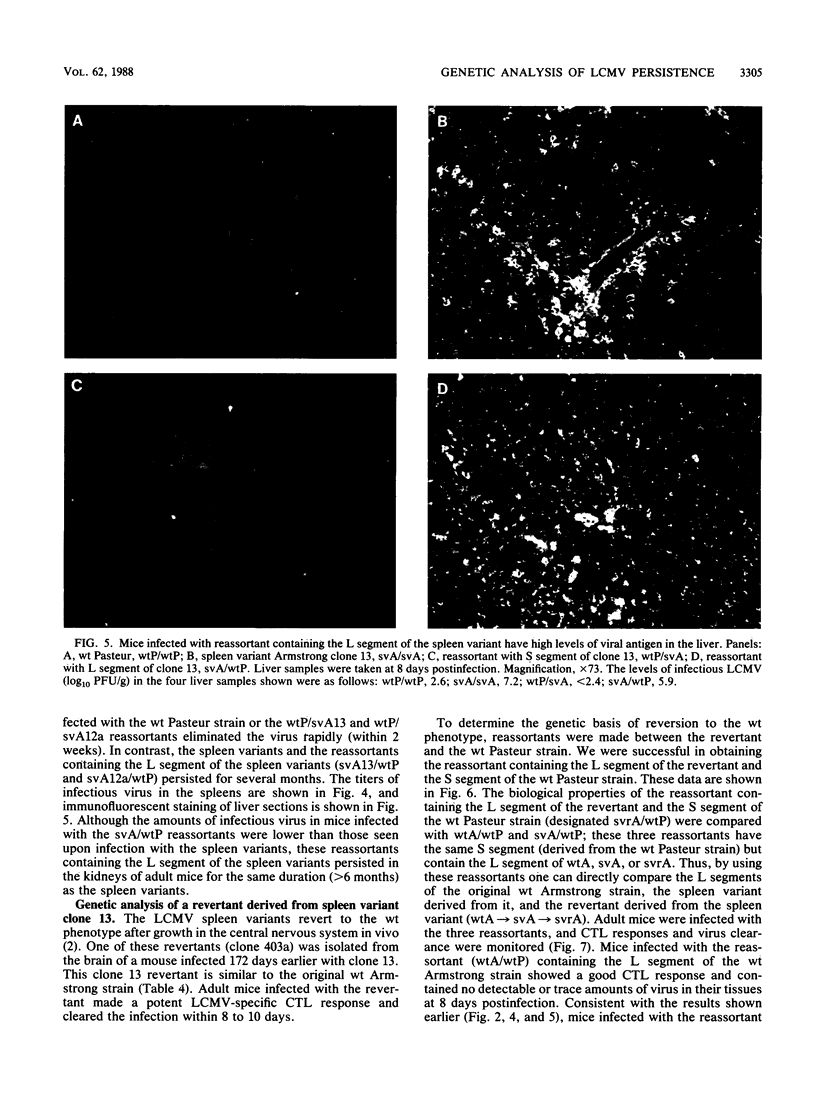
Viral variants with different biological properties are present in the central nervous systems (CNS) and lymphoid tissues of mice persistently infected with lymphocytic choriomeningitis virus (LCMV). Viral isolates from the CNS are similar to the original Armstrong LCMV strain and induce potent virus-specific T-cell responses in adult mice, and the infection is rapidly cleared. In contrast, LCMV isolates derived from spleens of carrier mice cause persistent infections in adult mice. This chronic infection is associated with low levels of antiviral T-cell responses. In this study, we genetically characterized two independently derived spleen variants by making recombinants (reassortants) between the spleen isolates and wild-type (wt) LCMV and showed that the ability to persist in adult mice and the associated suppression of T-cell responses segregates with the large (L) RNA segment. In addition, we analyzed a revertant (isolated from the CNS) derived from one of the spleen variants. By comparing the biological properties of three reassortants that contained the same S segment but had the L segment of either the original wt Armstrong LCMV, the spleen variant derived from it, or the CNS revertant derived from the spleen variant, we were able to show unequivocally that biologically relevant mutations occurred in the L segment not only during generation of the spleen variant from wt LCMV but also in reversion of the spleen variant to the wt phenotype. Thus, our results showed that (i) genetic alterations in the L genomic segment were involved in organ-specific selection of viral variants, and (ii) these mutations profoundly affected the ability of LCMV to cause chronic infections in adult mice.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Jamieson B. D., Porter D. D. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987 Dec;61(12):3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Oldstone M. B. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988 May 1;167(5):1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H. Ambisense RNA genomes of arenaviruses and phleboviruses. Adv Virus Res. 1986;31:1–51. doi: 10.1016/S0065-3527(08)60261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Jamieson B. D., Butler L. D., Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987 Dec;61(12):3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Biochemical evidence of recombination within the unsegmented RNA genome of aphthovirus. J Virol. 1982 Jan;41(1):66–77. doi: 10.1128/jvi.41.1.66-77.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Baric R. S., Makino S., Keck J. G., Egbert J., Leibowitz J. L., Stohlman S. A. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985 Nov;56(2):449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P. J., Buchmeier M. J., Dutko F. J., Oldstone M. B. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol. 1985 Mar;53(3):966–968. doi: 10.1128/jvi.53.3.966-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P. J., Buchmeier M. J., Oldstone M. B. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J Virol. 1985 Sep;55(3):704–709. doi: 10.1128/jvi.55.3.704-709.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski V., Matsuura Y., Bishop D. H. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 1985 Sep;3(2):101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Singh M. K., Fuller-Pace F. V., Buchmeier M. J., Southern P. J. Analysis of the genomic L RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Dec;161(2):448–456. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Singh M. K., Riviere Y., Jacoby D. R., Buchmeier M. J., Oldstone M. B. Molecular characterization of the genomic S RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Mar;157(1):145–155. doi: 10.1016/0042-6822(87)90323-0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]