Abstract
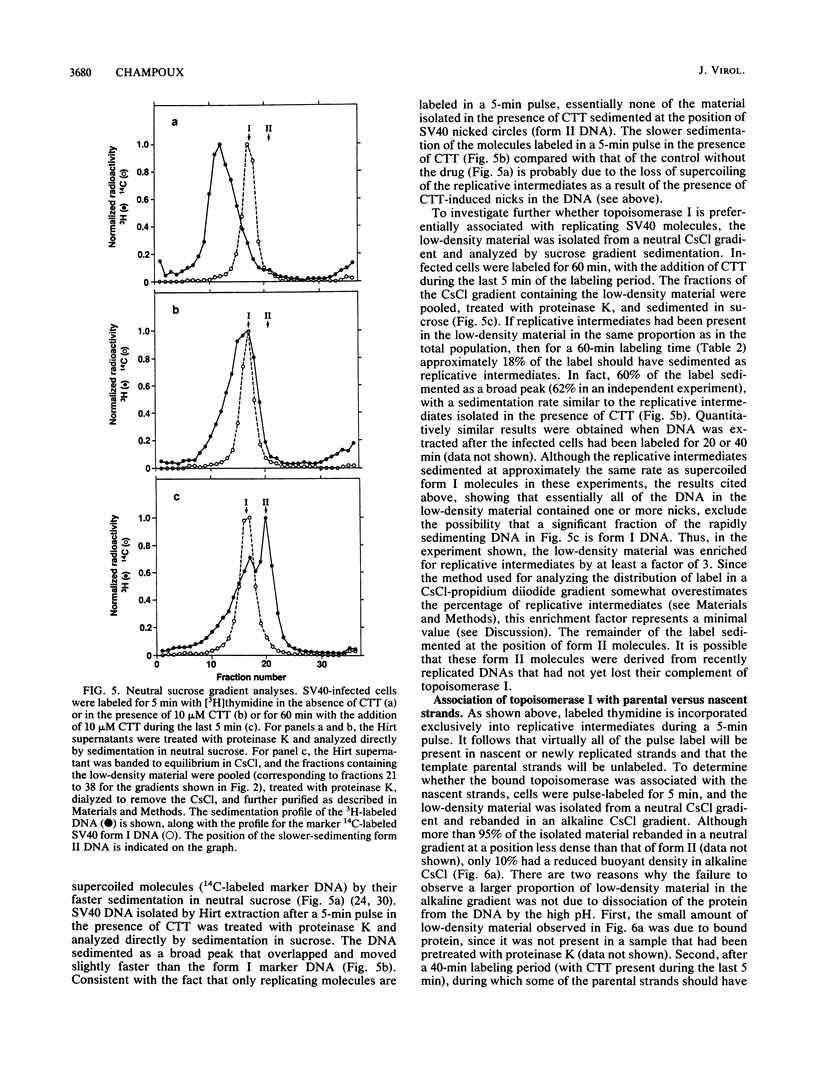
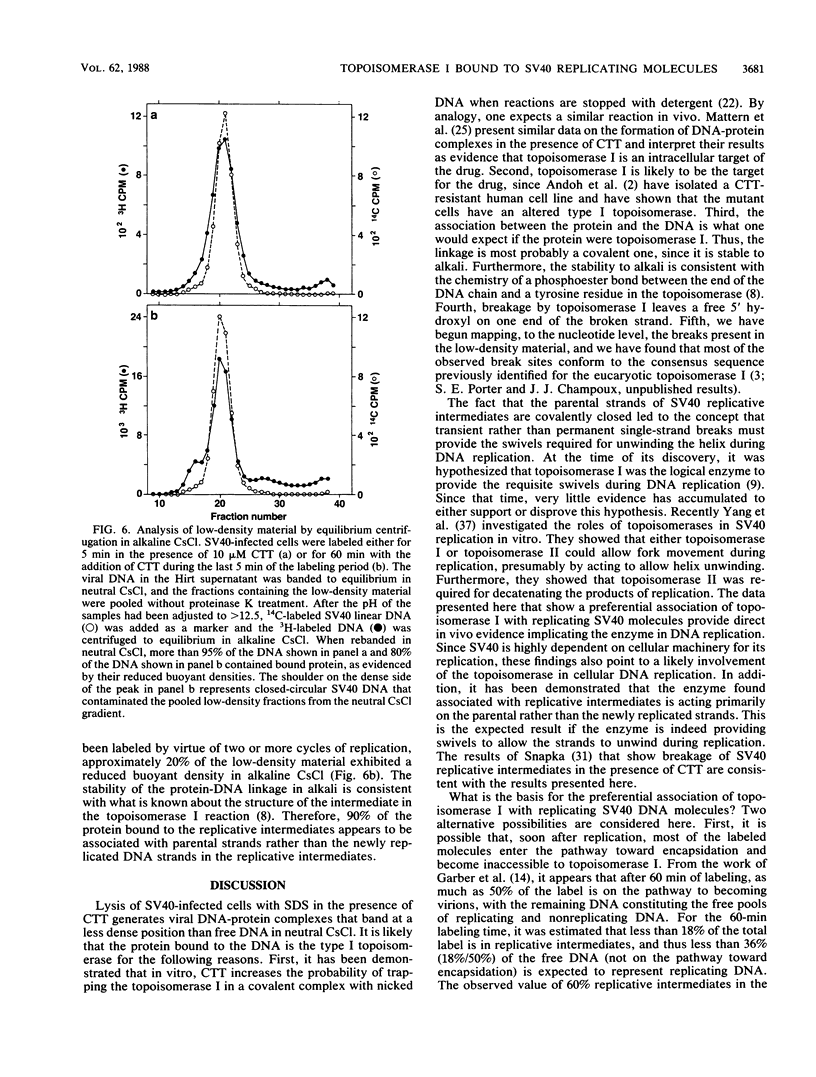
Detergent extraction of simian virus 40 (SV40) DNA from infected monkey CV-1 cells, after a brief exposure to the drug camptothecin, yields covalent complexes between topoisomerase I and DNA that band with reduced buoyant densities in CsCl. The following lines of evidence indicate that the enzyme is preferentially associated with SV40 replicative intermediates. First, the percentage of the isolated labeled viral DNA that exhibited a reduced buoyant density is inversely proportional to the length of the labeling period and approximately parallels the percentage of replicative intermediates for each labeling time (5 to 60 min). Second, after labeling for 60 min, the isolated low-density material was found to be enriched for replicative intermediates as measured by sedimentation in neutral sucrose. Third, analysis of extracted viral DNA by equilibrium centrifugation in CsCl-propidium diiodide gradients that separate replicating molecules from completed form I DNA revealed that camptothecin pretreatment specifically caused the linkage of topoisomerase I to replicating molecules. In addition, analysis of the low-density material obtained under conditions when only the newly synthesized strands of the replicative intermediates were labeled showed that the enzyme was associated almost exclusively with the parental strands. Taken together, these observations indicate that topoisomerase I is involved in DNA replication, and they are consistent with the hypothesis that the enzyme provides swivels to allow the helix to unwind. The observed bias in the distribution of topoisomerase I on intracellular SV40 DNA could be the result of rapid encapsidation of replicated molecules that precludes the association of topoisomerase I with the DNA or, alternatively, the result of a specific association of the enzyme with replicative intermediates.
Full text
PDF

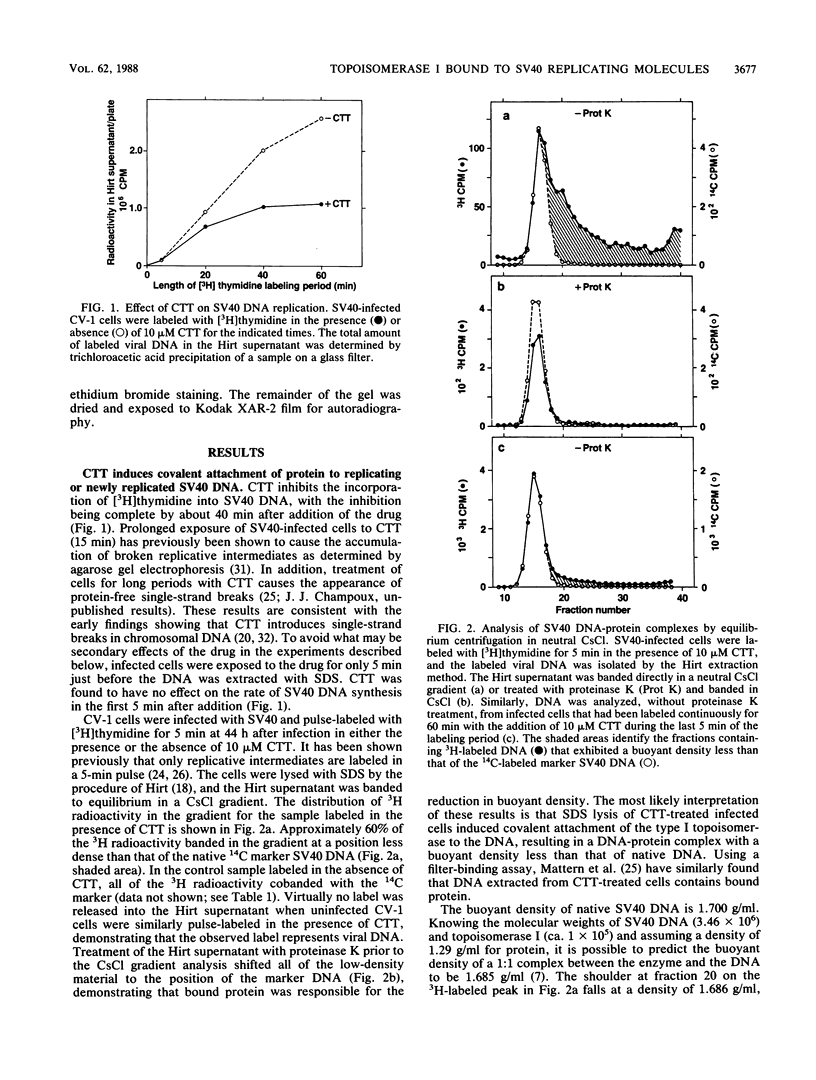
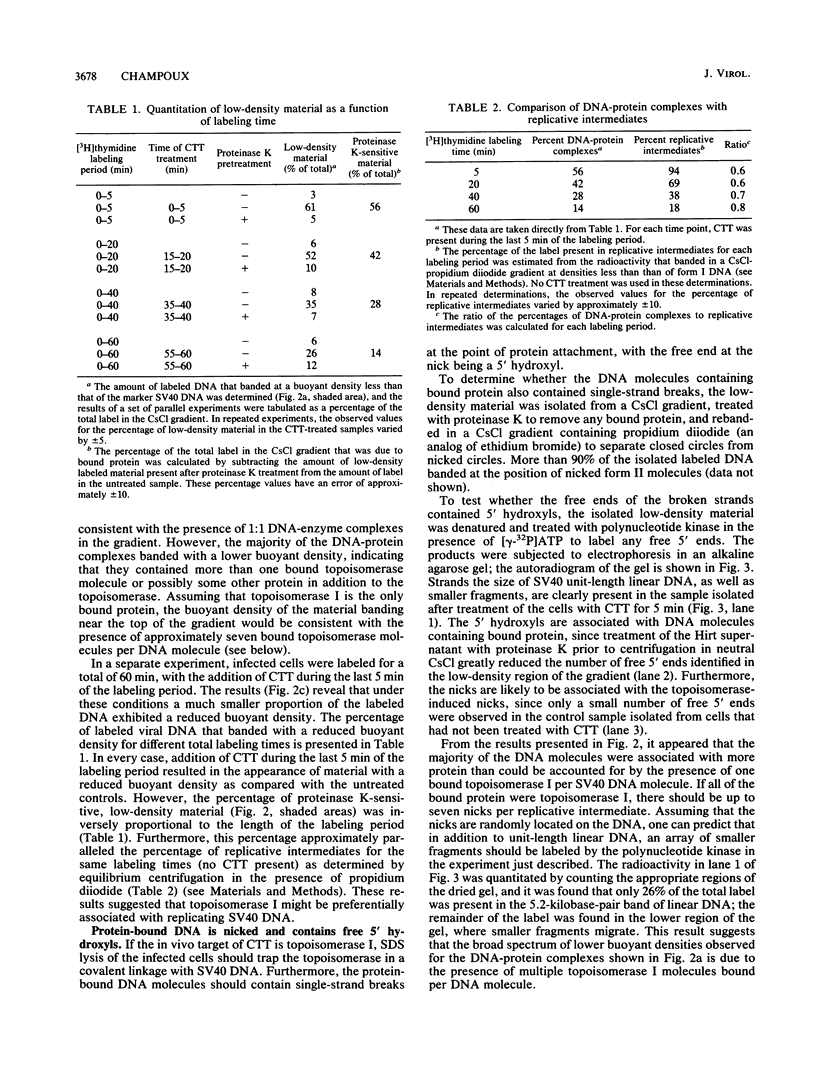
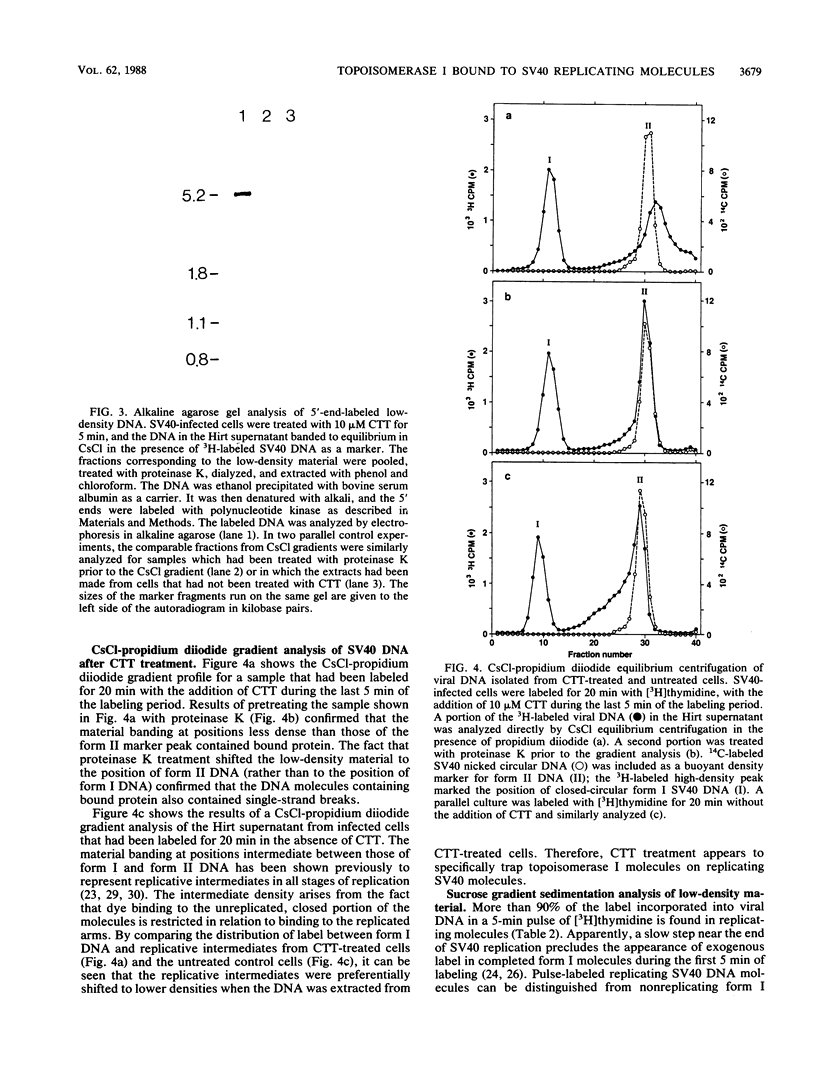


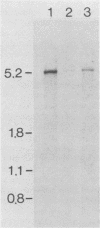
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Penman S. Induction of alkali labile links in cellular DNA by camptothecin. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1048–1054. doi: 10.1016/0006-291x(73)91512-x. [DOI] [PubMed] [Google Scholar]
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Burgess R. R., Champoux J. J. Nucleotide sequence preference at rat liver and wheat germ type 1 DNA topoisomerase breakage sites in duplex SV40 DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3097–3114. doi: 10.1093/nar/12.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Champoux J. J. Cutting of M13mp7 phage DNA and excision of cloned single-stranded sequences by restriction endonucleases. Methods Enzymol. 1983;101:90–98. doi: 10.1016/0076-6879(83)01007-1. [DOI] [PubMed] [Google Scholar]
- Bonven B. J., Gocke E., Westergaard O. A high affinity topoisomerase I binding sequence is clustered at DNAase I hypersensitive sites in Tetrahymena R-chromatin. Cell. 1985 Jun;41(2):541–551. doi: 10.1016/s0092-8674(85)80027-1. [DOI] [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. DNA is linked to the rat liver DNA nicking-closing enzyme by a phosphodiester bond to tyrosine. J Biol Chem. 1981 May 25;256(10):4805–4809. [PubMed] [Google Scholar]
- Champoux J. J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc Natl Acad Sci U S A. 1972 Jan;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Priming of superhelical SV40 DNA by Escherichia coli RNA polymerase for in vitro DNA synthesis. Biochemistry. 1975 Jan 28;14(2):307–316. doi: 10.1021/bi00673a017. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3800–3804. doi: 10.1073/pnas.74.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann G., Pflugfelder G., Steiner E. K., Javaherian K., Howard G. C., Wang J. C., Elgin S. C. Drosophila DNA topoisomerase I is associated with transcriptionally active regions of the genome. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6958–6962. doi: 10.1073/pnas.81.22.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. The detection and characterization of multiple forms of SV40 nucleoprotein complexes. Virology. 1978 Oct 15;90(2):305–316. doi: 10.1016/0042-6822(78)90315-x. [DOI] [PubMed] [Google Scholar]
- Garg L. C., DiAngelo S., Jacob S. T. Role of DNA topoisomerase I in the transcription of supercoiled rRNA gene. Proc Natl Acad Sci U S A. 1987 May;84(10):3185–3188. doi: 10.1073/pnas.84.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Elgin S. C. Localization of specific topoisomerase I interactions within the transcribed region of active heat shock genes by using the inhibitor camptothecin. Mol Cell Biol. 1987 Jan;7(1):141–148. doi: 10.1128/mcb.7.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Pflugfelder G., Wang J. C., Lis J. T. Topoisomerase I interacts with transcribed regions in Drosophila cells. Cell. 1986 Feb 14;44(3):401–407. doi: 10.1016/0092-8674(86)90461-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Horwitz S. B. Intracellular degradation of HeLa and adenovirus type 2 DNA induced by camptothecin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):723–727. doi: 10.1016/0006-291x(71)90476-1. [DOI] [PubMed] [Google Scholar]
- Horwitz S. B., Chang C. K., Grollman A. P. Studies on camptothecin. I. Effects of nucleic acid and protein synthesis. Mol Pharmacol. 1971 Nov;7(6):632–644. [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Mong S. M., Bartus H. F., Mirabelli C. K., Crooke S. T., Johnson R. K. Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured L1210 cells. Cancer Res. 1987 Apr 1;47(7):1793–1798. [PubMed] [Google Scholar]
- Mayer A., Levine A. J. DNA replication in SV40-infected cells. 8. The distribution of replicating molecules at different stages of replication in SV40-infected cells. Virology. 1972 Nov;50(2):328–338. doi: 10.1016/0042-6822(72)90384-4. [DOI] [PubMed] [Google Scholar]
- Muller M. T. Quantitation of eukaryotic topoisomerase I reactivity with DNA. Preferential cleavage of supercoiled DNA. Biochim Biophys Acta. 1985 Mar 20;824(3):263–267. doi: 10.1016/0167-4781(85)90057-0. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Roman A., Champoux J. J., Dulbecco R. Characterization of the replicative intermediates of polyoma virus. Virology. 1974 Jan;57(1):147–160. doi: 10.1016/0042-6822(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapka R. M. Topoisomerase inhibitors can selectively interfere with different stages of simian virus 40 DNA replication. Mol Cell Biol. 1986 Dec;6(12):4221–4227. doi: 10.1128/mcb.6.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spataro A., Kessel D. Studies on camptothecin-induced degradation and apparent reaggregation of DNA from L1210 cells. Biochem Biophys Res Commun. 1972 Aug 7;48(3):643–648. doi: 10.1016/0006-291x(72)90396-8. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell. 1984 Apr;36(4):1081–1088. doi: 10.1016/0092-8674(84)90058-8. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Schütz G. Camptothecin-induced in vivo topoisomerase I cleavages in the transcriptionally active tyrosine aminotransferase gene. Cell. 1987 Sep 25;50(7):1109–1117. doi: 10.1016/0092-8674(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]