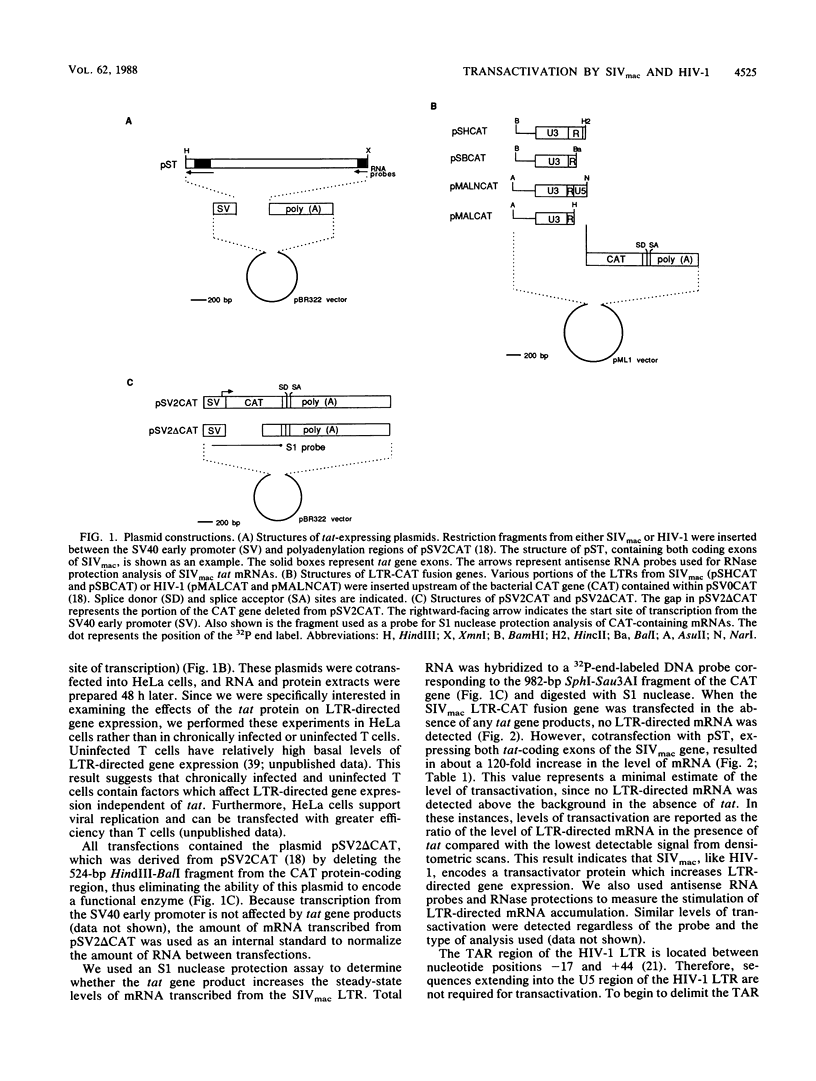
Abstract
Simian immunodeficiency virus from rhesus macaques (SIVmac), like human immunodeficiency virus type 1 (HIV-1), encodes a transactivator (tat) which stimulates long terminal repeat (LTR)-directed gene expression. We performed cotransfection assays of SIVmac and HIV-1 tat constructs with LTR-CAT reporter plasmids. The primary effect of transactivation for both SIVmac and HIV-1 is an increase in LTR-directed mRNA accumulation. The SIVmac tat gene product partially transactivates an HIV-1 LTR, whereas the HIV-1 tat gene product fully transactivates an SIVmac LTR. Significant transactivation is achieved by the product of coding exon 1 of the HIV-1 tat gene; however, inclusion of coding exon 2 results in a further increase in mRNA accumulation. In contrast, coding exon 2 of the SIVmac tat gene is required for significant transactivation. These results imply that the tat proteins of SIVmac and HIV-1 are functionally similar but not interchangeable. In addition, an in vitro-generated mutation in SIVmac tat disrupts splicing at the normal splice acceptor site at the beginning of coding exon 2 and activates a site approximately 15 nucleotides downstream. The product of this splice variant stimulates LTR-directed gene expression. This alternative splice acceptor site is also used by a biologically active provirus with an efficiency of approximately 5% compared with the upstream site. These data suggest that a novel tat protein is encoded during the course of viral infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Beaver B., Jagodzinski L., Ensoli B., Kanki P. J., Albert J., Fenyo E. M., Biberfeld G., Zagury J. F., Laure F. New human and simian HIV-related retroviruses possess functional transactivator (tat) gene. Nature. 1987 Aug 6;328(6130):548–550. doi: 10.1038/328548a0. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Separate regulatory elements for the heat-inducible and ovarian expression of the Drosophila hsp26 gene. Cell. 1985 Dec;43(3 Pt 2):737–746. doi: 10.1016/0092-8674(85)90247-8. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Dorsett D. L., Keshet I., Winocour E. Quantitation of a simian virus 40 nonhomologous recombination pathway. J Virol. 1983 Oct;48(1):218–228. doi: 10.1128/jvi.48.1.218-228.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D., Deichaite I., Winocour E. Circular and linear simian virus 40 DNAs differ in recombination. Mol Cell Biol. 1985 Apr;5(4):869–880. doi: 10.1128/mcb.5.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Guyader M., Montagnier L., Baltimore D., Muesing M. A. The specificity of the human immunodeficiency virus type 2 transactivator is different from that of human immunodeficiency virus type 1. EMBO J. 1987 Dec 1;6(12):3755–3760. doi: 10.1002/j.1460-2075.1987.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Collalti E., Ratner L., Gallo R. C., Wong-Staal F. A molecular clone of HTLV-III with biological activity. Nature. 1985 Jul 18;316(6025):262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Kong L. I., Lee S. W., Kumar P., Taylor M. E., Arya S. K., Shaw G. M. Relation of HTLV-4 to simian and human immunodeficiency-associated viruses. Nature. 1987 Nov 12;330(6144):184–186. doi: 10.1038/330184a0. [DOI] [PubMed] [Google Scholar]
- Hauber J., Cullen B. R. Mutational analysis of the trans-activation-responsive region of the human immunodeficiency virus type I long terminal repeat. J Virol. 1988 Mar;62(3):673–679. doi: 10.1128/jvi.62.3.673-679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Perkins A., Heimer E. P., Cullen B. R. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6364–6368. doi: 10.1073/pnas.84.18.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Kornfeld H., Kanki P. J., Essex M., Mullins J. I. Cross-reactivity to human T-lymphotropic virus type III/lymphadenopathy-associated virus and molecular cloning of simian T-cell lymphotropic virus type III from African green monkeys. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9754–9758. doi: 10.1073/pnas.83.24.9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Alroy J., Essex M. Isolation of T-lymphotropic retrovirus related to HTLV-III/LAV from wild-caught African green monkeys. Science. 1985 Nov 22;230(4728):951–954. doi: 10.1126/science.2997923. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Kurth R., Becker W., Dreesman G., McLane M. F., Essex M. Antibodies to simian T-lymphotropic retrovirus type III in African green monkeys and recognition of STLV-III viral proteins by AIDS and related sera. Lancet. 1985 Jun 8;1(8441):1330–1332. doi: 10.1016/s0140-6736(85)92818-1. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., McLane M. F., King N. W., Jr, Letvin N. L., Hunt R. D., Sehgal P., Daniel M. D., Desrosiers R. C., Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985 Jun 7;228(4704):1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Yetz J. M., Letvin N. L. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Li Y., Naidu Y. M., Butler C. V., Ochs M. F., Jaenel G., King N. W., Daniel M. D., Desrosiers R. C. Comparison of simian immunodeficiency virus isolates. Nature. 1988 Feb 18;331(6157):619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld H., Riedel N., Viglianti G. A., Hirsch V., Mullins J. I. Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature. 1987 Apr 9;326(6113):610–613. doi: 10.1038/326610a0. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Wong-Staal F. Demonstration of virus-specific transcriptional activator(s) in cells infected with HTLV-III by an in vitro cell-free system. Cell. 1986 Oct 10;47(1):29–35. doi: 10.1016/0092-8674(86)90363-6. [DOI] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Transcriptional but not translational regulation of HIV-1 by the tat gene product. Nature. 1988 Apr 7;332(6164):551–553. doi: 10.1038/332551a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Campbell K., Haseltine W. A. Construction of recombinant murine retroviruses that express the human T-cell leukemia virus type II and human T-cell lymphotropic virus type III trans activator genes. J Virol. 1986 Jan;57(1):379–384. doi: 10.1128/jvi.57.1.379-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Goh W. C., Dayton A. I., Lippke J., Haseltine W. A. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986 Feb 13;319(6054):555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel L. J., Ratner L., Josephs S. F., Derse D., Feinberg M. B., Reyes G. R., O'Brien S. J., Wong-Staal F. Transactivation induced by human T-lymphotropic virus type III (HTLV III) maps to a viral sequence encoding 58 amino acids and lacks tissue specificity. Virology. 1986 Jan 15;148(1):226–231. doi: 10.1016/0042-6822(86)90419-8. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Kalderon D., Roberts B. L., Colledge W. H., Edge M., Gillett P., Markham A., Paucha E., Richardson W. D. The nuclear location signal. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):43–58. doi: 10.1098/rspb.1985.0078. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Wright C. M., Felber B. K., Paskalis H., Pavlakis G. N. Expression and characterization of the trans-activator of HTLV-III/LAV virus. Science. 1986 Nov 21;234(4779):988–992. doi: 10.1126/science.3490693. [DOI] [PubMed] [Google Scholar]





