Abstract
Meiotic chromosome segregation occurs in Drosophila oocytes on an acentrosomal spindle, which raises interesting questions regarding spindle assembly and function. One is how to organize a bipolar spindle without microtubule organizing centers at the poles. Another question is how to orient the chromosomes without kinetochore capture of microtubules that grow from the poles. We have characterized the mei-38 gene in Drosophila and found it may be required for chromosome organization within the karyosome. Nondisjunction of homologous chromosomes occurs in mei-38 mutants primarily at the first meiotic division in females but not in males where centrosomes are present. Most meiotic spindles in mei-38 oocytes are bipolar but poorly organized, and the chromosomes appear disorganized at metaphase. mei-38 encodes a novel protein that is conserved in the Diptera and may be a member of a multigene family. Mei-38 was previously identified (as ssp1) due to a role in mitotic spindle assembly in a Drosophila cell line. MEI-38 protein localizes to a specific population of spindle microtubules, appearing to be excluded from the overlap of interpolar microtubules in the central spindle. We suggest MEI-38 is required for the stability of parallel microtubules, including the kinetochore microtubules.
MEIOSIS is a special type of cell division that produces haploid gametes from diploid parental cells. One round of chromosome replication is followed by two rounds of chromosome segregation. Fusion of two gametes during sexual reproduction restores the diploid chromosome complement. Proper chromosome segregation during meiosis is crucial for preventing aneuploidy, embryonic lethality, reductions in fertility, and birth defects. In Drosophila oocytes, and the oocytes of many other animals, meiotic spindles are assembled in the absence of centrosomes, which are at the center of the microtubule organizing centers at the poles of the canonical mitotic spindle (Matthies et al. 1996). In oocytes and other acentrosomal systems, it is believed that the chromosomes trigger spindle formation by capturing or nucleating microtubules (Theurkauf and Hawley 1992; McKim and Hawley 1995). These microtubules are then bundled and sorted to generate two poles in a process that involves interactions with a variety of motor proteins (Matthies et al. 1996; Walczak et al. 1998). The activities of many motors in acentrosomal spindle formation have been studied in activated Xenopus oocyte extracts (Karsenti and Vernos 2001).
The Drosophila oocyte is a good model for studying the mechanism of spindle assembly in the absence of centrosomes because of the combined benefits of genetics and cytology (Doubilet and McKim 2007). In particular, Drosophila mutants affecting these processes can be isolated and analyzed using genetic and cytological techniques. Analysis of several Drosophila segregation mutants has led to a model for spindle assembly that is based on the idea that the microtubules initially accumulate around the chromosomes. Motor proteins such as non-claret disjunctional bundle microtubules and, possibly through minus-end-directed movement, taper the fibers toward the poles (Theurkauf and Hawley 1992). In contrast, plus-end-directed motors like Subito bundle antiparallel microtubules within the central spindle and link the two half spindles (Jang et al. 2005). In addition to motor proteins, spindle-pole-associated (MSPS, TACC) (Cullen and Ohkura 2001) and kinetochore proteins (ALD) (Gilliland et al. 2007) have been characterized that are critical for acentrosomal meiosis. Little is known about how these proteins interact with the motor proteins to generate a bipolar, acentrosomal spindle. Most of these proteins are also expressed and function in mitotic cells although it is unclear what fraction of proteins involved in mitotic spindle assembly are also involved in meiotic spindle assembly. Furthermore, the mutant phenotype of genes might differ substantially in oocytes and mitotic cells due to the presence or absence of centrosomes.
In this article we report on a nonmotor protein, MEI-38, with an important function during acentrosomal meiosis. A single mei-38 allele was isolated by Baker and Carpenter (1972) in a screen for mutants with elevated levels of X chromosome nondisjunction. We have characterized the mei-38 null mutant phenotype and the gene's protein product. In the absence of mei-38, nondisjunction of homologous chromosomes at meiosis I is elevated and the metaphase chromosomes appear disorganized. In contrast, chromosome segregation at meiosis II and in male meiosis is not noticeably affected. While the most severe mutant phenotype is observed in female meiosis, loss of MEI-38 protein also caused spindle assembly defects in mitotic cells. MEI-38 is a novel protein which localizes to meiotic and mitotic microtubules. Interestingly, MEI-38 localizes to most microtubules with the exception of the antiparallel microtubules of the central spindle. The function of MEI-38 may be to stabilize kinetochore microtubules which in turn are important for interacting with homologous chromosomes at metaphase I of female acentrosomal meiosis.
MATERIALS AND METHODS
Genetic methods:
The frequency of X chromosome nondisjunction (X-ND) was determined by crossing y/y females to C(1;Y), v f B; C(4)RM, ci ey/0 or y w Hw/BSY males and calculated as 2(X-ND progeny)/total progeny, where total progeny = [2(X-ND progeny) + (regular progeny)]. In crosses involving C(4)RM, fourth chromosome nondisjunction (4-ND) was also detected and the frequency was calculated as [(4-ND progeny) + 2(simultaneous 4- and X-ND progeny)/total progeny]. If the fourth chromosome was not marked in the females, by svspa-pol, only fourth chromosome loss was measured and this number was doubled for the calculation. X chromosome crossing over was measured by crossing y mei-38 w/y mei-38 cv m f·y+ females to C(1;Y), v f B; C(4)RM, ci ey/0 males and scoring the male progeny. The y+ marker is a duplication of the wild-type yellow gene attached to the short right arm of the X chromosome, making it a marker for the centromere. This cross can also detect if chiasmata fail to direct segregation of homologs. If a crossover bivalent nondisjoins at meiosis I, then in 50% of the meiosis II divisions a recombinant chromatid will segregate into the same cell as a nonrecombinant chromatid carrying all of the recessive markers. The resulting female will have two maternal X chromosomes (diplo-X) and be homozygous for all of the markers distal to the crossover site. X-Y nondisjunction (X-Y-ND) in the male germline was measured by crossing y mei-38/y+Y males to y w; C(4)RM, ci ey/0 females. The nondisjunction frequency was calculated as (X-Y-ND progeny)/{(X-Y-ND progeny) + (regular progeny)}.
Nondisjunction on the second chromosome was tested by crossing y mei-38; al dp b pr cn c px sp/+ + + + + females to males carrying compound chromosomes [+/Y; C(2)EN, b sp]. In crosses to C(2)EN males, only progeny that inherit two second chromosomes from their mother survive. Progeny that do not inherit a second chromosome from their mother because of nondisjunction are not recovered because C(2)EN is transmitted poorly through the male germline. As in the X chromosome experiment, if a second chromosome crossover bivalent nondisjoins, in 50% of the second meiotic divisions a recombinant chromatid will segregate into the same product as a nonrecombinant chromatid carrying all of the recessive markers and be observed as a recombinant in the progeny.
Genetic screen for deletion alleles of mei-38:
Flies carrying a P element inserted close to mei-38 were crossed to a source of transposase (Δ2-3). Specifically, y w P{w+}/Y; Δ2-3, Sb/+ males were crossed to y/FM7, y w B females and excisions of the P element (= y w P{w−}/FM7, y w B) were detected in the progeny by the loss of the white+ marker gene. In some cases, the P elements also carried a y+ marker and we screened for loss of this marker. Individual white-eyed and/or yellow-bodied females were crossed to y mei-381/Y males and the P{w−}/ mei-381 progeny were crossed to assay for X chromosome nondisjunction. Those lines with elevated frequency of nondisjunction (>1%) were retested and stocks made for further analysis. The extent of the deletions was determined by PCR and sequencing.
Two insertions containing FRT sites in the same orientation, PBac{RB}e04351 (2A4) and P{XP}d04500 (2B1), were used to make a deletion that included rab27 but not the CG14781 coding region (Parks et al. 2004). Three-day-old P{XP}d04500/PBac{RB}e04351; P{70FLP} larvae in vials were heat-shocked in a water bath at 37° for 1 hr and then the adult females were crossed to FM7,w/y+ Y males. The female white-eyed progeny were then crossed with FM7, w B/y+ Y males to make Df/FM7, w B stocks. The deletion used in this study was homozygous lethal and confirmed by PCR to delete rab27.
Construction of transgenes:
There are two Rab27 transcripts, Rab27-RB and Rab27-RC. PCR was performed using cDNAs LP09977 (Rab27-RB) or GH21159 (Rab27-RC) as templates and the clones were confirmed by sequencing. Fragments containing the full-length coding regions were cloned into the pENTR4 Gateway vector (Invitrogen) using EcoRI and SalI. For CG14781 (mei-38), PCR was performed using cDNA RE11617 as template and primers to fuse the coding region in frame at the N terminus. The PCR product was cloned and confirmed by sequencing and then subcloned into pENTR4 with EcoRI and XhoI. The expression vectors were made using the clonase system to transfer the inserts from pENTR4 into pPWH (T. Murphy, personal communication) following the instructions for the LR Clonase II enzyme (Invitrogen). This vector places the insert under the control of the UASP promoter (Rorth 1998) and fuses it to three copies of the HA epitope tag. The expression vectors were sent to Model System Genomics (Duke University, Durham, NC) for embryo injection.
UASP-based trangenes were expressed in the germline by crossing to a Gal4 driver under the control of the nanos promoter, P{GAL4∷VP16-nos.UTR}MVD1 (Van Doren et al. 1998). To test for rescue, females of the genotype y w mei-38; P{GAL4∷VP16-nos.UTR}MVD1/P{UASP:mei-38HA} were constructed and crossed to test for nondisjunction as described above. These females were also used to detect MEI-38 protein by Western blot or immunofluorescence to detect the HA epitope tag.
Confocal microscopy:
Stage 14 oocytes were collected from 3- to 7-day-old yeast-fed females and fixed as described previously (Theurkauf and Hawley 1992; McKim et al. 1993). Oocytes were stained for DNA with Hoechst and for spindles with anti-α-tubulin conjugated to FITC (Sigma monoclonal antibody DM1A). Additional primary antibodies were rat-anti HA “high affinity” (Roche, clone 3F10), rat anti-SUB at 1:75 (Jang et al. 2005), INCENP (1:400) (C. Wu, unpublished data), MEI-S332 (1:1000) (Moore et al. 1998) with Cy3 or Cy5 conjugated secondary antibodies preadsorbed against a range of mammalian serum proteins including mouse and rabbit (Jackson Labs). Images were collected on a Leica TCS SP confocal microscope with a 63X, N.A. 1.3 lens. Images are shown as maximum projections of complete image stacks followed by cropping in Adobe Photoshop.
RESULTS
Meiosis I nondisjunction is elevated in mei-38 mutant females:
Baker and Carpenter (1972) isolated a single allele of mei-38 in a screen for elevated levels of X chromosome nondisjunction in females. The frequency of X chromosome nondisjunction in mei-38 mutant females is ∼8% but we did not detect nondisjunction in mei-38 mutant males (Table 1). The same frequency of nondisjunction was observed in mei-381/Df females (Table 1), suggesting mei-381 is a null allele. Nondisjunction can be caused by a failure to form chiasmata between homologs. In mei-38 mutants, however, crossing over on the X chromosomes was similar to wild-type controls (Table 2). This suggests that nondisjunction occurs despite the presence of chiasmata.
TABLE 1.
Nondisjunction in mei-38 mutants
| Genotype (♀ unless otherwise noted) | Regular progenya | Nondisjunction progeny (X/4)b | Nondisjunction (X/4) (%) |
|---|---|---|---|
| mei-381 | 1362 | 74/84 | 9.8/5.6 |
| mei-381 ♂ | 974 | 5/0 | 0.5 |
| mei-381/Df(1)S39 | 1446 | 62/76 | 7.9/4.8 |
| mei-381/Df(1)FDD-0225927 | 551 | 0 | 0 |
| mei-381 noda/mei-381+ | 1326 | 46/78 | 6.5/5.5 |
| mei-381 noda | 2589 | 127/2032 | 8.9/71.4 |
| mei-381 mei-2187/+ mei-2181 | 1492 | 150/89 | 28.7/4.2 |
| mei-381 mei-2187/mei-381 mei-2181 | 2551 | 981/769 | 43.5/17.0 |
| ald1/aldC3 | 1342 | 60 | 8.2 |
| mei-381; ald1/aldC3 | 680 | 90 | 20.9 |
Normal X and fourth chromosome segregation.
The first number is the X chromosome nondisjunction progeny and the second is the fourth chromosome nondisjunction progeny. If there is only one number, it is for X chromosome nondisjunction. See materials and methods for calculating the frequency of nondisjunction.
TABLE 2.
Crossing over on the X chromosome in mei-38 females
| Genetic interval (distance in cM)
|
||||||
|---|---|---|---|---|---|---|
| Female genotype | w–cv or pn–cva | cv–m | m–f | f–y+b | Total map (cM) | Total progeny |
| y w mei-38/y mei-38 cv m f·y+ | 10.9 | 22.2 | 16.5 | 7.5 | 57.1 | 921 |
| y mei-38/y pn cv m f·y+ | 12.7 | 24.7 | 14.6 | 4.5 | 56.5 | 1237 |
In the mei-38 homozygote, the w–cv interval was measured, while in the mei-38/+ experiment, the pn–cv interval was measured, which is only ∼1 cM larger (Lindsley and Zimm 1992).
The y+ marker is tightly linked to the centromere and the f–y+ interval includes the X chromosome centromere.
Since crossing over is not reduced in mei-38 mutants, it is likely that nondisjunction involves homolog pairs that are joined by chiasmata. In the experiment shown in Table 2 to score crossing over, the mei-38 mutant mothers gave rise to 53 female progeny that inherited two maternal X chromosomes, of which 14 were homozygous for at least one of the recessive markers, indicating nondisjunction of a chiasmate bivalent. The majority of these females (10) were homozygous for the distal marker white, consistent with a crossover followed by nondisjunction at meiosis I (the remaining 4/14 could be similarly explained if there was a double crossover). Since there are four possible products from the segregation of sister chromatids at meiosis II, only 1/4 of the zygotes from nondisjunction of chiasmate bivalents will be homozygous for a distal marker. Thus, the 14/53 progeny is consistent with most nondisjunction events involving chiasmate bivalents. None of the 53 females was yellow, and since y+ is a centromere marker, this indicates that nondisjunction of sister chromatids was not detected.
This result was confirmed for an autosome by crossing mei-38 females to C(2)EN males and examining the segregation of a genetically marked second chromosome. In these crosses, only the progeny that received two second chromosomes from the mother survived (materials and methods). In the control, no progeny were recovered from 45 mei-38/+; al dp b pr cn c px sp/+ females crossed to C(2)EN males, indicating a low frequency of second chromosome nondisjunction. This level of autosomal nondisjunction is consistent with a previous experiment (Rasooly et al. 1991) where only 10 progeny were recovered from 900 wild-type females. In contrast, from 99 mei-38; al dp b pr cn c px sp/+ females crossed to C(2)EN males, 142 progeny were recovered (1.4 per female parent), indicating that second chromosome nondisjunction was elevated in mei-38 mutants. Of these 142 nondisjunction progeny, 37 were homozygous for at least one of the recessive markers and therefore must have resulted from nondisjunction of chiasmate bivalents. Indeed, 33/37 of these progeny were homozygous for distal markers (either al or sp, or both), which results if there is a crossover followed by nondisjunction at meiosis I and normal segregation at meiosis II (the remaining 4/37 could be similarly explained if there was a double crossover). As for the X chromosome, we expected to observe only 1/4 of the chiasmate nondisjunction events, thus, 37/142 progeny is consistent with most nondisjunction events involving chiasmate bivalents. If sister chromatids were nondisjoining, we would have recovered progeny homozygous for centromere proximal markers like pr or cn, and these were not found. These data show that, like the X chromosome, autosomal nondisjunction in a mei-38 female occurs predominantly at meiosis I.
An additional test for nondisjunction of sister chromatids was to cross mei-38; CyO, CyO/+ females to C(2)EN males. CyO is a multiply inverted balancer chromosome that prevents the recovery of crossovers involving chromosome 2. Nondisjunction of chromosome 2 homologs would result in Curly wing progeny (e.g., +/CyO) whereas nondisjunction of sister chromatids would result in straight wing progeny (i.e., +/+). In the control, only three Curly wing progeny were recovered when 123 mei-38/+; CyO/+ females were crossed to C(2)EN males. In contrast, 345 exceptional progeny were recovered when 130 mei-38/mei-38; CyO/+ females were crossed to C(2)EN males (2.7 per parent) and 343/345 were Cy. The preponderance of Cy progeny indicates that most nondisjunction resulted from the failure to segregate homologs while the two Cy+ progeny indicate that sister chromatid nondisjunction occurs at a relatively low frequency in mei-38 mutant females.
mei-38 mutants affect meiotic spindle organization and chromosome behavior:
It is common for mutants that cause nondisjunction of chiasmate bivalents to have defects in spindle organization at meiosis I (McKim et al. 2002). Spindle assembly begins in mature stage 14 oocytes following nuclear envelope breakdown. In wild-type females, the microtubules initially assemble around the chromosomes, which are condensed into a single mass or karyosome (Theurkauf and Hawley 1992). The microtubules are then bundled and tapered into a bipolar spindle with the karyosome in the center. For the purposes of characterizing the mutant phenotype, we separately classified the chromosome and spindle phenotypes (Table 3).
TABLE 3.
Effect of mei-38 on chromosome and spindle morphology
| Spindle (%)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of oocytes | Chromosome (%)
|
Abnormal
|
||||||||
| Genotype | Round | Elongated | Disorganized | Normal | Frayed | Monopolar | Pole not tapered | Tubulin weak between poles | Other | |
| Wild type | 24 | 92 | 8 | 96 | 4 | |||||
| mei-381 | 24 | 50 | 8 | 42 (33 uneven) | 42 | 4 | 17 | 21 | 21 | |
| ald1/C3 | 21 | 62 | 5 | 33 (29 uneven) | 71 | 10 | 14 | 5 | ||
| mei-381; ald1/C3 | 30 | 63 | 7 | 30 (30 uneven) | 0 | 17 | 43 | 37 | 27 | 3 |
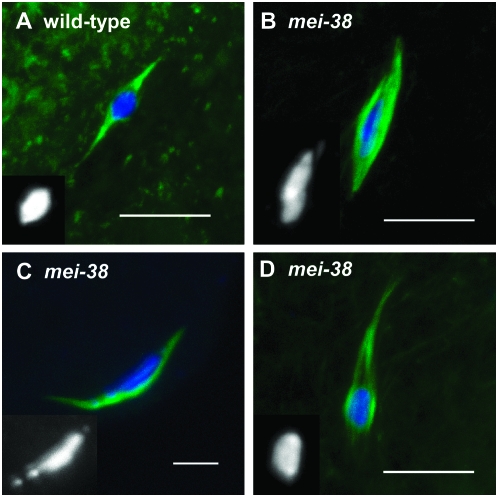
In wild-type controls, 100% of the oocytes had normal chromosome organization (a single round or oval karyosome) and 96% showed normal bipolar spindles (Figure 1A). Within the round and symmetric karyosome, the homologous centromeres of each bivalent are oriented toward the poles (e.g., Dernburg et al. 1996; Giunta et al. 2002). In mei-38 mutants, 42% of the oocytes showed disorganization of the chromosomes within the karyosome (Figures 1, B and C, and 2C). In most of these (33%), the karyosome was disorganized and the chromosomes were unevenly distributed in the center of the spindle. mei-38 mutants also had spindle organization defects in 58% of the oocytes, including monopolar spindles (Figure 1D, Table 3). A particularly striking phenotype in 21% of the oocytes was a drastic reduction or absence of microtubules between the poles and the karyosome while the central spindle remained intact (Figure 2B).
Figure 1.—
Spindle and chromosome organization in stage 14 oocytes. Tubulin (green) and DNA (blue and inserts) were stained in mature stage 14 oocytes. A normal bipolar spindle in wild type (A) compared to mei-381 mutant oocytes showing (B and C) disorganized karyosomes where chromosomes are not symmetrically arranged in a karyosome or (D) a monopolar spindle. Bar, 10 μm.
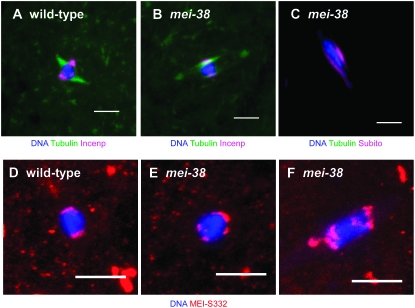
Figure 2.—
Localization of spindle- and centromere-associated proteins in stage 14 oocytes. Wild-type oocytes (A and D) and mei-381 mutant oocytes (B, C, E, and F) are shown. Oocytes were stained for Incenp (A and B), Subito (C) (all in magenta), and MEI-S332 (red). Subito and Incenp show identical staining patterns in wild-type oocytes (Jang et al. 2005), thus only a wild type with Incenp staining (A) is shown here. Tubulin staining is green in A and B and DNA is in blue. Note the uneven distribution of MEI-S332 signals in B even though the karyosome looks normal. Bar, 5 μm.
We have previously proposed that the central spindle plays an important role in spindle assembly (Jang et al. 2005). This structure is most likely composed of antiparallel microtubules and several proteins including the Kinesin 6 motor protein Subito and passenger proteins Incenp and Aurora B. This structure appears to be intact in mei-38 mutants since prominent microtubule, Subito and Incenp staining were observed (Figure 2, B and C). The only apparent effect of the mei-38 mutant on the localization of these proteins was that in some oocytes Subito and Incenp staining was more spread out along the spindle microtubules than in wild type (Figure 2C). It is possible this is a consequence of the chromosome organization defects since the central spindle tends to associate with the karyosome and is longer if the karyosome is stretched or separates.
It is important to note that although there were differences from wild type in the spindle organization of mei-38 mutants, bipolar spindles were still the predominant configuration. In Figure 1, B and C, for example, the chromosomes in the karyosome appear to be separating on a bipolar metaphase spindle. In many cases, defects in karyosome organization were observed even in the absence of overt spindle defects. To investigate the organization of the centromeres, we stained wild-type and mei-38 mutant oocytes for MEI-S332, which is the Drosophila homolog of the centromere protein Shugoshin (Moore et al. 1998). In wild-type metaphase I, the centromeres were usually clustered together and evenly separated on opposite sides of the karyosome (9/10 spindles), indicating the bivalents had properly oriented (Figure 2D). In mei-38 mutants, this clustering was frequently disrupted, resulting in a separation, dispersal, or uneven distribution of the MEI-S332 signals (7/10 spindles) (Figure 2, E and F). It is noteworthy that MEI-S332 staining revealed abnormal chromosome organization in an otherwise normal looking karyosome (Figure 2E).
mei-38 is required for the achiasmate chromosome segregation:
There is a robust system to segregate achiasmate chromosomes in Drosophila female meiosis (Hawley and Theurkauf 1993; Xiang and Hawley 2006). As described above, however, nondisjunction events in mei-38 mutants often involve chiasmate homologs. These data do not, however, rule out an effect on the achiasmate system. The maximum frequency of nondisjunction attributable to achiasmate X chromosomes is quite low (∼2%, Zhang and Hawley 1990), because in wild-type females 95% of the X chromosomes have at least one chiasma. Effects on the achiasmate system are most easily detected by comparing nondisjunction frequencies in the presence and absence of crossing over. In the autosomal nondisjunction experiments described above, more progeny per female were recovered from mei-38; CyO/+ females (2.7 per parent), in which the majority of second chromosomes were achiasmate due to crossover suppression by the balancer, than from mei-38; al dp b pr cn c px sp/+ females (1.4 per parent), in which crossing over was not suppressed. These results suggest that mei-38 is required for achiasmate segregation.
Two additional experiments confirmed that mei-38 mutants affect the achiasmate system. First, we made a double mutant with mei-218, in which the majority of chromosomes lack chiasmata (Carpenter and Sandler 1974). In mei-218 homozygous females, the frequency of nondisjunction (28.7%, Table 1) was similar to previous experiments (McKim et al. 1996). Importantly, this frequency of nondisjunction is lower than expected if achiasmate homologs in a mei-218 mutant segregated randomly (Baker and Hall 1976). In the mei-38 mei-218 double mutant, the frequency of X chromosome nondisjunction (43.5%) was significantly elevated, suggesting that the achiasmate chromosomes were segregating randomly. This result is similar to the frequency of X chromosome nondisjunction in a nod mei-218 double mutant (data not shown), in which the absence of NOD protein results in loss of the achiasmate segregation system, suggesting that mei-38 is required for the segregation of achiasmate chromosomes. Fourth chromosome nondisjunction was also elevated in the mei-38 mei-218 mutant, but this is not necessarily indicative of a defect in achiasmate segregation. Most mutants that increase nondisjunction, due to either a defect in spindle assembly or crossover formation, cause elevated levels of fourth chromosome nondisjunction (Baker and Hall 1976). Fourth chromosome nondisjunction may occur in crossover defective mutants because the system is overloaded with multiple pairs of achiasmate chromosomes.
Second, we examined nondisjunction in y mei-381/y mei-381/y+Y females. In XXY females, two achiasmate X chromosomes tend to segregate from the single Y chromosome (Grell 1976). For example, when we crossed y/y/y+Y; mei-W681/mei-W681 females to y w/BSY males, X chromosome nondisjunction was higher (51.9%, N = 237) than in y/y females (34.7%, N = 62) and 95% of the events involved the two X chromosomes segregating from the Y (N = 83). mei-W68 mutants have no crossing over due to the absence of double-strand breaks but the achiasmate system is unaffected (McKim and Hayashi-Hagihara 1998). Similarly, Carpenter and Sandler (1974) showed that in mei-9/mei-9/Y females, in which crossing over is reduced by >90%, 88% of the nondisjunction events involved the two X chromosomes segregating from the Y. In y mei-381/y mei-381/y+Y females, X chromosome nondisjunction was increased relative to y mei-381/y mei-381 females (16.4%, N = 1915), and only 66% of the events involved the two X chromosomes segregating from the Y (N = 157). In both mei-W68 and mei-38 mutants, the presence of the Y chromosome increased nondisjunction relative to normal (y/y) females, but in mei-38 females the low frequency of XX from Y segregation events indicates the achiasmate segregation system is defective. We have made similar observations when examining simultaneous X and second chromosome nondisjunction. When the second chromosome nondisjoins in crossover defective mutants, XX ↔ 22 events are the most frequent class. mei-38 mutants reduce the frequency of these events (data not shown).
These genetic experiments show that achiasmate chromosome segregation is defective in mei-38 mutants. This was not, however, reflected in increased severity of spindle defects. For example, the spindle and chromosome defects in mei-38; CyO/+ oocytes were no different than in mei-38 oocytes without the balancer (data not shown), indicating that the presence of univalents did not affect spindle structure. Similarly, the spindles observed in the mei-38 mei-28 double mutant, which lacks chiasmata, were not different than either single mutant. Double-mutant oocytes in metaphase had spindle and chromosome organization defects typical of mei-38 single mutants. In addition, and similar to mei-218 single-mutant oocytes, approximately half of the double-mutant oocytes had precociously entered anaphase, which occurs when chiasmata are absent (McKim et al. 1993). These results suggest that the increases in nondisjunction in the presence of achiasmate chromosomes were caused by errors in chromosome organization at metaphase rather than more severe spindle defects.
Comparison of mei-38 to other meiotic mutants:
Some mutants which affect the achiasmate system, such as ald and Axs, have a unique effect on fourth chromosome nondisjunction (O'Tousa 1982; Zitron and Hawley 1989; Whyte et al. 1993). X and fourth chromosome nondisjunction are not independent. Gametes resulting from simultaneous X and fourth chromosome nondisjunction are more common than expected and XX;O and O;44 gametes are more common than XX;44 and O;O gametes. mei-38 mutants also showed higher than expected levels of simultaneous X and fourth chromosome nondisjunction (5- to 10-fold more common that expected if independent events), but there was no preference for XX;O and O;44 gametes (data not shown). These results are consistent with mei-38 affecting segregation patterns of the achiasmate system.
To characterize the genetic interaction of mei-38 with other genes known to be involved in achiasmate chromosome segregation, we constructed double mutants with nod and ald. nod encodes a chromokinesin required for achiasmate chromosome segregation (Afshar et al. 1995). The low frequency of X chromosome nondisjunction in nod single mutants (∼2%, Zhang and Hawley 1990) is due to its effects being specific to the achiasmate system. This is also the reason for the exceptionally high frequency of fourth chromosome nondisjunction in nod mutants; this chromosome is always achiasmate. The mei-38 nod double mutant had an additive phenotype (Table 1). The frequency of X chromosome nondisjunction was similar to mei-38 while the frequency of fourth chromosome nondisjunction was similar to nod. These results can be explained if achiasmate X chromosome segregation requires both mei-38 and nod while chiasmate X chromosome segregation requires only mei-38.
Ald encodes the Drosophila ortholog of MPS1, which is required for the mitotic spindle assembly checkpoint (Gilliland et al. 2005). It is also required for chromosome segregation during meiosis and interestingly, some of our results from the analysis of mei-38 are similar to ald mutants. Both mutants have a similar level of nondisjunction (Table 1), affect both chiasmate and achiasmate chromosomes, and have relatively mild meiosis I spindle-organization defects (Table 3). Similar to our results with mei-38, ald mutant oocytes exhibit chromosome organization defects at meiosis I (Gilliland et al. 2005, 2007). Because ald null homozygotes are lethal, we used a heterozygote for a hypomorph, ald1, and a null allele, aldC3 to construct a double mutant with mei-38. This double mutant had a significantly higher frequency of nondisjunction compared to the single mutants (Table 1) and was accompanied by an increased frequency of spindle or chromosome abnormalities. There were no spindles classified as normal in the double mutant. In addition to spindles with a mei-38-specific phenotype, such as weak tubulin staining, there was a significant increase in monopolar spindles (Table 3). Therefore, the more severe nondisjunction phenotype in the double mutant correlates with the failure to maintain the chromosomes in the middle of the metaphase bipolar spindle.
mei38 encodes a novel protein:
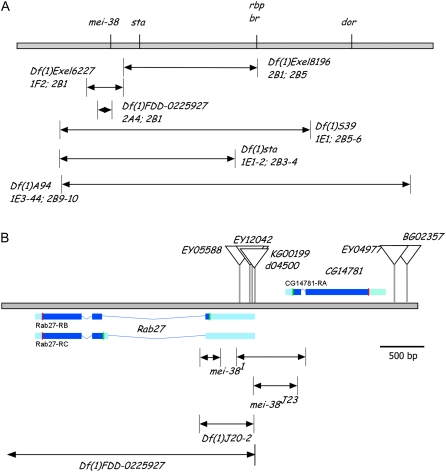
Genetic mapping was used to position mei-38 near the left end of the X chromosome (Figure 3A). Most critical was that mei-381 failed to complement Df(1)Exel6227 but complemented Df(1)Exel8196, which localized mei-38 to the region between 1F2 and 2B1. Sequencing of candidate genes in the region revealed two closely linked deletions that affected the adjacent genes Rab27 and CG14781 (Figure 3B). These deletions affect the coding regions of CG14781 and one of the two isoforms of Rab27. To determine which gene was responsible for the mei-38 mutant phenotype, we took two approaches: excision of flanking P-element insertions to induce deletions of each gene and generating transgenic lines expressing the wild-type form of each gene. Results from both approaches pointed to CG14781 as the gene associated with the meiotic nondisjunction phenotype.
Figure 3.—
Genetic mapping and cloning of mei-38. (A) Deficiencies (arrows) in the mei-38 region shown relative to the genetic map (top line). The cytological breakpoints of each deficiency are indicated at the bottom of its name. (B) Physical map of the mei-38 region. Triangles mark the positions of each transposon insertion. The blue boxes show the transcription units with the coding regions in dark blue and start and stop codons shown with green and red vertical lines, respectively (from Gelbart et al. 1997). At the bottom are the two regions deleted in the original mei-381 mutant and three deletions generated in this study. Df(1)FDD-0225927 was generated through recombination between two FRT sites, one in P{XP}d04500 and the other in PBac{RB}e04351, located ∼10 kb distal to Rab27.
Five P elements were used to generate deletions, three in the 5′-UTR of Rab27 and two located 3′ to CG14781 (Figure 3B). Excision of the two P elements 3′ to CG14781 failed to generate any useful deficiencies. Using three P elements inserted within the Rab27 5′-UTR, several deletions were isolated that failed to complement mei-38 for the nondisjunction phenotype and each of these also deleted CG14781. For example, excision of P-element P{SUPor-P}KG00199 yielded mei-38J23, which deleted part of exon 1 including the start codon of CG14781. mei-38J23 failed to complement mei-381, confirming that mei-38 is one of the two genes, but it also removed the intervening region between the two genes, failing to rule out Rab27. However, another excision, J20-2, which deletes part of Rab27 but leaves CG14781 intact, complemented mei-381, suggesting CG14781 is mei-38. To specifically test the phenotype of a Rab27 null allele, we used a site-directed method described in Parks et al. (2004) to generate a deletion between P{XP, w+}d04500 and PBac{RB, w+}e04351, the latter an insertion located ∼10 kb distal to Rab27 (Figure 3). Both insertions contain FRT sites in the same orientation. When FLPase was expressed, recombination between the two FRT sites generated a deletion (Df(1)FDD-0225927), which removed Rab27. This deletion was confirmed by PCR, is a recessive lethal, and complemented mei-381, showing that mutations in Rab27 and mei-38 affect different genes and that mei-38 is CG14781.
These results were confirmed with transgenes expressing either Rab27 or CG14781. cDNA sequences of each Rab27 isoform and CG14781 (Figure 3) were cloned into pPHW (T. Murphy, personal communication), which is based on the UASP vector (Rorth 1998) and fuses each coding region to three copies of the HA epitope tag and places them under the control of a promoter with multiple copies of the UAS sequence. These transgenes were expressed by crossing the transgenics to flies carrying GAL4 under the control of the nanos promoter (GAL4∷VP16-nos.UTR) (Van Doren et al. 1998), which we have used previously to express genes in oocytes (Jang et al. 2007). Each pair of transgene and GAL4 driver was crossed into a homozygous mei-381 background and tested for their effects on X chromosome nondisjunction. The constructs expressing the Rab27 isoforms did not rescue whereas a construct expressing CG14781 (P{UASP:mei-38HA}8) completely rescued the mei-38 mutant nondisjunction phenotype (Table 4). These results confirm that CG14781 is mei-38.
TABLE 4.
Transgene rescue of X chromosome nondisjunction in mei-38 mutants by CG14781
| Genotypea | Regular progeny | Nondisjunction progeny | Nondisjunction (%) |
|---|---|---|---|
| mei-38; nosGal4/P{UASP:mei-38HA}8 | 4296 | 8 | 0.4 |
| mei-38; nosGal4/P{UASP:Rab27HA}GHB-5 | 864 | 19 | 4.2 |
| mei-38; nosGal4/P{UASP:Rab27HA}LPB-10 | 1066 | 50 | 8.6 |
P{UASP:mei-38} is the transgene carrying the CG14781 coding region. nosGal4 = P{GAL4∷VP16-nos.UTR}MVD1.
The predicted MEI-38 protein is 325 amino acids and ∼37 kDa. Blast searches revealed MEI-38 orthologs in other Drosophila species and the mosquito Aedes aegypti (supplemental Figure S1). Orthologs in more distant species were not detected. Interestingly, however, mei-38 appears to be a member of a multigene family, with similarities in protein sequence primarily in the last half of the protein (supplemental Figure S1). MEI-38 is 50% identical and 70.8% similar to Drosophila melanogaster CG15395 and 34.7% identical and 59.7% similar to CG5781 over a 72-amino-acid region toward the end of the protein. The function of these genes and whether they are partially redundant with mei-38 is not known.
MEI-38 is a spindle-associated protein:
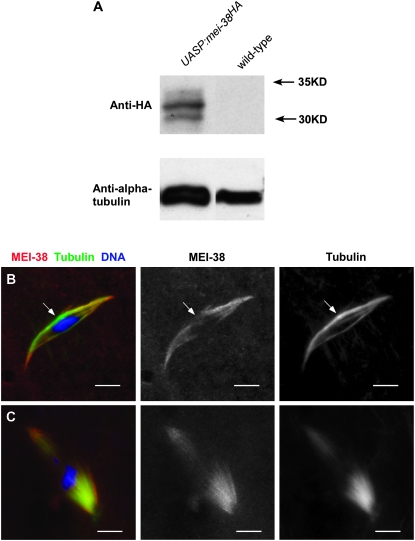
The P{UASP:mei-38HA}8 transgene which rescued the mei-38 mutant phenotype had three copies of the HA epitope tag fused to the coding region at the amino terminus. Using an antibody against the HA tag, we detected MEI-38 protein on Western blots and by immunofluorescence in oocytes (Figure 4). The HA antibody detected at least three bands on a Western blot, suggesting there may be post-translational modification of MEI-38. Indeed, Bodenmiller et al. (2007) mapped at least three phosphorylation sites in MEI-38. Immunostaining of stage 14 oocytes expressing P{UASP:mei-38HA}8 showed that MEI-38 localized to a subpopulation of spindle microtubules (Figure 4B). There are two populations of microtubules visible on the wild-type metaphase I arrested spindle. The first are the kinetochore microtubules, which extend from the poles to the chromosomes. The second are the interpolar microtubules, which extend from the poles and overlap in the central spindle rather than make contact with the chromosomes. MEI-38 staining appeared on most microtubules with the notable exception of those in the central spindle region.
Figure 4.—
Localization of MEI-38. (A) Western blot of ovary protein from females expressing HA-tagged MEI-38 protein or wild-type females using antibodies to the HA epitope or α-tubulin as a control. (B) Immunolocalization of MEI-38 (red) relative to tubulin staining (green) and DNA (blue) in stage 14 oocytes. MEI-38 colocalizes with microtubule staining except in the central spindle (arrow). (C) MEI-38 colocalizes with all microtubule staining in sub1/sub131 oocytes. Bar, 5 μm.
Subito localizes to the antiparallel microtubules of the central spindle at meiotic metaphase (Jang et al. 2005). In contrast, MEI-38 localizes predominantly to the parallel microtubules, many of which interact with the chromosomes (Figure 4B). We noted earlier that Subito was present in the central spindle in mei-38 mutants, although the region of staining was expanded in some oocytes. In a complementary experiment, MEI-38 was still localized to microtubules in sub1/sub131 mutant stage 14 oocytes (Figure 4C). As opposed to wild-type oocytes, MEI-38 colocalized with all microtubules in sub mutants. This confirmed that in wild type, MEI-38 localizes to all microtubules except those that depend on Subito. Interestingly, MEI-38 spindle staining was reproducibly more intense in sub1/sub131 compared to wild-type oocyte spindles, suggesting that in a sub mutant the MEI-38–microtubule interaction was more stable or there were more MEI-38 binding sites available.
MEI-38 is a mitotic protein:
Using the P{tubP-GAL4}LL7 driver, which expresses GAL4 in most dividing cells (Lee and Luo 1999), we observed expression of P{UASP:mei-38HA}8 in mitotically dividing larval brain cells. MEI-38 protein was found to colocalize with the mitotic spindle microtubules (supplemental Figure S2). Consistent with the data from oocytes, these results suggest that MEI-38 protein associates with microtubules. Interestingly, MEI-38 was absent from the midzone of mitotic anaphase, which may be mechanistically similar to its absence from the central spindle of meiotic metaphase since both are composed of interpolar microtubules and Subito protein. A similar localization pattern has been reported for a GFP-MEI-38 fusion protein in Drosophila S2 cells (Goshima et al. 2007). These authors identified mei-38 (referred to as ssp1) because RNAi of S2 cells resulted in monastral (γ-tubulin at only one pole), monopolar, and short spindles. The two MEI-38 paralogs in the Drosophila genome that we identified did not have a spindle phenotype in their RNAi screen.
To determine if mi-381 mutants had defects in mitosis similar to those observed following RNAi in S2 cells, we examined the mitotically dividing cells of the larval brain in mei-38 mutants. We did not detect an increase in aneuploidy or precocious sister chromatid separation in the mitotically dividing brain cells of mei-38 mutant larvae (data not shown). This lack of an effect on chromosome segregation in mitotically dividing cells is consistent with the observation that mei-38 mutants are viable with no noticeable effect on viability (data not shown). However, several lines of evidence suggest MEI-38 does have a role in mitosis as well as meiosis. We observed some of the same spindle organization defects in mitotic cells noted by Goshima et al. (2007). We found examples of mei-38 mutant brain cells with only a single pole, three poles, uneven γ-tubulin staining at the poles, abnormal spindle morphology, or a gap between the pole and spindle microtubules (supplemental Figure S3). Furthermore, double-mutant studies suggest that the function of MEI-38 in mitosis may be redundant with other proteins. Among the progeny of mei-38/Y; sub131/CyO males crossed to mei-38/FM7, sub1/CyO females, the ratio of mei-38/mei-38; sub1/sub131 to mei-38/FM7; sub1/sub131 females was much lower than the expected 1:1 (2:115) and the two surviving mei-38/mei-38; sub1/sub131 females lacked oocytes. These results suggest that, in the absence of both MEI-38 and Subito, mitosis is compromised, to such a degree as to cause lethality.
DISCUSSION
Baker and Carpenter (1972) performed one of the first screens for meiotic mutants. This screen was remarkably successful, generating alleles of nod (a chromokinesin) (Zhang et al. 1990; Afshar et al. 1995), mei-41 (ATR) (Hari et al. 1995), mei-9 (XPF/Rad1) (Sekelsky et al. 1995), and mei-218 (Mcm related) (McKim et al. 1996) and mei-352/Klp3A (Page and Hawley 2005). Baker and Carpenter (1972) also recovered one allele of mei-38. We have shown that loss of mei-38 does not affect crossing over but does compromise chromosome segregation. Homologous chromosomes in mei-38 mutant females fail to segregate at the first meiotic division at a high frequency. Similarly, observations made by Goshima et al. (2007) and ourselves show that MEI-38 also has a role in mitosis. With the cloning of mei-38, essentially all of the genes identified by Baker and Carpenter (1972) have now been cloned.
MEI-38 is a microtubule-associated protein required for chromosome organization but not a bipolar spindle:
mei-38 encodes a protein with an interesting localization pattern. During meiosis and mitosis, MEI-38 colocalizes with microtubules in a pattern that is complementary to Subito. These observations are similar to Goshima et al. (2007) who tagged mei-38 at the N terminus with GFP and observed localization to most spindle microtubules in S2 cells. While Subito localizes to the central spindle, presumably the region of microtubules in antiparallel overlap, MEI-38 appears to be excluded from this region. MEI-38 has a preference for the parallel microtubules that include those that interact with the chromosomes, the kinetochore microtubules. These kinetochore microtubules stain with reduced intensity in mei-38 mutants, suggesting that MEI-38 localization is required for the stability of the kinetochore microtubules.
Several genes thought to be involved in organizing the meiotic spindle have been identified because the mutants cause tripolar or frayed meiotic spindles (Doubilet and McKim 2007). The microtubule stability defects in mei-38 mutants apparently do not dramatically affect bipolar spindle organization since tripolar spindles were not observed. The absence of tripolar spindles in mei-38 mutants can be explained by a model which emphasizes a role for the central spindle in acentrosomal spindle assembly (Jang et al. 2005). The meiotic spindle of metaphase I-arrested oocytes has a prominent region of interpolar microtubules in the center of the spindle. On the basis of the phenotype of mutants that affect this structure (e.g., subito), it has been proposed that the central spindle is required to organize a stable bipolar spindle. In contrast, kinetochore microtubules may not be essential for bipolar spindle formation (Doubilet and McKim 2007; Jang et al. 2007), which could explain why MEI-38 and the kinetochore microtubules it affects are not required to establish a bipolar spindle.
The relationship of MEI-38 to achiasmate chromosome segregation:
Drosophila has two systems of chromosome segregation that are defined by different classes of mutants. In crossover defective mutants, the absence of chiasmata causes nondisjunction. However, the achiasmate segregation system functions in these mutants as shown by the observation that four achiasmate chromosomes will segregate 2:2 regardless of homology (Baker and Hall 1976). In contrast, mutations in nod cause achiasmate chromosomes to segregate randomly, although chiasmate chromosomes are not affected. At the intersection of these two classes are genes that affect both systems and that are typically involved in spindle structure and function. An example is subito, which is required for normal spindle structure and the segregation of both chiasmate and achiasmate chromosomes (Giunta et al. 2002).
Several lines of evidence suggest that mei-38 is required for achiasmate chromosome segregation. First, the frequency of X and autosome nondisjunction in mei-38 mutants is increased when crossing over on just these chromosomes is reduced. Second, secondary nondisjunction, the situation in XXY females where the two X chromosomes segregate from the Y (Bridges 1916; Cooper 1948), occurs with less efficiency in mei-38 females. These results suggest achiasmate segregation is defective in mei-38 mutants; but there is a caveat. Given that the small fourth chromosomes always segregate by the achiasmate system, the larger chromosomes (X and the autosomes) must be more sensitive to mei-38. As described in results, the effects of mei-38 on fourth chromosome nondisjunction could be indirect and a secondary consequence of the misbehavior of the larger chromosomes.
Carpenter (1973) argued that achiasmate segregation was a two-step process. For example, in XXY females the first step is orientation which commits both X chromosomes to segregate to the same pole. The second step is ensuring the disjunction of the one Y chromosome from the two X chromosomes. Expanding on this work, Xiang and Hawley (2006) suggested that XXY pairing is established in early prophase, which can lead to XX from Y segregation if the X chromosomes are achiasmatic. Coorientation by a chiasma, however, acts to dissolve the association of the two X chromosome centromere regions with the Y before bipolar spindle formation. mei-38 is probably defective in the second step. Since nondisjunction is increased in XXY relative to XX mei-38 females, the Y can cause both X chromosomes to orient toward the same pole, but disjunction from the Y is defective. Other genes required for the second but not the first step of this process include nod (Carpenter 1973).
The first commitment step is defined by the ald mutation, which affects the pairing of chromosomes in the achiasmate system (O'Tousa 1982). Ald is a centromere protein and the ortholog of the human Mps1 checkpoint protein (Gilliland et al. 2005). Interestingly, ald and mei-38 mutants have some similarities in their mutant phenotypes. In both mutants, the most severe cytological defects are in maintaining karyosome organization while genetic data show both mutants cause nondisjunction of chiasmate and achiasmate bivalents. Live imaging of ald mutant oocytes indicates that metaphase arrest is not maintained and the chromosomes move precociously toward the poles (Gilliland et al. 2007). Our observations also suggest that the mei-38 mutant karyosome is more dynamic than wild type. There is an important difference, however, since ald does not disrupt secondary nondisjunction patterns while mei-38 does. In addition, the frequency of the monopolar spindle phenotype was dramatically increased in the mei-38; ald double mutant and there was a synergistic effect on nondisjunction. These results are consistent with Carpenter's model for the achiasmate system. While both genes contribute to maintaining the chromosomes in the center of the spindle, ald functions in the first step (commitment and orientation) and mei-38 functions in the second step (disjunction and segregation). A checkpoint model could also be relevant and explain the synergistic effect of the double mutant. mei-38 mutants may cause defects in chromosome organization, which can be corrected in a process that depends on the checkpoint activity of the ALD protein.
The role of MEI-38 in chiasmate chromosome segregation:
To extend the two-step model for achiasmate segregation to chiasmate segregation, we suggest there are two possible reasons for the nondisjunction in mei-38 mutants. The first is that homologous centromeres fail to orient. The second is that properly oriented homologs fail to move to opposite poles. The most striking cytological phenotypes of mei-38 mutants are weakened kinetochore microtubule staining, a disorganized or mispositioned karyosome, and the irregular organization of centromeres. All these phenotypes are consistent with loss of MEI-38 affecting the function or stability of the microtubules that interact with the chromosomes. While defects in orienting homologous centromeres cannot be ruled out, we propose that a defect in kinetochore microtubules in mei-38 mutants, such as in their stability or structure, leads to errors in kinetochore attachment and bivalent organization. This could include instability of microtubules leading to disassembly and reassembly of spindle poles. To be consistent with the conclusions from the examination of achiasmate segregation, homologs may orient correctly in mei-38 mutants but then fail to segregate correctly because of the failure to maintain the attachment of the kinetochores to the microtubules. The disorganized MEI-S332 staining could be due to the disruption of kinetochore microtubules, allowing the spreading of the centromeres from their normal tight clustering. This model could also explain the presence of monopolar spindles in mei-38 mutants, which could occur because the balance of forces that keep the chromosomes in the middle of the spindle are destabilized, sometimes allowing movement of the karyosome toward one pole.
The mutant phenotypes of mei-38 are most severe at meiosis I, although it is clear that MEI-38 also contributes to mitosis. This is similar to several other mutants, such as sub (Cesario et al. 2006) and ncd (Endow et al. 1994), which have their strongest effects in meiosis but also have a role in mitosis. Surprisingly, defects in chromosome segregation in these mutants during female meiosis II and male meiosis have not been observed. The likely explanation is twofold. First, in most cases only genetic assays have been performed, which may not be sensitive to mild disruptions of spindle structure. Second, meiosis I might be the most critical time for these functions. In this regard, it appears that establishing and maintaining bipolar orientation of the homologs, rather than building a bipolar spindle, might be the most difficult aspect of performing meiosis without centrosomes. Indeed, chromosome orientation may occur in a process that is distinct from bipolar spindle formation, and kinetochore microtubule interactions may be dispensable for bipolar spindle assembly in acentrosomal meiotic cells.
Acknowledgments
We are grateful to Li Nguyen for technical assistance and Jeff Cesario and Sarah Radford for comments on the manuscript. K. McKim also recognizes Larry Sandler, Adelaide Carpenter, and Bruce Baker, who had the foresight to perform genetic screens for meiotic mutants in Drosophila. Some stocks used in this study were obtained from the Bloomington Stock Center. This work was supported by a grant from the National Institutes of Health (GM 067142) to K.M.
References
- Afshar, K., N. R. Barton, R. S. Hawley and L. S. B. Goldstein, 1995. DNA binding and meiotic chromosomal localization of the Drosophila Nod kinesin-like protein. Cell 81 129–138. [DOI] [PubMed] [Google Scholar]
- Baker, B. S., and A. T. C. Carpenter, 1972. Genetic analysis of sex chromosomal meiotic mutants in Drosophila melanogaster. Genetics 71 255–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, B. S., and J. C. Hall, 1976. Meiotic mutants: genetic control of meiotic recombination and chromosome segregation, pp. 351–434 in The Genetics and Biology of Drosophila, Vol. 1a, edited by M. Ashburner and E. Novitski. Academic Press, New York.
- Bodenmiller, B., J. Malmstrom, B. Gerrits, D. Campbell, H. Lam et al., 2007. PhosphoPep–a phosphoproteome resource for systems biology research in Drosophila Kc167 cells. Mol. Syst Biol. 3 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges, C. B., 1916. Non-disjunction as proof of the chromosome theory of heredity. Genetics 1 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, A. T. C., 1973. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73 393–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, A. T. C., and L. Sandler, 1974. On recombination-defective meiotic mutants in Drosophila melanogaster. Genetics 76 453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario, J. M., J. K. Jang, B. Redding, N. Shah, T. Rahman et al., 2006. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J. Cell Sci. 119 4770–4780. [DOI] [PubMed] [Google Scholar]
- Cooper, K. W., 1948. A new theory of secondary non-disjunction in female Drosophila melanogaster. Proc Natl Acad Sci USA 34 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, C. F., and H. Ohkura, 2001. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3 637–642. [DOI] [PubMed] [Google Scholar]
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 85 135–146. [DOI] [PubMed] [Google Scholar]
- Doubilet, S., and K. S. McKim, 2007. Spindle assembly in the oocytes of mouse and Drosophila–similar solutions to a problem. Chromosome Res. 15 681–696. [DOI] [PubMed] [Google Scholar]
- Endow, S. A., R. Chandra, D. J. Komma, A. H. Yamamoto and E. D. Salmon, 1994. Mutants of the Drosophila ncd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. J. Cell Sci. 107 859–867. [DOI] [PubMed] [Google Scholar]
- Gelbart, W. M., M. Crosby, B. Matthews, W. P. Rindone, J. Chillemi et al., 1997. FlyBase: a Drosophila database. The FlyBase consortium. Nucleic Acids Res. 25 63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland, W. D., S. M. Wayson and R. S. Hawley, 2005. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr. Biol. 15 672–677. [DOI] [PubMed] [Google Scholar]
- Gilliland, W. D., S. E. Hughes, J. L. Cotitta, S. Takeo, Y. Xiang et al., 2007. The multiple roles of Mps1 in Drosophila female meiosis. PLoS Genet. 3 e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta, K. L., J. K. Jang, E. M. Manheim, G. Subramanian and K. S. McKim, 2002. subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 160 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., R. Wollman, S. S. Goodwin, N. Zhang, J. M. Scholey et al., 2007. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell, R. F., 1976. Distributive pairing, pp. 436–486 in The Genetics and Biology of Drosophila, Vol. 1a, edited by M. Ashburner and E. Novitski. Academic Press, New York.
- Hari, K. L., A. Santerre, J. J. Sekelsky, K. S. McKim, J. B. Boyd et al., 1995. The mei-41 gene of D. melanogaster is a structural and function homolog of the human ataxia telangiectasia gene. Cell 82 815–821. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., and W. E. Theurkauf, 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 9 310–317. [DOI] [PubMed] [Google Scholar]
- Jang, J. K., T. Rahman and K. S. McKim, 2005. The kinesin-like protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol. Biol. Cell 16 4684–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J. K., T. Rahman, V. S. Kober, J. Cesario and K. S. McKim, 2007. Misregulation of the kinesin-like protein Subito induces meiotic spindle formation in the absence of chromosomes and centrosomes. Genetics 177 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti, E., and I. Vernos, 2001. The mitotic spindle: a self-made machine. Science 294 543–547. [DOI] [PubMed] [Google Scholar]
- Lee, T., and L. Luo, 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22 451–461. [DOI] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Matthies, H. J., H. B. McDonald, L. S. Goldstein and W. E. Theurkauf, 1996. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J Cell Biol 134 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, K. S., and R. S. Hawley, 1995. Chromosomal control of meiotic cell division. Science 270 1595–1601. [DOI] [PubMed] [Google Scholar]
- McKim, K. S., J. K. Jang, W. E. Theurkauf and R. S. Hawley, 1993. Mechanical basis of meiotic metaphase arrest. Nature 362 364–366. [DOI] [PubMed] [Google Scholar]
- McKim, K. S., J. B. Dahmus and R. S. Hawley, 1996. Cloning of the Drosophila melanogaster meiotic recombination gene mei-218: a genetic and molecular analysis of interval 15E. Genetics 144 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, K. S., and A. Hayashi-Hagihara, 1998. mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev. 12 2932–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, K. S., J. K. Jang and E. A. Manheim, 2002. Meiotic recombination and chromosome segregation in Drosophila females. Annu. Rev. Genet. 36 205–232. [DOI] [PubMed] [Google Scholar]
- Moore, D. P., A. W. Page, T. T. Tang, A. W. Kerrebrock and T. L. Orr-Weaver, 1998. The cohesion protein MEI-S332 localizes to condensed meiotic and mitotic centromeres until sister chromatids separate. J. Cell Biol. 140 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa, J., 1982. Meiotic chromosome behavior influenced by mutation-altered disjunction in Drosophila melanogaster females. Genetics 102 503–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, S. L., and R. S. Hawley, 2005. The Drosophila meiotic mutant mei-352 is an allele of klp3A and reveals a role for a kinesin-like protein in crossover distribution. Genetics 170 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36 288–292. [DOI] [PubMed] [Google Scholar]
- Rasooly, R. S., C. M. New, P. Zhang, R. S. Hawley and B. S. Baker, 1991. The lethal(1)TW-6cs mutation of Drosophila melanogaster is a dominant antimorphic allele of nod and is associated with a single base change in the putative ATP-binding domain. Genetics 129 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth, P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78 113–118. [DOI] [PubMed] [Google Scholar]
- Sekelsky, J. J., K. S. McKim, G. M. Chin and R. S. Hawley, 1995. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics 141 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W. E., and R. S. Hawley, 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren, M., A. L. Williamson and R. Lehmann, 1998. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8 243–246. [DOI] [PubMed] [Google Scholar]
- Walczak, C. E., I. Vernos, T. J. Mitchison, E. Karsenti and R. Heald, 1998. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8 903–913. [DOI] [PubMed] [Google Scholar]
- Whyte, W. L., H. A. Irick, T. Arbel, G. Yasuda, R. L. French et al., 1993. The genetic analysis of achiasmate segregation in Drosophila melanogaster. III. The wild-type product of the Axs gene is required for the meiotic segregation of achiasmate homologs. Genetics 134 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y., and R. S. Hawley, 2006. The mechanism of secondary nondisjunction in Drosophila melanogaster females. Genetics 174 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., and R. S. Hawley, 1990. The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics 125 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., B. A. Knowles, L. S. B. Goldstein and R. S. Hawley, 1990. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell 63 1053–1062. [DOI] [PubMed] [Google Scholar]
- Zitron, A. E., and R. S. Hawley, 1989. The genetic analysis of distributive segregation in Drosophila melanogaster. I. Isolation and characterization of Aberrant X segregation (Axs), a mutation defective in chromosome partner choice. Genetics 122 801–821. [DOI] [PMC free article] [PubMed] [Google Scholar]