Abstract
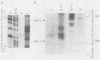
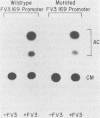
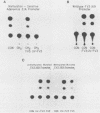
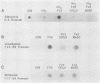
Methylation of critical sites within the promoter region of eucaryotic genes has been shown to inhibit transcription by RNA polymerase II. However, although the large DNA virus frog virus 3 (FV3) has a highly methylated genome, it uses host RNA polymerase II for at least the immediate-early stage of transcription. We have previously shown that an FV3-induced trans-acting protein allows transcription from adenovirus promoters inactivated by methylation. Since FV3 immediate-early genes are transcribed in the absence of de novo protein synthesis, it appears that the virus-induced trans-acting protein that allows transcription from methylated templates is not required for transcription of the immediate-early FV3 genes, possibly because they are not methylated in critical regulatory sequences. In this study, we used site-directed mutagenesis to alter the three CpG dinucleotide sequences in the promoter region of an immediate-early FV3 gene and thereby created sites recognized by bacterial methylases. Transient-expression assays demonstrated that neither the mutations nor methylation of the mutated sites inhibited transcription from the FV3 promoter in FV3-infected cells. These findings support the hypothesis that the immediate-early genes of FV3 do not contain methylatable sites in regions critical for transcription. The function of the virus-induced trans-acting protein that can override the inhibitory effect of methylation may therefore be to facilitate the transcription of methylated delayed-early or late FV3 genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Goorha R. Frog virus 3 requires RNA polymerase II for its replication. J Virol. 1981 Jan;37(1):496–499. doi: 10.1128/jvi.37.1.496-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Granoff A., Willis D. B., Murti K. G. The role of DNA methylation in virus replication: inhibition of frog virus 3 replication by 5-azacytidine. Virology. 1984 Oct 15;138(1):94–102. doi: 10.1016/0042-6822(84)90150-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., Naegele R. F. Nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus (PCDV). Virology. 1970 Jan;40(1):170–174. doi: 10.1016/0042-6822(70)90390-9. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Yisraeli J., Cedar H. Effect of regional DNA methylation on gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2560–2564. doi: 10.1073/pnas.82.9.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel D., Doerfler W. N6-methyldeoxyadenosine residues at specific sites decrease the activity of the E1A promoter of adenovirus type 12 DNA. J Mol Biol. 1986 May 20;189(2):371–375. doi: 10.1016/0022-2836(86)90518-8. [DOI] [PubMed] [Google Scholar]
- Kruczek I., Doerfler W. Expression of the chloramphenicol acetyltransferase gene in mammalian cells under the control of adenovirus type 12 promoters: effect of promoter methylation on gene expression. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7586–7590. doi: 10.1073/pnas.80.24.7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. P., Aubertin A. M., Kirn A. Expression of frog virus 3 early genes after ultraviolet irradiation. Virology. 1982 Oct 30;122(2):402–410. doi: 10.1016/0042-6822(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Cartwright E. M., Brownlee G. G., Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. II. The GC-rich region. Cell. 1978 Apr;13(4):717–725. doi: 10.1016/0092-8674(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Naegele R. F., Granoff A. Viruses and renal carcinoma of Rana pipiens. XI. Isolation of frog virus 3 temperature-sensitive mutants; complementation and genetic recombination. Virology. 1971 May;44(2):286–295. doi: 10.1016/0042-6822(71)90260-1. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. I., Maxam A. M. Detection of 5-methylcytosine in DNA sequences. Nucleic Acids Res. 1978 May;5(5):1479–1485. doi: 10.1093/nar/5.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Stein R., Sciaky-Gallili N., Razin A., Cedar H. Pattern of methylation of two genes coding for housekeeping functions. Proc Natl Acad Sci U S A. 1983 May;80(9):2422–2426. doi: 10.1073/pnas.80.9.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. P., Granoff A., Willis D. B. Trans-activation of a methylated adenovirus promoter by a frog virus 3 protein. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7688–7692. doi: 10.1073/pnas.83.20.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B. DNA sequences required for trans-activation of an immediate-early frog virus 3 gene. Virology. 1987 Nov;161(1):1–7. doi: 10.1016/0042-6822(87)90164-4. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. DNA methyltransferase induced by frog virus 3. J Virol. 1984 Jan;49(1):86–91. doi: 10.1128/jvi.49.1.86-91.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XI. A ts mutant of frog virus 3 that is defective in late transcription. Virology. 1979 Oct 30;98(2):328–335. doi: 10.1016/0042-6822(79)90556-7. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. Nongenetic reactivation of frog virus 3 DNA. Virology. 1979 Oct 30;98(2):476–479. doi: 10.1016/0042-6822(79)90572-5. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Frog virus 3 DNA is heavily methylated at CpG sequences. Virology. 1980 Nov;107(1):250–257. doi: 10.1016/0042-6822(80)90290-1. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IX. Two temporal classes of early viral RNA. Virology. 1978 May 15;86(2):443–453. doi: 10.1016/0042-6822(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. trans activation of an immediate-early frog virus 3 promoter by a virion protein. J Virol. 1985 Nov;56(2):495–501. doi: 10.1128/jvi.56.2.495-501.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D., Foglesong D., Granoff A. Nucleotide sequence of an immediate-early frog virus 3 gene. J Virol. 1984 Dec;52(3):905–912. doi: 10.1128/jvi.52.3.905-912.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]