Abstract
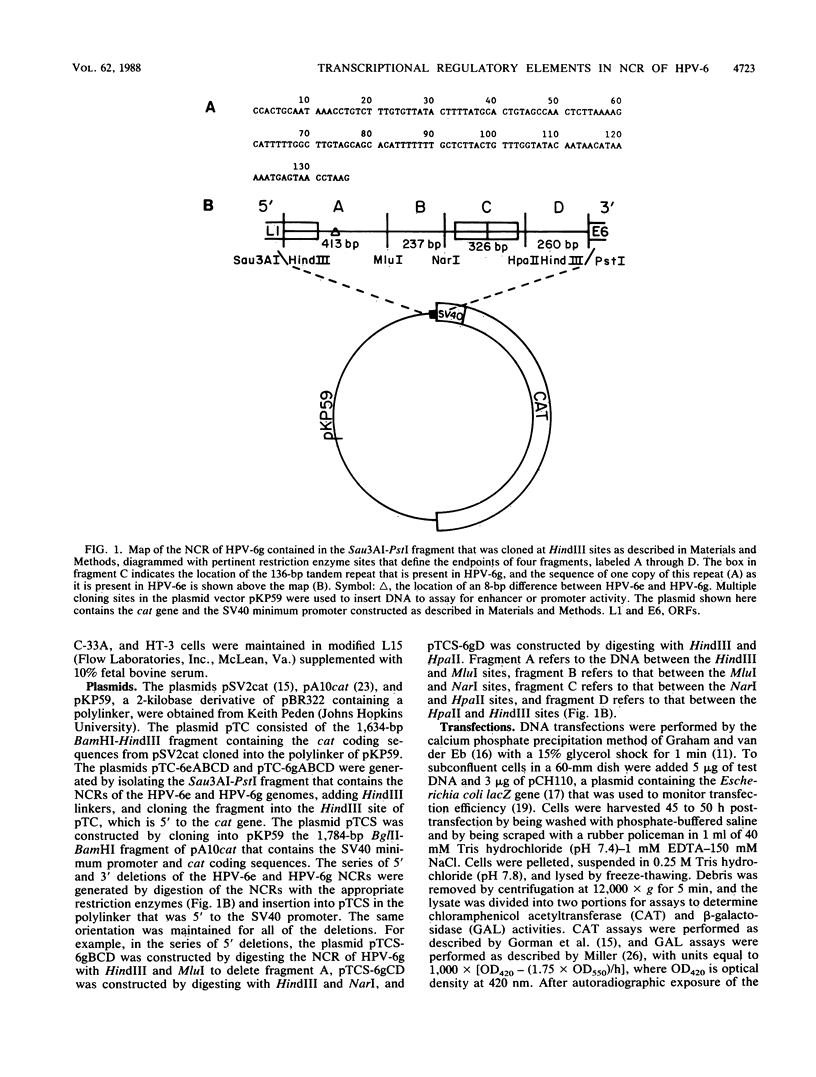
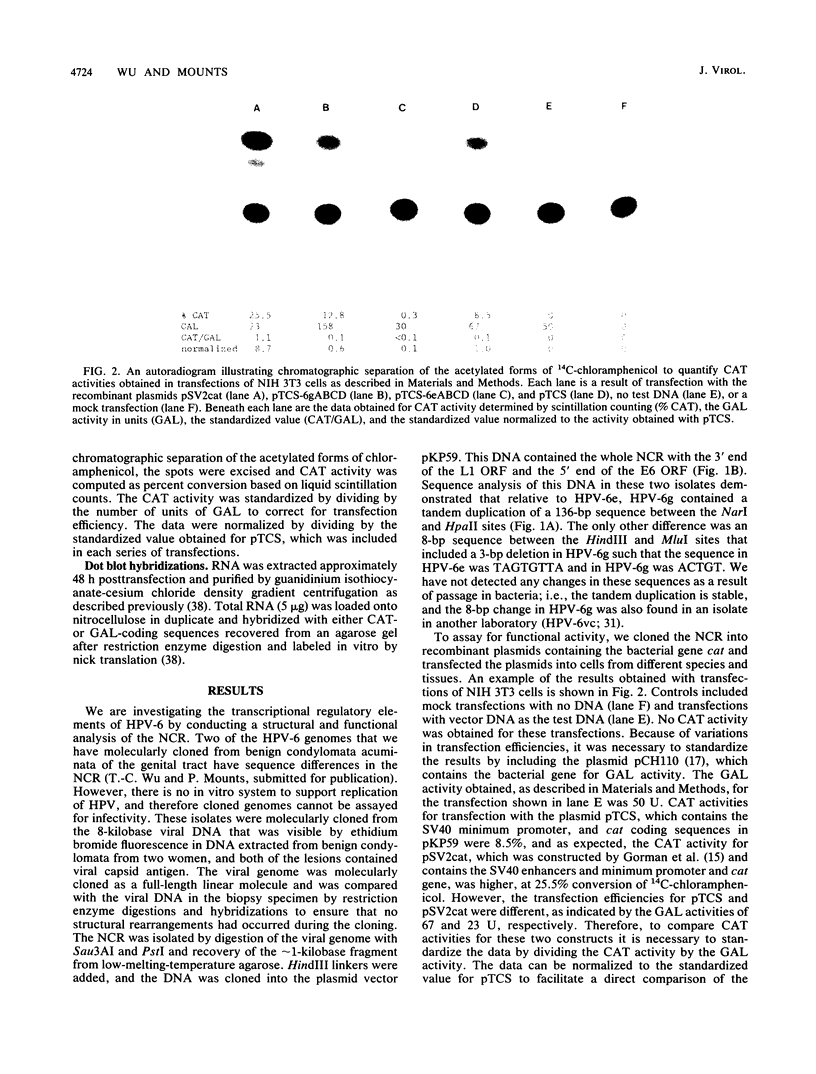
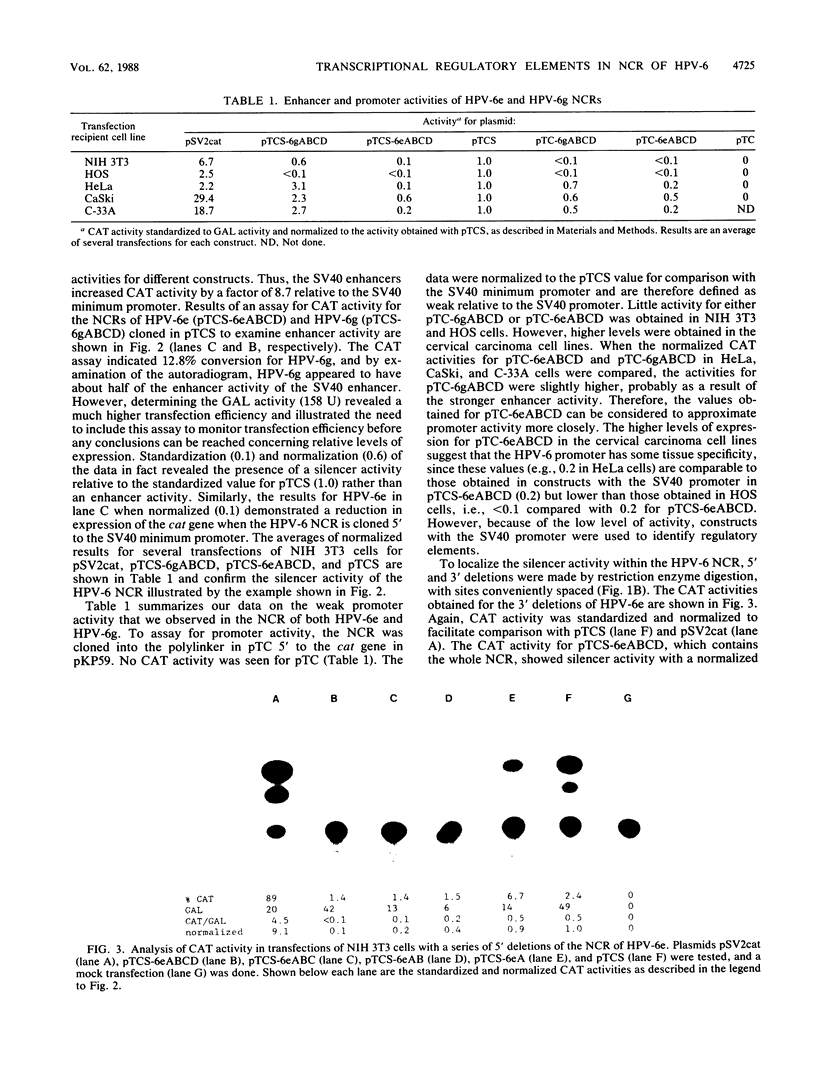
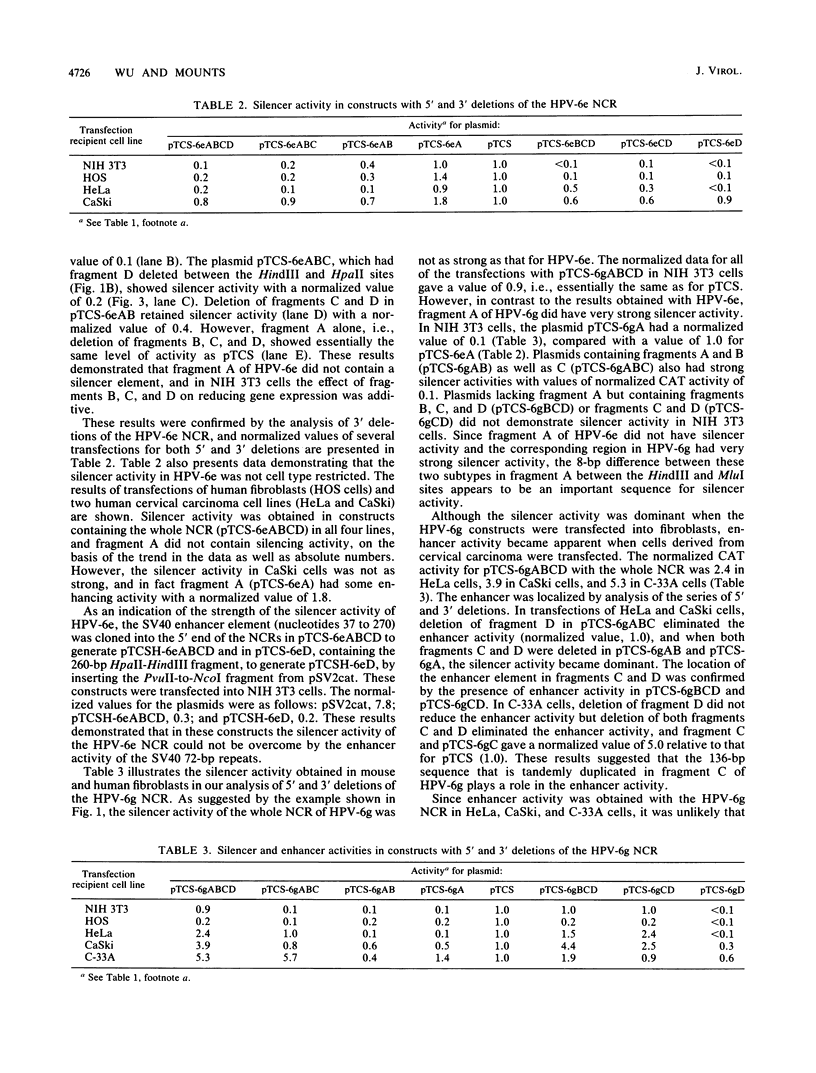
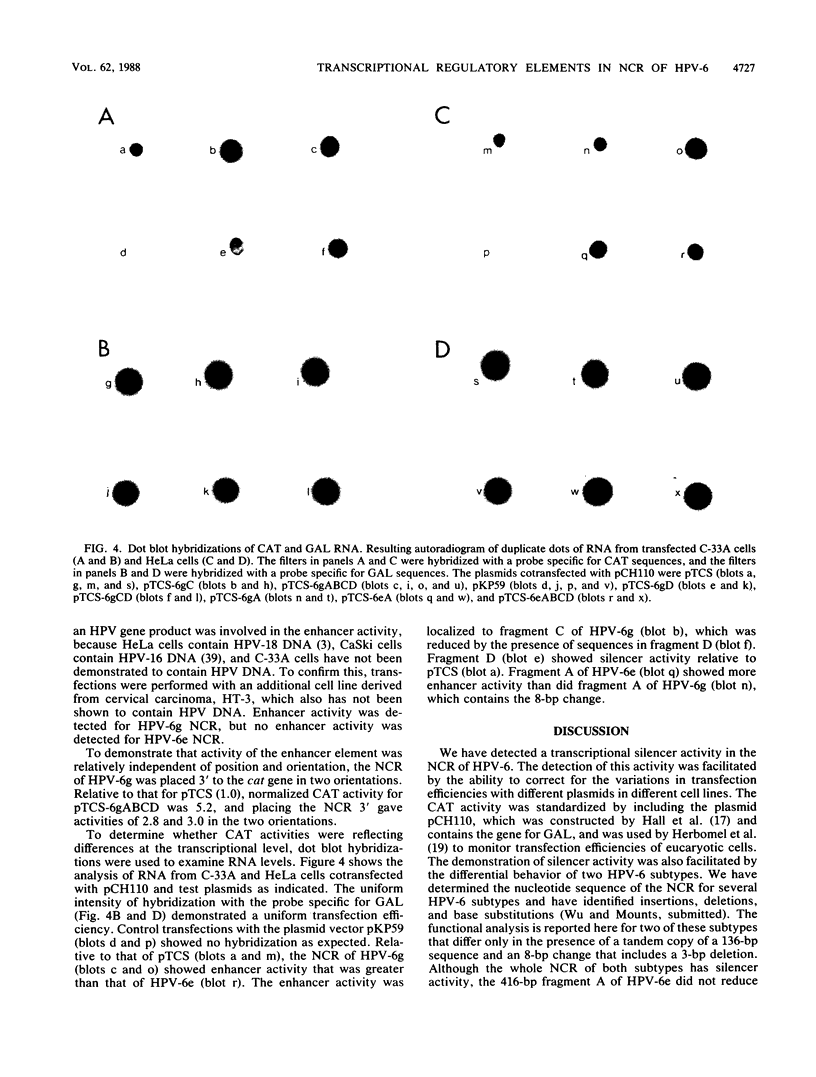
We have identified three elements in the noncoding region of human papillomavirus type 6 (HPV-6) that regulate transcription when assayed in recombinant plasmids containing the bacterial gene for chloramphenicol acetyltransferase. One was a silencer that reduced expression in both a species- and tissue-dependent manner. The second was an enhancer element that was tissue specific. The third was a weak promoter that showed some tissue specificity. These elements have been localized within the noncoding region by analysis of 5'-to-3' and 3'-to-5' deletions with two HPV-6 subtypes, HPV-6e and HPV-6g. HPV-6g differs from HPV-6e by the presence of an additional copy in tandem of a 136-base-pair (bp) sequence and by an 8-bp sequence containing a 3-bp deletion. Silencer activity, assayed in plasmids with the simian virus 40 minimum promoter which were transfected into NIH 3T3 cells, could not be overcome by the enhancer activity of the simian virus 40 72-bp repeats. The 413-bp fragment of A of HPV-6g showed silencer activity, while the corresponding HPV-6e fragment containing the 8-bp change did not. Enhancer activity of HPV-6g was localized to fragment C of 326 bp which contains the 136-bp repeat. Dot blot hybridizations reflected relative chloramphenicol acetyltransferase activities and demonstrated enhancer and silencer activities at the RNA level. Analysis of the interaction of these activities in naturally occurring variants should provide information on tissue specificity and regulation of gene expression of HPVs and may provide information on the mechanism of action of transcriptional regulatory elements in eucaryotic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson A. L., Brandsma J., Steinberg B., Winkler B. Verrucous carcinoma of the larynx. Possible human papillomavirus etiology. Arch Otolaryngol. 1985 Nov;111(11):709–715. doi: 10.1001/archotol.1985.00800130041003. [DOI] [PubMed] [Google Scholar]
- Bergstrom L. Laryngeal papillomatosis: recurrence after 33-year remission. Laryngoscope. 1982 Oct;92(10 Pt 1):1160–1163. [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Cripe T. P., Haugen T. H., Turk J. P., Tabatabai F., Schmid P. G., 3rd, Dürst M., Gissmann L., Roman A., Turek L. P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987 Dec 1;6(12):3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Giri I., Thierry F., Yaniv M. Papillomavirus genomes: sequences and consequences. J Invest Dermatol. 1984 Jul;83(1 Suppl):7s–11s. doi: 10.1111/1523-1747.ep12281115. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Bernard H. U., Seedorf K., Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987 Dec 1;6(12):3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hawley-Nelson P., Androphy E. J., Lowy D. R., Schiller J. T. The specific DNA recognition sequence of the bovine papillomavirus E2 protein is an E2-dependent enhancer. EMBO J. 1988 Feb;7(2):525–531. doi: 10.1002/j.1460-2075.1988.tb02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Broker T. R., Chow L. T. Enhancers and trans-acting E2 transcriptional factors of papillomaviruses. J Virol. 1987 Aug;61(8):2599–2606. doi: 10.1128/jvi.61.8.2599-2606.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- McBride A. A., Schlegel R., Howley P. M. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988 Feb;7(2):533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Mounts P., Kashima H. Association of human papillomavirus subtype and clinical course in respiratory papillomatosis. Laryngoscope. 1984 Jan;94(1):28–33. doi: 10.1002/lary.5540940106. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Howley P. M. Transcriptional trans-activation by the human papillomavirus type 16 E2 gene product. J Virol. 1987 May;61(5):1630–1638. doi: 10.1128/jvi.61.5.1630-1638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. F., Groff D. E., Chirikjian J. G., Lancaster W. D. Isolation and characterization of a novel human papillomavirus type 6 DNA from an invasive vulvar carcinoma. J Virol. 1986 Jan;57(1):353–356. doi: 10.1128/jvi.57.1.353-356.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. F., Lancaster W. D., Han P., Lopez C. The noncoding region of HPV-6vc contains two distinct transcriptional enhancing elements. Virology. 1986 Dec;155(2):545–556. doi: 10.1016/0042-6822(86)90215-1. [DOI] [PubMed] [Google Scholar]
- Steinberg B. M., Topp W. C., Schneider P. S., Abramson A. L. Laryngeal papillomavirus infection during clinical remission. N Engl J Med. 1983 May 26;308(21):1261–1264. doi: 10.1056/NEJM198305263082104. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Shamanski F. L., Blair D., Vande Woude G. Human recipient cell for oncogene transfection studies. Mol Cell Biol. 1987 Mar;7(3):1280–1284. doi: 10.1128/mcb.7.3.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Heard J. M., Dartmann K., Yaniv M. Characterization of a transcriptional promoter of human papillomavirus 18 and modulation of its expression by simian virus 40 and adenovirus early antigens. J Virol. 1987 Jan;61(1):134–142. doi: 10.1128/jvi.61.1.134-142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Mounts P. Transcriptional activity of human papillomavirus type 6 in respiratory tract papillomata. J Gen Virol. 1988 Jul;69(Pt 7):1671–1682. doi: 10.1099/0022-1317-69-7-1671. [DOI] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M., Gissmann L., zur Hausen H. Molecular cloning of viral DNA from human genital warts. J Virol. 1981 Dec;40(3):932–935. doi: 10.1128/jvi.40.3.932-935.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]