Abstract
Until recently, gait was generally viewed as a largely automated motor task, requiring minimal higher-level cognitive input. Increasing evidence, however, links alterations in executive function and attention to gait disturbances. This review discusses the role of executive function and of attention in healthy walking and gait disorders while summarizing the relevant, recent literature. We describe the variety of gait disorders that may be associated with different aspects of executive function, and discuss the changes occurring in executive function as a result of aging and disease as well the potential impact of these changes on gait. The attentional demands of gait are often tested using dual tasking methodologies. Relevant studies in healthy adults and patients are presented, as are the possible mechanisms responsible for the deterioration of gait during dual tasking. Lastly, we suggest how assessments of executive function and attention could be applied in the clinical setting as part of the process of identifying and understanding gait disorders and fall risk.
Keywords: gait, executive function, attention, Parkinson’s disease, Alzheimer’s disease, aging
INTRODUCTION
The relationship between higher-level cognitive function and gait disturbances has received considerable attention in recent years. Gait is no longer considered as merely an automated motor activity that utilizes minimal higher-level cognitive input. Instead, the multi-faceted neuropsychological influences on walking and the interactions between the control of mobility and related behaviors are increasingly appreciated. This is manifest in part by an individual’s awareness of a destination, the ability to appropriately control the limb movements that produce gait, and the ability to navigate within often complex environs in order to successfully reach the desired location. Studies on cognitive function and gait now include many areas of research, ranging from physiology and biomechanics to brain mapping, physics and neuropsychology. For example, imaging studies have demonstrated frontal and parietal activity during locomotion1, 2. This review covers the importance and relevance of two specific cognitive functions, executive function and attention, to the performance of gait during normal walking, as well as in aging and in pathological conditions. We review the physiology underlying these cognitive processes, describe the clinical findings and consequences of these relationships and discuss the physiological mechanisms that are brought into play. Finally, we summarize the implications of these associations for the daily lives of individuals affected by impaired function of one or more or these elements and provide suggestions for applying these insights to augment the diagnosis of gait disorders in the clinic.
This review article is based on a systematic literature search for reviews and trials reported in English in the electronic databases of Medline and Psychinfo up to April, 2007. Relevant studies were identified by first searching using the combinations of the following key words: gait (or walking), cognition (or cognitive), dual task, divided attention, executive function, and aging. We also examined “related articles” and relevant cited works.
EXECUTIVE FUNCTION
Executive function (EF) refers to a variety of higher cognitive processes that use and modify information from many cortical sensory systems in the anterior and posterior brain regions to modulate and produce behavior3-6. These integrative functions include both cognitive and behavioral components that are necessary for effective, goal-directed actions and for the control of attentional resources which are at the basis of the ability to manage independent activities of daily living5, 7, 8.
Lezak divided EF into four major components: volition, planning, purposive action and effective performance (action monitoring)5. Others also explicitly include cognitive inhibition as an EF component7-9. Impairment of one or more of these components of EF may impact one’s ability to walk efficiently and safely. Poor self-awareness of limitations, an aspect of volition, might result in an increased risk of falling10. Impaired planning skills could result in getting lost or choices that produce inefficient pathways or unnecessary effort to arrive at a destination. Table 1 summarizes the main components of EF and their potential effects on gait.
Table 1.
Executive function (EF) components and their possible effects on gait disorders: A theoretical view
| EF Component | Description of Component | Effect on Gait (when this component is impaired) |
|---|---|---|
| Volition | The capacity for intentional behavior, for formulation of a goal or intention, and for initiation of activity5. | Loss of mobility due to reduced motivation. Decreased inner drive to move. May be mistaken for bradykinesia. |
| Self-awareness | The ability to place oneself (psychologically and physically) in the physical environment and the on-going situation5. | Careless walking: Poor or inaccurate estimation of one’s physical limitations may lead to inappropriate evaluation of environmental hazards and increase the risk of falling. |
| Planning | “The identification and organization of the steps and elements needed to carry out an intention”5. This may rely on other cognitive skills such as the ability to conceptualize changes from present circumstances, conceiving alternatives, weighing and making choices, controlling impulses and using memory5, 14. | Deficits in decision-making abilities while walking in a complex environment. Inefficient, faulty or even risky choices. Losing the way or wasting time or effort to arrive at the desired destination. |
| Response inhibition | Allows one to ignore irrelevant sensory inputs, overcome primary reflexes, and filter out distractions in order to solve problems and respond discriminatively to important features in the environment129,14. This ability is closely related to selective attention. | When walking in complex, everyday environments, response inhibition allows one to focus on gait and give it the appropriate attention and priority, despite numerous distractions. |
| Response monitoring | Enables one to compare ongoing actions with an internal plan and to detect errors130,129. This skill facilitates decision making and the flexible adjustment of behavior129. | This EF component may also be important for walking in complex environments. Demented patients may walk too fast, increasing their risk of falls, because of reduced inhibition 10. Performance on classical tests of response inhibition and response monitoring, the Stroop and the Go No-Go tests, have been associated with gait variability27,26. |
| Attention / Dual Tasking | The ability to appropriately allocate attention among tasks that are performed simultaneously. | See Tables 2a and 2b. |
The Normal Anatomy and Physiology of Executive Function
EF is traditionally associated with the frontal lobes and related brain networks. The area of the prefrontal lobe and, in particular, the dorsolateral prefrontal cortex (DLPFC, Brodmann’s area 9) and the cingulate cortex (e.g., the anterior cingulate: ACC, Brodmann’s areas 24,32) have been related to the cognitive aspects of EF5, 7-9 . Patients with frontal damage frequently display impairments in cognitive functions attributed to EF, although activation of other brain areas, such as the parietal lobe, association areas and subcortical areas, including the limbic areas, are also attributed to EF5, 7-9, 11. In general, the anterior parts of the frontal lobes are involved with aspects of self-regulation, such as inhibition and self awareness, whereas the dorsal parts are involved with reasoning processes.
Neuroimaging studies attempting to localize the activity of EF report inconsistent findings. Collete et al. reviewed investigations that explored the neural substrates of EF, focusing on specific aspects such as response inhibition or dual task coordination11. They found that different EF tasks not only activated different frontal and parietal areas, they also activated other areas of the brain. This supports the hypothesis that EF is based on a network of anterior and posterior cerebral areas and is not localized only to the frontal cortex11. Stuss and Alexander argued that the common use of the term “frontal function” (or “frontal syndrome”) as a synonym for EF is not accurate, because of the many methodological problems in the studies that tried to explore associations between the two12. In support of that claim, a meta-analysis found that three classical tests of EF (i.e., the Wisconsin Card Sorting, the Stroop, and the verbal fluency tests) were sensitive to frontal lobe damage, but other areas of the brain could also lead to poor performance in these tests13. The authors suggested “that the frontal lobes participate to a greater extent than other areas of the brain in functions considered to be executive”13. Thus, patients who do not have a frontal lesion might demonstrate impairments in components of EF and display “cognitive” gait disorders. In summary, the frontal lobes and closely related networks play a critical role in EF, but other areas also contribute to this cognitive domain.
EF and the Aging Process
The frontal lobes are apparently highly susceptible to age-associated changes8, 14, 15. These include lesions of diffused white matter which might affect fronto-striatal circuits and cause, among other things, impairment in EF16. A meta-analysis by Gunning-Dixon and Raz revealed that white matter hyper-intensities on magnetic resonance imaging (MRI) were associated with a decline of processing speed, EF, and memory, but not with the level of general intelligence17. Other studies support these findings and emphasize the vulnerability of EF to white matter lesions18-20. Additional brain pathology, such as loss of dendritic branching (especially in the prefrontal cortex)21 and changes in the gray matter (mainly tissue loss) are also associated with decline in performance on EF tests16, 17, 22. Age-associated decline in dopaminergic activity in the frontal areas is also related to poorer performance on executive tasks19, 21, 22. As mentioned above, it is, however, important to keep in mind that EF changes may be the result of alterations in areas other than just the frontal lobe.
In parallel to these anatomical changes, neuropsychological studies demonstrated impaired EF in generally healthy elderly subjects. This includes difficulties in problem solving that requires flexible thinking and cognitive shifting, impaired response inhibition, and impaired creative thinking15. There is, however, a great variability of these frontal brain changes among aging individuals in terms of the magnitude, time of performance, and the influence of education and lifestyle22,16. Although it is generally agreed that there is an overall cognitive slowing with aging, and that there is a decline in some aspects of EF, such as mental flexibility, abstract thinking and attention, this does not necessarily reach the level of “dysfunction” and there is no consensus regarding the precise pattern of altered executive function that results from age-associated changes5. Based on the literature and our clinical experience, we suggest that executive function is generally persevered in healthy and normal aging, although some components, like attention, show subtle decline. Therefore, in a clinical setting, the determination of EF impairment should be carried out with caution, since decline in some EF domains should not lead to sweeping generalizations. Further, conclusions on EF status should not be made on the basis of neuropsychological test scores alone, but should also include the individual’s abilities to carry out activities of daily living (ADL) that tax EF successfully. Meal preparation and grocery shopping are two common activities of daily living that typically rely on EF.
Correlation of EF Testing and Gait Measures
Several investigations have attempted direct study of the relationships between EF and gait abilities. In the InChanti study, over 900 non-demented older adults (mean age 74.6 ± 6.7 yrs, MMSE 25.5± 2.8)walked at a self-selected speed and at a fast speed over an obstacle course23. To assess EF, subjects also performed the Trail Making Test (TMT), a classic test of EF. Briefly, the TMT is a visuomotor timed task that is used routinely in clinical evaluations and has a dimension of cognitive flexibility. The test consists of two parts: TMT-A and TMT-B. TMT-A is a simple visual-scanning task that requires one to draw a line connecting consecutive numbers from 1 to 25. TMT-B adds a dimension of cognitive flexibility by requiring the subject to draw a line connecting numbers and letters in an alternating sequence. Subjects in the InChanti study were divided into three groups on the basis of their performance on the TMT: poor, intermediate and good23. Poor and intermediate performance on the TMT was associated with decreased gait speed on the obstacle course (see Figure 1), although the mean speeds in all three groups were within normal limits. The authors concluded that EF is critical in complex gait situations. A follow-up study in this cohort also found associations between the effects of other dual tasks on walking performance and TMT scores24.
Figure 1.
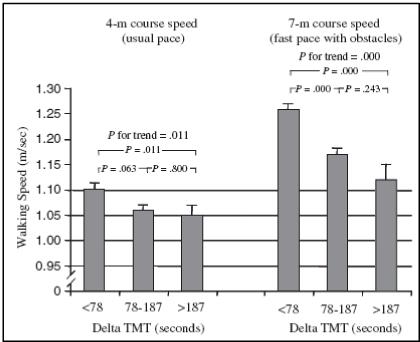
Association between gait speed and performance on the TMT in the InCHIANTI study. Subjects with poor and intermediate performance in the TMT (i.e., large increase when going from TMT-A to TMT-B) had decreased gait speed, especially on the obstacle course. From Ble et al.23.
Similar findings were reported by Holtzer et al.25. They demonstrated associations between performance on a cognitive test battery and gait speed. The cognitive battery assessed speed of processing, attention, memory, language and EF. Both EF and memory were correlated with gait speed under dual task conditions, while verbal IQ was not. Those authors suggested that gait in the elderly is a complex task requiring higher control of executive processing and memory. Springer et al.26 and Yogev et al.27 reported weak associations between EF and gait variability under usual walking conditions that became stronger under dual tasks conditions among elderly non-demented fallers and among patients with Parkinson’s disease, while such associations were not observed in healthy young adults. Similarly, Hausdorff et al. showed that better gait performance (e.g., higher gait speed, lower stride time variability) were associated with better scores on a “catch game”, a complex motor task, but this association did not exist for finger tapping, a relatively simple motor task.28 Associations between EF and performance of balance and mobility were also observed in stroke patients, even after adjustment for age, quadriceps strength of the paretic side and current physical activity level29.
The above mentioned studies demonstrate how gait is related to EF. It appears that this association is stronger if the locomotor task is more challenging (e.g., obstacle course) and/or if the normal gait pattern is already altered (e.g., in patient populations). The results of the InChianti and other studies suggest that the reliance on EF increases as the locomotor task becomes more difficult and challenging. Still, many questions remain about these relationships. For example, while there is some evidence suggesting that a decline in EF ability may actually contribute to alterations in walking abilities, cause and effect has not been definitively demonstrated. In addition, most studies correlated only selected parameters of gait (e.g., speed or gait variability) with selected features of EF. Although associations were observed, the inclusion of a wider array of measures may provide a broader picture of the interdependence between gait and EF and may help to define if and how specific EF properties affect specific aspects of walking.
ATTENTION
Definition of Attention
Attention may be considered a specific example of EF7, 30. The term covers a “number of different processes that are related aspects of how the organism becomes receptive to stimuli and how it may begin processing incoming or attended-to excitation, whether internal or external”5. There is, however, “no single and clear-cut definition of attention”5. Posner et al. view attention as anatomical network whose “primary purpose is to influence the operation of other brain networks”31. Attention can be classified into separate functions, including focused or selective, sustained, divided and alternating, although these distinctions are somewhat artificial. Selective attention, which enables filtering of stimulus information32 and suppression of distractors, is commonly referred to as “concentration”5. Sustained attention refers to the ability to maintain attention to a task over a period of time5, 32. Divided attention refers to the ability to carry out more than one task at the same time and alternating attention refers to rapid shifting of attention from one task to another5, 32. Here we focus primarily on divided attention. This type of attention plays an important role in walking in multi-tasking and changing situations, serves as a common tool for examining the attentional demands of various tasks including walking, and has clinical implications for fall risk.
Models of Dual Tasking
During the past two decades, many studies have investigated whether gait requires attention. Dual tasking is the most popular method for testing this question. This involves challenging attentional capacities, specifically the ability to divide attention. A priori, if gait is automatic and does not require attention, then simultaneous execution of an additional task should not affect gait or the performance of the other task. Before we review the studies that provide evidence that gait is indeed an attention-demanding, high-level, controlled task, we first briefly summarize the neuropsychology theories that have been proposed to explain why there are dual tasking costs in other situations.
Difficulties in the simultaneous performance of two or more tasks have led to the development of several neuropsychological theories on human information processing. Explanations generally revolve around the capacity-sharing theory, the bottleneck theory or the multiple resource models theory. The capacity-sharing theory posits that attentional resources are limited in capacity, and so the performance of two attention-demanding tasks will cause deterioration of at least one of the tasks. When the time between the presentation of two or more stimuli is reduced, the time of processing will be increased because of the limitations of the shared capacity33. This theory assumes that it is possible to voluntarily allocate capacity to a specific task, even when both tasks are over-learned and largely automatic. Thus, the performance of an additional task during walking alters gait (e.g., stability, speed) or the execution of the second task or both. The bottleneck theory proposes that if two tasks are processed by the same neural processor or networks, a bottleneck is created in the processing of information. The processing of the second task will be delayed until the processor is free from processing the first task. This explains delays in the reaction times of the second task as a function of the temporal gap in the presentation of the two stimuli. Some investigators suggest that a delay may occur only at the stage of response selection, while others postulate that delay in processing can occur at any stage33, 34. According to this theory, performance of another task during walking might result in a slowed gait or delayed performance of the second cognitive task, but only if the neural networks involved in the two processes overlap.
The multiple resource models suggest that processing may need a number of resources35-37. One of these theories claims that if two tasks do not share common resources, dual task interference will not occur. For example, walking while performing a cognitive task might not cause any changes, but a second motor task which shares the same resources as walking will. Conversely, the cross-talk theory posits that if both tasks are from a similar domain and use the same neuronal populations, they will not disturb each other35-37. Neuroimaging investigations (e.g., PET, fMRI) have shown that during dual tasking activity is found in the anterior cingulate cortex and prefrontal areas including the inferior frontal gyrus (which is not surprising, since activity of these area is attributed to EF)38-43. Such studies have been interpreted to support all three models and at present, there is no consensus on the theory that best explains human information processing and dual tasking costs.
Dual Tasking Costs in Healthy Adults
The effects of dual tasking on gait have been studied in various populations including healthy young and older adults, as well as in patients suffering from neurologic disease (e.g., post stroke, brain injuries, idiopathic fallers, Parkinson’s disease, and Alzheimer’s disease). Table 2 summarizes those investigations which have utilized dual tasking to assess gait in these populations. As seen in Table 2a, most studies of healthy adults report that the performance of a second task influences gait. Healthy young adults generally walked more slowly when they were asked to walk and perform another task. Even healthy children exhibit a slowing of gait during dual tasking44. Among healthy adults, dual tasking often caused a decline in the performance of the second task and often a reduced gait speed, however, other aspects of gait generally remained intact (e.g., gait variability) in healthy adults. Those few studies that reported no effect on gait in young adults may have used only a low cognitive demand dual task or perhaps prioritization was explicitly given to gait. Either way, from Table 2a, it is clear that gait depends on attention even in healthy adults who have intact locomotor and cognitive function; the “dual task cost” is non-zero in healthy adults.
Table 2.
| a: The effect of dual tasking on gait of healthy young and older adults | |||||
|---|---|---|---|---|---|
| Authors and Year | Subjects | Dual Task(s) Used | Effect on Gait | Effect on other Task(s) | Main Findings |
| Lajoie et al. 199350 | HY (n=6) |
Response to auditory stimuli | No | Yes | ↑ RT, but no effect on gait (e.g., swing time and gait speed) |
| Ebersbach et al. 199549 | HY (n=10) |
1.Digit span (memory) 2. Fine motor (opening and closing buttons) 3.Combination of 1+2 4. Finger tapping |
Yes | Yes | Reduction of gait speed in task 4, increase in double support in task 3 |
| Lajoie et al. 1996131 | HY HO (n=16) |
Response to auditory stimuli | No | Yes | ↑ RT, especially in the elderly. Elderly walked slower with short stride length |
| Lindenberger et al. 200057 | HY HMA HO (n=140) |
Memorization | Yes | Yes | Age caused increase in dual task cost (in gait speed, accuracy and memorization). Training improved gait speed and accuracy, but age differences remained. |
| Li et al. 200153 | HY HO (n=77) |
Memorization | Yes | Yes | Dual task costs were greater in the older adults. Dual task costs for elderly were greater for memorization than walking speed. |
| Bloem et al. 200179 | HY HO (n=63) |
Set of functional tasks of increasing difficulty, e.g., walking through obstacles, holding a tray | Yes | Yes | As task complexity increased, more motor errors occurred. In complex postural tasks, subjects, especially young subjects, gave priority to motor components over cognitive tasks. |
| Abernethy et al. 200251 | HY (n=11) |
Response to a visual stimuli | No | Yes | ↑ RT |
| Sparrow et al. 200255 | HY HO (n=24) |
1.response to a auditory stimuli 2.response to a visual stimuli |
No | Yes | ↑ RT, especially in the elderly, but no effect of attentional load on gait speed |
| Weerdesteyn et al. 2003132 | HY (n=10) |
Auditory Stroop test | Yes | Not measured | Performing cognitive task while walking and avoiding obstacles resulted in larger rates of failure to avoid touching an obstacle and smaller horizontal crossing swing velocities |
| Gage et al. 2003133 | HY HO (n=31) |
Verbal response to an auditory stimulus | No | Yes | ↑ RT in young adults, especially in single support, compared to double support phase. ↑ RT when walking in a threatening condition for both groups, more for the elderly group, but gait did not change. |
| Schordt et al. 200354 | HO (n=21) |
“1-back” task: subjects were presented with series of numbers and had to state the previously presented number. | No | Yes | Decline in the cognitive task but not in gait speed or step length). When required to step over an obstacle and perform cognitive task, toe obstacle distance was greater and obstacle heel distance was reduced |
| Bootsma-Van der Wiel et al. 200377 | HY HO (n=380) |
Verbal fluency | Yes | Yes | Performance of the verbal fluency test while walking did not predict prospective falls better than single task |
| Shkuratova et al. 2004134 | HY HO (n=40) |
Transferring coins from right pocket to left pocket | Yes | Not measured | Both groups decreased stride length and increased cadence, to similar degrees. |
| Beauchet et al. 2005135 | HY (n=49) |
Counting back from 50 | Yes | Yes | Gait speed decreased and stride time variability increased. |
| Grabiner & Trot 200586 | HY (n=15) |
Stroop test | Yes | Yes | Step width variability decreased. |
| Regnaux et al. 2006136 | HY (n=11) |
1.Simple task: reaction to sensory stimuli 2.Complex task: respond only to weak stimuli |
No | Yes | For both tasks, ↑ RT while walking compared to sitting. No change in gait pattern was evident. |
| Dubost et al. 200656 | HO (n=45) |
Verbal fluency | Yes | Not measured | Gait speed reduced and stride time variability increased |
| Sparrow et al. 200655 | HY HO (n=20) |
Response to a visual stimuli | No | Yes | ↑ RT, especially in the elderly. |
| HOllman et al. 2006137 | HY HMA HO (n=60 |
Spelling 5 letters words backwards | Yes | Yes | Older subjects walked significantly slower and increased gait variability in the dual task condition, compared to their normal walking and to young and middle age subjects. |
| Faulkner et al. 2006138 | Community dwelling elderly (n=217 |
1. Response to a auditory stimuli 2. Visuospatial decision task |
Yes | Yes | ↑ RT when walking. Poor performance in dual tasking was associated with slower walking and weaker quadriceps strength |
| Coppin et al. 200624 | Community dwelling elderly (n=737) |
1.walking while talking 2.walking while picking up an object 3.carrying a large package 4.walking over obstacles 5. walking while wearing a weighted vest |
Yes | Not measured | Subjects with poor performance in EF tests (TMT) walked slower in tasks 2 and 4 but not the other tasks. |
| Faulkner et al. 200784 | Community dwelling elderly (n=377 |
1. response to a auditory stimuli 2. visuospatial decision task |
Yes | Yes | Poor performance in dual tasking was associated with higher odds of recurrent falls. |
| Beauchet et al. 200790 | HO (n=187) |
Counting back from 50 | Not measured | Yes | Better performance in the counting task while walking was associated with higher risk of falling. |
| Van Iersel et al. 2007139 | HO (n= 59) |
1. Serial 7 subtractions 2. Serial 13 subtractions 3. Verbal fluency |
Yes | Not measured | Reduced gait velocity, increased stride variability and body sway, especially when performing the verbal fluency task. |
| Verghese J et al. 200792 | HO (n=189) |
Recitation of alternate letters of the alphabet | Yes | No | Subjects who gave priority to the cognitive task reduced gait speed and cadence, compared to subjects who paid attention to both walking and the cognitive task. |
| b: The effects of dual tasking on gait in patient populations | |||||
|---|---|---|---|---|---|
| Authors and year | Subjects | Dual Task(s) used | Effect on gait | Effect on other task(s) | Main findings |
| Lundin-ollson et al. 199776 | Frail elderly (n=58) |
Conducting a conversation | Yes | Not measured | Subjects who stopped walking while talking were at a greater risk of falling in the follow-up period. |
| de-Hoon et al. 2003140 | Frail elderly (n=17) |
Answering a question | Yes | Yes | 8 out of 17 stopped in order to answer. These subject also had reduced gait speed and larger trunk angular displacement |
| Beauchet et al. 200580 | Frail elderly (n=16) |
1.Verbal fluency 2. Counting back from 50 |
Yes | Yes | Increased stride time for both tasks, increased variability in the arithmetic task |
| Toulotte al 200681 | Elderly fallers and non-fallers (n=40) |
Holding a glass of water | Yes | Not measured | Gait (speed, stride and step time, single support time) deteriorated in fallers, but not in non-fallers. |
| Springer et al. 200626 | Young adults, elderly fallers and non-fallers (n=60) |
1.Listening to text 2.Listening+ phoneme monitoring 3. Serial 7 subtractions |
Yes | Yes | Gait speed reduced in all 3 groups. Gait variability increased only in the fallers. Gait variability was associated with EF. |
| Wellmon et al. 2006141 | Elderly subjects who used assistive device and controls who did not (n=105) |
Voice reaction time | Not measured | Yes | ↑RT in subjects who used assistive device when walking, compared to normal walking and compared to subjects who do not use assistive devices. |
| Toulotte et al. 2006110 | Elderly non-fallers and fallers (n=16) |
not mentioned Subjects trained for 3 months |
Not reported | Not reported | Significant improvement in balance measures (e.g. unipedal test, single support time) as well in gait speed, in both groups (fallers and non fallers) in both conditions (single task and dual task) |
| Morris et al. 199663 | PD patients (n=54) |
1.Reciting sentences in increasing level of difficulty 2. Repeat the days of the week backwards |
Yes | Not measured | The improvement in gait (e.g., stride length) achieved by training with external cues was abolished when subjects performed secondary tasks. |
| Camicioli 199871 | PD patients with and without freezing and healthy elderly (n=38) |
Verbal fluency | Yes | Not measured | Freezers walked slower and made more steps during dual task. Effect remained also after taking anti-parkinsonian medication in the freezers group, although improvement was observed. |
| Bond and Morris 200070 | PD patients and healthy elderly (n=24) |
1. Walking with a tray 2.Walking with a tray with plastic glasses |
Yes | Not measured | During task 2, PD patients, but not controls, walked significantly slower with a shorter stride length. |
| Bloem et al. 200187 | PD patients and healthy young adults and elderly (n=90) |
Set of functional tasks in increasing difficulty, e.g., walking through obstacles, holding a tray. | Yes | Yes | In addition to slow performance, PD patients gave priority to the cognitive tasks rather than gait. |
| O’shea et al. 200274 | PD patients and healthy elderly (n=30) |
1. Transferring coins from right pocket to left pocket 2. Serial 3 subtractions |
Yes | Yes | When performing both tasks, gait (e.g., speed, stride length, double support time) deteriorated in the PD group, but not in the control group. |
| Hausdorff et al. 200373 | PD patients (n=10) |
Serial 7 subtractions | Yes | Not measured | Increase in gait variability |
| Yogev et al. 200527 | PD patients and healthy elderly (n=58) |
1.Listening to text 2.Listening+phoneme monitoring 3.Serial 7 subtractions |
Yes | Yes | Gait speed was lower in both groups, however gait variability increased only in the PD patients group. |
| Canning et al. 200591 | PD patients (n=12) |
Carrying a tray with four empty glasses of wine | Yes | Not measured | When instructed to direct attention to walking, gait speed and stride length improved significantly. |
| Rochester et al. 2005142 | PD patients and healthy elderly (n=30) |
Functional test including stand up, walk to the kitchen, pick up a tray+ glasses | Yes | Not measured | Gait speed significantly decreased in the PD patients, but not in controls. Stride length significantly decreased in the PD patients. In the controls, stride length decreased only in the most difficult task. |
| Gallety and Brauer 200572 | PD patients and healthy elderly (n=32) |
1. Serial 3 subtractions 2. Verbal fluency 3. Pressing a button a smany times as possible |
Yes | Yes | Stride length decreased in the PD patients in tasks 1 and 2, but not in task 3. |
| Yogev et al. 2006143 | PD patients, idiopathic fallers and healthy elderly (n=47) |
Serial 7 subtractions | Yes | Yes | Gait asymmetry significantly increased in the dual task condition for both PD patients and idiopathic fallers, but for the healthy elderly. |
| Sheridan et al. 2003144 | Alzheimer’s disease patients (n=28) |
Forward digit span | Yes | Not Measured | Gait speed significantly decreased and gait variability significantly increased. |
| Camicioli et al. 2006145 | Alzheimer’s disease patients with and without extra pyramidal signs (n=42) |
Counting from 1 by ones | Yes | No | Stride time,and swing time variability increased with the dual task in both groups to a similar degree.) |
| Allali et al. 2007146 | Alzheimer’s disease and vascular dementia patients (n= 16) |
Forward and backwards counting | Yes | Yes | Both tasks, but especially backwards counting, caused increased stride time and stride time variability. |
| Pettersson et al. 2007147 | Alzheimer’s disease, Mild cognitive Impairment (MCI) and healthy controls (n= 27) |
Verbal fluency | Yes | Not measured | Patients with Alzheimer’s, but not MCI e significantly reduced their gait speed, more so than in healthy controls. |
| Bowen et al. 2001148 | Post stroke patients (n=11) |
Give one of two verbal responses to two verbal stimuli | Yes | Not measured | Gait speed decreased and double support time increased |
| Cockburn et al. 2003149 | Post stroke patients (n=10) |
Verbal fluency | Yes | Yes | Patients followed over time (1-9 months) during rehab. Although decrement in stride duration or cognitive task or both still existed, there was improvement with time in dual tasking abilities |
| Hyndman and Ashbum 200478 | Post stroke patients (n=63) |
Conducting a conversation | Yes | Not measured | Among those who were prospectively identified as fallers, 62% stopped walking while talking, but only 38% of those who could walk and talk fell. |
| Regnaux et al. 2005150 | Post stroke patients and healthy controls (n=28) |
Reaction to sensory stimuli | No | Yes | ↑RT while walking in the post stroke group as compared to sitting and standing reaction times and as compared to controls. |
| Canning et al. 2006106 | Post stroke patients and healthy young and elderly (n=60) |
1. Holding a filled glass 2. Verbal color classification task 3. Combination of 1+2 |
Yes | Not measured | Both post stroke patients and elderly adults showed similar decrease in gait speed and cadence as a function of task difficulty. Such trends did not exist for the young. |
| Lord et al. 2006151 | Post stroke patients (n=27) |
Dual tasks tested in 3 settings (clinic, suburban street, shopping mall): 1.Stepping over an obstacle 2. Responding by “yes” or “no” to whether a number is odd or even |
Yes | No | The environmental conditions (shopping mall versus clinic) affected gait speed, but not the secondary task. |
| Hyndman et al. 2006152 | Post stroke patients and healthy controls (n=60) |
Memorization (7-item shopping list) | Yes | Yes | Both groups slowed down, but walk time and decreased cognitive recall were greater for people with stroke and reduced stride length distinguished fallers from non-fallers. |
| Yang et al. 2006153 | Post stroke patients and healthy controls (n=45) |
1. Buttoning 2. Carrying a tray with glasses |
Yes | Not measured | Post stroke subjects community ambulators whose gait was similar to controls reduced their gait speed and stride length and increased their gait asymmetry, when performing secondary tasks, more so than in healthy controls. |
| Haggard et al. 2000154 | Brain injuries (post stroke, head injuries, others) and healthy controls (n=50) |
1.Word generation by category 2. Arithmetic task 3. Verbal paired association monitoring 4. Visuospatial decision task |
Yes | Yes | Significant reduction in stride duration and decrement in cognitive task in the brain injury group, but not in controls. |
| Catena et al. 2006155 | Subjects with grade II concussion and healthy controls (n=28) |
1.Questions and answers that included spelling 5 letters words backwards, subtraction by 7’s, reciting the months of the year in reverse order 2.Pressing a button as fast as possible in reaction to audible cue |
Yes | No | Gait speed was reduced in both groups. Subjects with concussion had a wider step width and greater sway when crossing obstacles compared to healthy controls. |
| Parker et al. 2006156 | Subjects with a concussion and healthy controls (n=30) |
1.Spelling 5 letters words backwards 2. Subtractions by 7 3. Reciting the months of the year in reverse order |
Yes | Not measured | When performing cognitive task, subjects with a concussion walked slower and swayed more compared to single task and compared to healthy controls, up to 4 weeks after the injury. |
| Vallee et al. 2006157 | Traumatic brain injury patients and healthy controls (n=18) |
The Stroop test +/- stepping over a narrow and wide obstacle | Yes | Yes | Despite their relatively normal gait speeds, patients with TBI walked significantly slower while stepping over the obstacles and while performing the most complex dual task. |
Tables 2a and 2b summarize studies published between 1993 through April, 2007 that report on the effects of dual tasking on gait. A systematic literature search was performed in the electronic database of Medline and Psychinfo. Relevant reports in English were identified using combination of the following key words: gait (or walking) AND cognition, gait AND dual task, gait AND divided attention. We also examined “related articles” and cited references. Case studies were not included. Otherwise all studies of dual tasking and gait that we found are included.
RT: Increased reaction times of the cognitive task. HY: healthy young adults. HO: healthy older adults. HMA: healthy middle-aged adults.
RT: Increased reaction times of the cognitive task. PD: Parkinson’s disease. Entries are grouped by patient group and year.
Dual Tasking Costs in Healthy Older Adults
With aging, structural changes of the brain occur, especially in the prefrontal areas, regions that have been associated with the EF and attentional systems5, 9, 18, 19. Therefore, it is not surprising that elderly subjects may show difficulties in dual tasking, in general, and when walking while performing another task, in particular8, 45-47. Woollacott and Shumway-Cook reviewed a large number of works published in the 1980’s and 1990’s on the relationship between attention and postural control in aging (as well in neurological patients)45. Most studies tested the attentional demands for maintaining standing balance in a variety of challenging situations, but only a few examined the effects of attentional loading on gait in the elderly48-50. These reports demonstrated that the performance of cognitive tasks while walking may increase the reaction times of the cognitive task or reduce gait speed, but other changes in the gait pattern were not observed. More recent studies support these earlier findings that both healthy young adults and healthy older adults walk more slowly when they simultaneously perform a cognitive task (see Table 2a). In addition, although there may be some deterioration in the performance of the cognitive task that tends to increase with aging, gait stability (as measured by gait variability) is generally not affected by dual tasking26, 51-55. Other reports, however, showed that even healthy elderly people might show signs of gait instability under dual tasking conditions. For example, Dubost et al. reported increased gait variability in healthy older adults who performed simple arithmetical tasks56 and Lindenberger et al. demonstrated that the dual task costs increased with aging, especially when walking through a complex course (i.e., reduction in gait speed, increased number of missteps when walking over a narrow route, as well as reduction in performance of the cognitive task)57. Thus, while there are a few exceptions, most studies in healthy older adults observe some “normal” strategies in response to dual tasking (e.g., reducing gait speed or decreasing the reaction time of the secondary cognitive task) without widespread changes to the gait pattern.
Dual Tasking Costs in Patients with Neurological Disease
Neurological patients have an especially difficult time walking while performing another task (see Table 2b. Typically, the dual tasking costs are larger than those seen in healthy, age-matched groups. This is likely due to a combination of factors. Most of the patient groups studied (e.g., post-stroke, Parkinson’s disease, and Alzheimer’s disease) have known deficits in EF and the ability to divide attention16, 58-62. At the same time, these patients also have an altered, less automatic walking pattern63-67. For example, Parkinson’s disease is characterized by gait impairments and loss of walking automaticity68, 69 that are accompanied by cognitive impairments of EF and attention62. As such, the effect of a cognitive challenge (attentional load) is easy to demonstrate: when attention resources of patients with Parkinson’s disease are allocated to more than one task, gait abnormalities increase. This is manifest as a slower gait speed, shorter strides, increased double support time, and increased stride-to-stride variability (see, for example, Figure 2)27, 63, 70-74. Similar findings are found in idiopathic fallers, Alzheimer’s disease, post stroke, and in patients with head injuries (Table 2b). During dual tasking, the gait of these patient groups suffers from a compounding of effects: cognitive and motor deficits challenge the locomotor system’s ability to walk and carry out another task.
Figure 2.
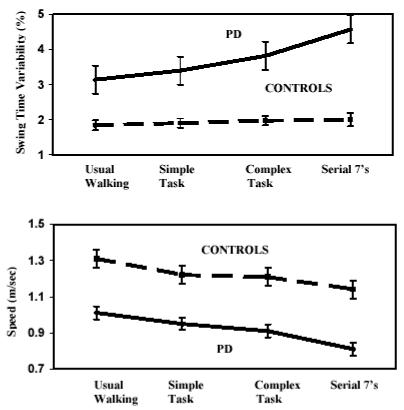
Common and distinct effects of secondary, dual tasks on the gait of patients with Parkinson’s disease and age-matched healthy controls. Above: Swing time variability and Below: Average gait speed. While all both groups slowed down during dual tasking, gait variability increased only in the patient group. From Yogev et al.27.
From this perspective, it’s understandable why dual task walking abilities may be able to reveal deficits not seen during single task walking and why dual tasking performance may be an especially sensitive predictor of fall risk. Dual tasking apparently increases the risk of falling among the frail elderly or those elderly who suffer from recurrent falls without any known organic reason (sometimes referred to as “idiopathic fallers”)75. In an elegant and very simple study, Lundin-Olsson et al. showed that older adults who could not “walk and talk” subsequently fell, while those subjects who could walk and talk were much less prone to future falls76. A few studies have found that dual tasking does not provide independent fall prediction information77, 78, perhaps because other factors also play a role (e.g., depression), however, several other investigations have also demonstrated that dual tasking severely affected gait parameters associated with fall risk in populations prone to falls, much more then in a healthy elderly cohort 26, 79-83. For example, Springer et al. reported that idiopathic fallers increase their gait variability when performing a cognitive task while walking, while a control group comprised of nonfallers did not26. Interestingly, this dual task cost was associated with poor performance in neuropsychological tests of attention and EF. In a study of 377 older adults, Faulkner et al. observed that dual tasking performance, both gait speed and the reaction time, were associated with fall risk84. Extrapolation from these findings suggests that to minimize fall risk in high-risk older adults, performance of other tasks should probably be minimized during walking.
Although there are differences in the dual tasks employed and the observed response, the clear message from Tables 2a and 2b is that gait uses attention, even in healthy adults, and, further, that the dual task costs generally increase as gait becomes less automatic, e.g., in patient populations.
Prioritization: Competition between Motor and Cognitive Demands
Simultaneous performance of two attention-demanding tasks not only causes a competition for attention, it also challenges the brain to prioritize the two tasks. Two areas of the brain are commonly mentioned in connection with the process of prioritization, the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC). Activation of both of these areas has been well-documented when subjects perform dual tasking38, 39. Williams suggests that the significance and relevance of concurrent information is determined by a core motivation to minimize danger and maximize pleasure85. In agreement with this view, Bloem et al. reported that healthy young adults and, to a certain degree, healthy elderly give priority to the stability of gait when walking and performing a cognitive task79. The unconscious, healthy “posture first” strategy might be one of the keys to avoiding hazards and preventing falls while walking. Consistent with this idea, when healthy adults performed a cognitive task while walking, quality of the task declined, but the gait pattern and gait stability did not, perhaps in order to avoid falling52, 54, 57. Similarly, Grabiner and Troy proposed that the decrease in step width variability observed in young healthy adults who performed a second task while walking reflects an increase in dynamic gait stability86.
These examples of appropriate prioritization of gait in healthy adults may be contrasted to the response to dual tasking in patient populations. Patients with Parkinson’s disease inappropriately use a “posture second” strategy and unnecessarily exacerbate their risk of falling in dual tasking situations87. Similarly, improper prioritization may partially explain why certain patients post-stroke are unable to follow a straight walking path88. Further, older adults with an increased risk of falling apparently prioritize the planning of future stepping actions over the accurate execution of ongoing movements, a decision strategy that contributes to increased likelihood of falls89. In addition, one prospective study found that compared to non-fallers, elderly fallers did better on a simple arithmetic task during walking90, perhaps because they inappropriately used a “posture second” strategy. In a sense, fall risk may be exacerbated by intrinsic motor impairment, a decline in EF and in the ability to divide attention, as well as inappropriate utilization of limited resources.
All these examples, however, refer to situations in which the subjects are not given any specific instructions regarding prioritization. In studies where the subjects were explicitly instructed to direct their attention to either gait or to the cognitive task, the overt prioritization resulted in a reduced dual task decrement for the prioritized task91, 92. The potential effects of interventions based on changing prioritization are discussed below.
Comments on Methodology
The application of dual tasking to evaluate the role of attention during walking is generally well-accepted. Nonetheless, specific implementation issues are not yet standardized. Some studies explicitly prioritize one task over the other, while many do not explicitly instruct the subject93. The choice of the second task used to evaluate the effects of dual tasks on walking also varies widely between studies and there is no consensus on which one is most appropriate and which optimally creates attentional loading. Many of the tasks used are borrowed from neuropsychological tests, such as the verbal fluency task or the Stroop test, while others have been created by the authors of a specific study. A method for quantifying the level of attention loading is also lacking. This makes it difficult to compare results across studies.
Some considerations should be taken into account when choosing a task. The task should be difficult enough to load the attentional system, but it should not cause undue stress or anxiety. Another concern is the individual’s ability to perform the dual task. Tasks that require mathematical skills (such as serial 7 subtractions) might create only minimal loading of attention if the subject is highly skilled with mathematical calculations whereas subjects who are not comfortable with such tasks might be severely affected by the performance of such a task. Similarly, a test of verbal fluency might be relatively difficult, causing severe attention loading, in a subject with language difficulties. One way to take into account these individual differences is to adjust the dual task performance relative to single task ability. The degree to which the loading of attention remains constant throughout the assessment of the dual task effect on gait should also be considered. Naming of words that start with a given letter may be relatively easy initially, but more difficult as the test progresses. On the other hand, assuming the subject has sufficient practice, the attention devoted to serial subtractions is not likely to change over time during a given test. When assessing the consistency of gait over time (e.g. variability and related measures), the consistency of the attention loading of the additional task should also be taken into account.
CLINICAL IMPLICATIONS
Assessment of EF and dual tasking abilities can provide the clinician with important information about gait disturbances and the risk of falling that might not be seen during a routine exam. Traditional, comprehensive neuropsychological testing of multiple cognitive domains typically takes many hours. More recently, computer-based tests have been developed to more quickly quantify reaction time and other features not typically evaluated using pen and paper tests94-99. Still, for practical purposes, it would be desirable to obtain an initial evaluation of such abilities without submitting the patient to exhaustive and time consuming test batteries that may also demand special equipment. Tables 3 and 4 summarize simple and quick assessment tools that can be used to evaluate EF at the bedside or in the clinic and tasks that can be used for dual tasking when directly assessing the effect on walking in such settings. These evaluations do not require special equipment, forms or extensive training of the examiner. Experience using these tests can give the clinician valuable insight into the degree to which the patient walks safely while encountering common everyday “dual tasks” and may assist in the prescription of therapeutic interventions and clinical recommendations about how to handle such situations. If deficits are suspected, more comprehensive testing may be appropriate. It is important to note, however, that these tests were not initially designed to assess EF in healthy elderly. For example, the CLOX was developed for studying patients with Alzheimer’s disease and the FABS was developed for evaluating patients with frontal lobe dysfunction. There is still work to be done in developing appropriate assessment tools for healthier subjects.
Table 3.
Relatively simple tests of executive function that can be used at the bedside and in the clinic
| Test Name | Brief Description of Test | Time to complete | Correlations with other Tests |
|---|---|---|---|
| Frontal Assessment Battery (FABS)158 | Six aspects of EF considered to relate to frontal lobe function are tested using a simple battery: conceptualization, mental flexibility, motor programming, sensitivity to interference, and inhibitory control. | 10 min | Wisconsin card sorting test (r= 0.77) Mattis Dementia Rating Scale (r=0.82) |
| The Executive Interview (EXIT25)159 | Bedside assessment of executive cognitive impairment that includes 25 items. Cut off point of 15 out of 50 discriminates non-demented elderly controls from subjects with cortical and non-cortical dementia. | 10 min | MMSE (r=-0.85) TMT part A (r=0.73) TMT part B (r=0.64) Test of sustained Attention and Tracking (r=0.83) |
| CLOX: an executive clock drawing task160 | This test discriminates between executive elements and non-executive elements of the task (free drawing vs. copying a clock). The authors suggest that clock drawing demands EF such as planning and abstract thinking. While CLOX does not evaluate specific EF components; it provides an overview of executive control. | A few minutes | EXIT25 (r=0.83) MMSE (r=0.85) |
EF: executive function. MMSE: Mini Mental State Exam. TMT: Trails Making Test.
Table 4.
Tasks that can be used to assess the effect of dual tasking on gait in the clinical setting
| Task | Description of Task | Outcome Measures | Limitations of task |
|---|---|---|---|
| Stop walking while talking76 | Subject walks while conducting a conversation with the examiner (for example, answering questions regarding one’s medical history) | Walking speed Number of stops (walking). | No standardized of questions. Examiner should be consistent with the questions and level of question difficulty. |
| Arithmetic task (digit span, backward counting, serial 3 or 7 subtractions)26, 27, 135, 144 | Subject walks while counting back from 100 (or any other number), or subtracting 3’s or 7’s. | Walking speed Number of stops Mistakes in calculations Number of calculations completed | Skill dependent: might be more difficult for some subjects than others. |
| Carrying a tray with filled glasses of water70, 91, 142, 153 | Subject walks while holding a tray with at least one filled glass of water. | Walking speed Number of stops Amount of water spilled | Involvement of upper extremities might affect the gait pattern. Difficulty is dependent on the amount of water in the glasses. |
| Verbal fluency56, 71, 77, 80, 139 | Subject walks while naming items that start with a certain letter or have a certain common characteristic (e.g., farm animals). | Walking speed Numbers of words generated. | 1) Skill dependent- might be more difficult for some subjects than others. 2) Subjects may generate most words in the initial part of the test. |
Possible Treatments
Treatment of an impaired ability to simultaneously perform another task while walking could greatly enhance quality of life and reduce the risk of falling. The dual tasking effects on walking are especially increased among patient populations who have gait impairment due to both motor and cognitive deficits. It follows, therefore, that treatment designed to reduce the dual tasking costs during walking should focus on one or both of these domains. Several potential strategies emerge.
Although there are changes in motor learning capabilities in older adults and in patients with neurological disease, the capacity to learn or re-learn motor tasks remains largely intact100-102. Thus, theoretically, training of dual tasking abilities should also be possible. This possibility has been examined in several neuropsychological studies103, 104. Two main strategies emerge in that context: the part-task strategy involves training each task separately while the whole-task strategy involves simultaneous training of both components of the dual task. Although the findings are somewhat controversial, dual tasks were found to be more than the sum of their parts and there appears to be added benefits to training of both tasks at the same time103. Furthermore, training dual tasking as a whole task was apparently critical for acquiring attentional control and task coordination strategies103. Consistent with this, an fMRI study found reduced activation brain areas that where initially involved with dual tasking processing after whole task training, interpreted as an “increase in neural efficiency”5, 105.
The possibility of improving gait while performing dual tasks using the whole-task or part-task strategies has not been well-studied. There is some evidence demonstrating that the dual tasking effects on postural control can be reduced with dual task training106-108 and one can extrapolate that similar effects should also be achievable for walking. Pilot data also support this idea109, 110. For example, Toulotte et al. trained 8 elderly non-faller and 8 elderly fallers twice weekly for 3 months110. Training included single and dual task exercises aimed to improve static and dynamic balance. Significant improvements in balance (e.g., unipedal stance time) and gait speed were observed in both groups in both single and dual task conditions. Another possibility is to training to alter inappropriate prioritization92. Strong evidence is currently lacking and many questions remain about efficacy and the design of optimal interventions to improve dual tasking during walking.
An alternative strategy focuses on improving cognitive function. There is some evidence suggesting that interventions designed to improve cognitive function in older adults and in patient populations achieve their goals and carryover to functional domains. For example, behavioral interventions and computer “games” improve cognitive function, including attention and executive function, in older adults and in patients with Parkinson’s disease100, 111-114. While the impact of such gains on gait has yet to be investigated, transfer to other everyday tasks (e.g., instrumental activities of daily114) is apparently successful.
Pharmacological therapy may be another way of affecting gait via the “cognitive” channel. A randomized-controlled study of gait in children with ADHD found that methylphenidate, the most widely used treatment option for patients with ADHD, reduced the dual task costs44. Studies in patients with Parkinson’s disease demonstrated that methylphenidate also improves EF, walking times, and gait speed while reducing gait variability115, 116 and similar findings have been observed in older adults117. The precise mechanisms whereby these improvements were achieved remain to be more firmly established. One possibility is that MPH inhibits pre-synaptic dopamine re-uptake and also inhibits the presynaptic norepinphrine transporter118. There is, however, evidence suggesting that these findings are result from the influence of the drug on cognitive function, and not a general “motor” or stimulant effect115. Some suggest that dopamine plays a key role in the attentional behavior and that the mesocortical dopaminergic system is essential for the function of the dorsal lateral pre-frontal cortex (DLPFC)119, 120, presumably the anatomical substrate of EF processes. Animal studies support the idea that optimal levels of dopamine contribute to focus of attention. Therefore the effect of MPH on dopamine could be critical both to motor and cognitive processes120. While there appears to be much potential for reducing dual tasking costs, all of the therapeutic options described have yet to be investigated fully and efficacy remains to be demonstrated in large scale controlled studies.
Perspectives and Conclusions
In this review, we have seen that a substantial body of evidence indicates that gait, even in healthy young adults, utilizes attention (recall Table 2a). From this perspective and understanding, it becomes easier to interpret somewhat surprising findings of recent epidemiological studies which linking walking and dementia, in two directions. Several large scale, longitudinal studies have demonstrated that subjects with gait abnormalities at baseline had a significantly increased risk of developing dementia as much as six to ten years later121-124; in other words, subtle gait changes predicted the subsequent development of dementia.. At the same time, results from four prospective longitudinal studies, sampling thousands of elderly subjects during a similar six to ten year follow-up, suggest that people who walk daily have a significantly reduced risk of developing dementia compared to those who do not walk or walk infrequently125-128. While the relationship between walking and cognitive function is clearly multi-factorial, all of these findings can be readily explained if gait makes use of and exercises EF and attention. It is possible that gait changes predict the development of dementia in part because gait depends on and is marker of EF and daily walking prevents the development of dementia because it makes use of EF (“use it or lose it”). Support for this view would be strengthened by longitudinal studies of the relationship between dual tasking gait abilities and the development of dementia, but the results from such reports are not yet available.
Recent studies have established the fact that attention demanding tasks change the walking pattern in all subjects, to some degree, and that alterations in EF, in particular attention, are associated with gait disturbances. A causal relationship between EF changes and gait changes remains to be more firmly proven, however, there is ample evidence demonstrating a relationship between these two seemingly disparate domains including numerous investigations which have documented changes in the gait pattern in response to dual tasking. This dependence is most notable in patient populations where the ability to compensate for an impaired gait is restricted to due a lack of “cognitive reserve.” To more fully evaluate gait abnormalities and fall risk, assessment of the EF and dual tasking walking abilities should, perhaps, become a part of the routine examination among neurological patients who suffer from a “double hit” on key functions that contribute to gait. Further work is needed to establish treatment options. Nonetheless, it seems likely that interventions that target cognitive function and those that train the ability to walk and perform another task may be successful at enhancing gait and reducing the dual tasking costs that impact on functional abilities and fall risk in many patient populations.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants AG-14100, RR-13622, HD-39838 and AG-08812, by the National Parkinson Foundation, and by the European Union Sixth Framework Program, FET contract n° 018474-2, Dynamic Analysis of Physiological Networks (DAPHNet). We thank Dr. A. Schweiger for invaluable discussions and E. Eshkol for editorial assistance.
REFERENCES
- 1.Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams R, Parsons O. Neuropsychology for clinical practice: etiology, assessment, and treatment of common neurologic disorders. American Psychological Association; Washington DC: 2003. [Google Scholar]
- 4.Fuster JM. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand Suppl. 1999;395:51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- 5.Lezak MD. Neuropsychological assessment. Oxford University Press, Inc.; New York: 1995. [Google Scholar]
- 6.Goethals I, Audenaert K, Van de WC, Dierckx R. The prefrontal cortex: insights from functional neuroimaging using cognitive activation tasks. Eur J Nucl Med Mol Imaging. 2004;31:408–416. doi: 10.1007/s00259-003-1382-z. [DOI] [PubMed] [Google Scholar]
- 7.Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz-Reuter PA. Cognitive neuropsychology of the aging brain. In: Park DC, Schawartz N, editors. Cognitive Aging: A Primer. Psychology Press, Taylor & francis; Philadelphia PA: 2000. pp. 93–114. [Google Scholar]
- 9.Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2000. [Google Scholar]
- 10.van Iersel MB, Verbeek AL, Bloem BR, Munneke M, Esselink RA, Rikkert MG. Frail elderly patients with dementia go too fast. J Neurol Neurosurg Psychiatry. 2006;77:874–876. doi: 10.1136/jnnp.2005.084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collette F, Hogge M, Salmon E, Van der LM. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 14.Craik FIM, Grady CL. Aging, memory, and frontal lobe functioning. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press, Inc.; New York: 2002. [Google Scholar]
- 15.Dorfman J. Problem solving, inhibition and frontal lobe function. In: Raz N, editor. The Other Side of the Error Term: Aging and Development as Model Systems in Cognitive Neuroscience. The Netherlands: Elsevier Science; Amsterdam: 1998. [Google Scholar]
- 16.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 18.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- 19.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 20.Shenkin SD, Bastin ME, Macgillivray TJ, et al. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis. 2005;20:310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- 21.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 22.Kramer AF. Cognitive plasticity and aging. In: Ross BH, editor. The Psychology of Learning and Motivation. 43 ed. Academic Press; NY: 2003. pp. 267–302. [Google Scholar]
- 23.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 24.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 26.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 27.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 28.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 29.Liu-Ambrose T, Pang MY, Eng JJ. Executive function is independently associated with performances of balance and mobility in community-dwelling older adults after mild stroke: implications for falls prevention. Cerebrovasc Dis. 2007;23:203–210. doi: 10.1159/000097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodruff-Pak DS, Papka M. Theories of neuropsychology and aging. In: Bengston VL, Schaie KW, editors. Handbook of theories of aging. Springer; New York: 1999. [Google Scholar]
- 31.Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Netw. 2006;19:1422–1429. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Rogers WA. Attention and aging. In: Park DC, Schwarz N, editors. Cognitive aging: A Primer. Psychology Press, Taylor & Francis Group; USA: 2006. pp. 57–71. [Google Scholar]
- 33.Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- 34.Ruthruff E, Pashler HE, Klaassen A. Processing bottlenecks in dual-task performance: structural limitation or strategic postponement? Psychon Bull Rev. 2001;8:73–80. doi: 10.3758/bf03196141. [DOI] [PubMed] [Google Scholar]
- 35.Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RA&TDL. Motor control and learning. Human Kinetics; Champaign.L.: 1999. [Google Scholar]
- 37.Sharon M. Organizers and non-organizers: a study on high organizing abilities and divided-attention capacity. Tcherikover Publishers; Tel-Aviv: 1997. [Google Scholar]
- 38.Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 40.Szameitat AJ, Schubert T, Muller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci. 2002;14:1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- 41.Schubert T, Szameitat AJ. Functional neuroanatomy of interference in overlapping dual tasks: an fMRI study. Brain Res Cogn Brain Res. 2003;17:733–746. doi: 10.1016/s0926-6410(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 42.Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M. On the neural basis of focused and divided attention. Brain Res Cogn Brain Res. 2005;25:760–776. doi: 10.1016/j.cogbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Collette F, Olivier L, Van der LM, et al. Involvement of both prefrontal and inferior parietal cortex in dual-task performance. Brain Res Cogn Brain Res. 2005;24:237–251. doi: 10.1016/j.cogbrainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Leitner Y, Barak R, Giladi N, et al. Gait in attention deficit hyperactivity disorder : Effects of methylphenidate and dual tasking. J Neurol. 2007 doi: 10.1007/s00415-006-0522-3. epub. [DOI] [PubMed] [Google Scholar]
- 45.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 46.Holtzer R, Stern Y, Rakitin BC. Age-related differences in executive control of working memory. Mem Cognit. 2004;32:1333–1345. doi: 10.3758/bf03206324. [DOI] [PubMed] [Google Scholar]
- 47.Holtzer R, Stern Y, Rakitin BC. Predicting age-related dual-task effects with individual differences on neuropsychological tests. Neuropsychology. 2005;19:18–27. doi: 10.1037/0894-4105.19.1.18. [DOI] [PubMed] [Google Scholar]
- 48.Chen HC, Schultz AB, Ashton-Miller JA, Giordani B, Alexander NB, Guire KE. Stepping over obstacles: dividing attention impairs performance of old more than young adults. J Gerontol A Biol Sci Med Sci. 1996;51:M116–M122. doi: 10.1093/gerona/51a.3.m116. [DOI] [PubMed] [Google Scholar]
- 49.Ebersbach G, Dimitrijevic MR, Poewe W. Influence of concurrent tasks on gait: a dual-task approach. Percept Mot Skills. 1995;81:107–113. doi: 10.2466/pms.1995.81.1.107. [DOI] [PubMed] [Google Scholar]
- 50.Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res. 1993;97:139–144. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- 51.Abernethy B, Hanna A, Plooy A. The attentional demands of preferred and non-preferred gait patterns. Gait Posture. 2002;15:256–265. doi: 10.1016/s0966-6362(01)00195-3. [DOI] [PubMed] [Google Scholar]
- 52.Gerin-Lajoie M, Richards CL, McFadyen BJ. The negotiation of stationary and moving obstructions during walking: anticipatory locomotor adaptations and preservation of personal space. Motor Control. 2005;9:242–269. doi: 10.1123/mcj.9.3.242. [DOI] [PubMed] [Google Scholar]
- 53.Li KZ, Lindenberger U, Freund AM, Baltes PB. Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci. 2001;12:230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- 54.Schrodt LA, Mercer VS, Giuliani CA, Hartman M. Characteristics of stepping over an obstacle in community dwelling older adults under dual-task conditions. Gait Posture. 2004;19:279–287. doi: 10.1016/S0966-6362(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 55.Sparrow WA, Bradshaw EJ, Lamoureux E, Tirosh O. Ageing effects on the attention demands of walking. Hum Mov Sci. 2002;21:961–972. doi: 10.1016/s0167-9457(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 56.Dubost V, Kressig RW, Gonthier R, et al. Relationships between dual-task related changes in stride velocity and stride time variability in healthy older adults. Hum Mov Sci. 2006;25:372–382. doi: 10.1016/j.humov.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- 58.Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain. 2001;124:1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- 60.Bedard MA, el Massioui F, Malapani C, et al. Attentional deficits in Parkinson’s disease: partial reversibility with naphtoxazine (SDZ NVI-085), a selective noradrenergic alpha 1 agonist. Clin Neuropharmacol. 1998;21:108–117. [PubMed] [Google Scholar]
- 61.Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. Executive dysfunction in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;60:91–93. doi: 10.1136/jnnp.60.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 63.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119( Pt 2):551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology. 1996;42:108–113. doi: 10.1159/000213780. [DOI] [PubMed] [Google Scholar]
- 65.Rochester L, Hetherington V, Jones D, et al. Attending to the task: interference effects of functional tasks on walking in Parkinson’s disease and the roles of cognition, depression, fatigue, and balance. Arch Phys Med Rehabil. 2004;85:1578–1585. doi: 10.1016/j.apmr.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 66.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 67.Visser H. Gait and balance in senile dementia of Alzheimer’s type. Age Ageing. 1983;12:296–301. doi: 10.1093/ageing/12.4.296. [DOI] [PubMed] [Google Scholar]
- 68.Giladi N, Nieuwboer A. Gait disturbances. In: Factor SA, Weiner WJ, editors. Parkinson’s Disease: Diagnosis and Medical Management Demos Medical Publishing. 2007. in press. [Google Scholar]
- 69.Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci. 2006;24:1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- 70.Bond JM, Morris M. Goal-directed secondary motor tasks: their effects on gait in subjects with Parkinson disease. Arch Phys Med Rehabil. 2000;81:110–116. doi: 10.1016/s0003-9993(00)90230-2. [DOI] [PubMed] [Google Scholar]
- 71.Camicioli R, Oken BS, Sexton G, Kaye JA, Nutt JG. Verbal fluency task affects gait in Parkinson’s disease with motor freezing. J Geriatr Psychiatry Neurol. 1998;11:181–185. doi: 10.1177/089198879901100403. [DOI] [PubMed] [Google Scholar]
- 72.Galletly R, Brauer SG. Does the type of concurrent task affect preferred and cued gait in people with Parkinson’s disease? Aust J Physiother. 2005;51:175–180. doi: 10.1016/s0004-9514(05)70024-6. [DOI] [PubMed] [Google Scholar]
- 73.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16:53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 74.O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. 2002;82:888–897. [PubMed] [Google Scholar]
- 75.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 76.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- 77.Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, van Exel E, Bloem BR, Westendorp RG. Walking and talking as predictors of falls in the general population: the Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1466–1471. doi: 10.1046/j.1532-5415.2003.51468.x. [DOI] [PubMed] [Google Scholar]
- 78.Hyndman D, Ashburn A. Stops walking when talking as a predictor of falls in people with stroke living in the community. J Neurol Neurosurg Psychiatry. 2004;75:994–997. doi: 10.1136/jnnp.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bloem BR, Valkenburg VV, Slabbekoorn M, Willemsen MD. The Multiple Tasks Test: development and normal strategies. Gait Posture. 2001;14:191–202. doi: 10.1016/s0966-6362(01)00141-2. [DOI] [PubMed] [Google Scholar]
- 80.Beauchet O, Dubost V, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? J Mot Behav. 2005;37:259–264. [PubMed] [Google Scholar]
- 81.Toulotte C, Thevenon A, Watelain E, Fabre C. Identification of healthy elderly fallers and non-fallers by gait analysis under dual-task conditions. Clin Rehabil. 2006;20:269–276. doi: 10.1191/0269215506cr929oa. [DOI] [PubMed] [Google Scholar]
- 82.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 83.Lundin-Olsson L, Nyberg L, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc. 1998;46:758–761. doi: 10.1111/j.1532-5415.1998.tb03813.x. [DOI] [PubMed] [Google Scholar]
- 84.Faulkner KA, Redfern MS, Cauley JA, et al. Multitasking: association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007;55:570–576. doi: 10.1111/j.1532-5415.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 85.Williams LM. An integrative neuroscience model of “significance” processing. J Integr Neurosci. 2006;5:1–47. doi: 10.1142/s0219635206001082. [DOI] [PubMed] [Google Scholar]
- 86.Grabiner MD, Troy KL. Attention demanding tasks during treadmill walking reduce step width variability in young adults. J Neuroengineering Rehabil. 2005;2:25. doi: 10.1186/1743-0003-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bloem BR, Valkenburg VV, Slabbekoorn M, van Dijk JG. The multiple tasks test. Strategies in Parkinson’s disease. Exp Brain Res. 2001;137:478–486. doi: 10.1007/s002210000672. [DOI] [PubMed] [Google Scholar]
- 88.Huitema RB, Brouwer WH, Hof AL, Dekker R, Mulder T, Postema K. Walking trajectory in neglect patients. Gait Posture. 2006;23:200–205. doi: 10.1016/j.gaitpost.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Chapman GJ, Hollands MA. Evidence that older adult fallers prioritise the planning of future stepping actions over the accurate execution of ongoing steps during complex locomotor tasks. Gait Posture. 2007;26:59–67. doi: 10.1016/j.gaitpost.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 90.Beauchet O, Dubost V, Allali G, Gonthier R, Hermann FR, Kressig RW. ‘Faster counting while walking’ as a predictor of falls in older adults. Age Ageing. 2007 doi: 10.1093/ageing/afm011. epub. [DOI] [PubMed] [Google Scholar]
- 91.Canning CG. The effect of directing attention during walking under dual-task conditions in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11:95–99. doi: 10.1016/j.parkreldis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Verghese J, Kuslansky G, Holtzer R, et al. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88:50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giordani B, Persd CC. Neuropsychological influences on gait in the elderly. In: Hausdorff JM, Alexander NB, editors. Gait disorders: evaluation and management. Taylor & Francis; Boca Raton: 2005. pp. 117–142. [Google Scholar]
- 94.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:4. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Egerhazi A, Berecz R, Bartok E, Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:746–751. doi: 10.1016/j.pnpbp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Levaux MN, Potvin S, Sepehry AA, Sablier J, Mendrek A, Stip E. Computerized assessment of cognition in schizophrenia: promises and pitfalls of CANTAB. Eur Psychiatry. 2007;22:104–115. doi: 10.1016/j.eurpsy.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Doniger GM, Zucker DM, Schweiger A, et al. Towards practical cognitive assessment for detection of early dementia: a 30-minute computerized battery discriminates as well as longer testing. Curr Alzheimer Res. 2005;2:117–124. doi: 10.2174/1567205053585792. [DOI] [PubMed] [Google Scholar]
- 98.Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schweiger A, Abramovitch A, Doniger GM, Simon ES. A clinical construct validity study of a novel computerized battery for the diagnosis of ADHD in young adults. J Clin Exp Neuropsychol. 2007;29:100–111. doi: 10.1080/13803390500519738. [DOI] [PubMed] [Google Scholar]
- 100.Witt K, Daniels C, Daniel V, Schmitt-Eliassen J, Volkmann J, Deuschl G. Patients with Parkinson’s disease learn to control complex systems-an indication for intact implicit cognitive skill learning. Neuropsychologia. 2006;44:2445–2451. doi: 10.1016/j.neuropsychologia.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 101.Behrman AL, Cauraugh JH, Light KE. Practice as an intervention to improve speeded motor performance and motor learning in Parkinson’s disease. J Neurol Sci. 2000;174:127–136. doi: 10.1016/s0022-510x(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 102.Kelly SW, Jahanshahi M, Dirnberger G. Learning of ambiguous versus hybrid sequences by patients with Parkinson’s disease. Neuropsychologia. 2004;42:1350–1357. doi: 10.1016/j.neuropsychologia.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Kramer AF, larish JL. Aging and Dual Task Performance. In: Rogers WA, Fisk AD, Walker N, editors. Aging and Skilled Performance. Lawrence Erlbaum Associates; Mahwah, NJ: 1996. pp. 83–112. [Google Scholar]
- 104.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Testing the limits of cognitive plasticity in older adults: Application to attentional control. Acta Psychol (Amst) 2006 doi: 10.1016/j.actpsy.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Erickson KI, Colcombe SJ, Wadhwa R, et al. Training-induced functional activation changes in dual-task processing: an FMRI study. Cereb Cortex. 2007;17:192–204. doi: 10.1093/cercor/bhj137. [DOI] [PubMed] [Google Scholar]
- 106.Canning CG, Ada L, Paul SS. Is automaticity of walking regained after stroke? Disabil Rehabil. 2006;28:97–102. doi: 10.1080/09638280500167712. [DOI] [PubMed] [Google Scholar]
- 107.Pellecchia GL. Dual-task training reduces impact of cognitive task on postural sway. J Mot Behav. 2005;37:239–246. doi: 10.3200/JMBR.37.3.239-246. [DOI] [PubMed] [Google Scholar]
- 108.Dault MC, Frank JS. Does practice modify the relationship between postural control and the execution of a secondary task in young and older individuals? Gerontology. 2004;50:157–164. doi: 10.1159/000076773. [DOI] [PubMed] [Google Scholar]
- 109.Silsupadol P, Siu KC, Shumway-Cook A, Woollacott MH. Training of balance under single-and dual-task conditions in older adults with balance impairment. Phys Ther. 2006;86:269–281. [PubMed] [Google Scholar]
- 110.Toulotte C, Thevenon A, Fabre C. Effects of training and detraining on the static and dynamic balance in elderly fallers and non-fallers: a pilot study. Disabil Rehabil. 2006;28:125–133. doi: 10.1080/09638280500163653. [DOI] [PubMed] [Google Scholar]
- 111.Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson’s disease. J Neurol Sci. 2006;248:115–119. doi: 10.1016/j.jns.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 112.Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith J, Siegert RJ, McDowall J, Abernethy D. Preserved implicit learning on both the serial reaction time task and artificial grammar in patients with Parkinson’s disease. Brain Cogn. 2001;45:378–391. doi: 10.1006/brcg.2001.1286. [DOI] [PubMed] [Google Scholar]
- 114.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29:15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 116.Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ben-Itzhak R, Hausdorff JM, Simon E, Giladi N. Methylphenidate improves cognition and reduces fall risk in elderly people with increased fall risk: Single dose, placebo controlled, double-blind study. Neurology. 2007;68:A14. [Google Scholar]
- 118.Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev Psychobiol. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- 120.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 121.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 122.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 123.Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229-230:89–93. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 124.Verghese J, Wang C, Holtzer R, Lipton R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007 doi: 10.1136/jnnp.2006.106914. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 126.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 127.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 128.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 129.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 130.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lajoie Y, Teasdale N, Bard C, Fleury M. Upright standing and gait: are there changes in attentional requirements related to normal aging? Exp Aging Res. 1996;22:185–198. doi: 10.1080/03610739608254006. [DOI] [PubMed] [Google Scholar]
- 132.Weerdesteyn V, Schillings AM, van Galen GP, Duysens J. Distraction affects the performance of obstacle avoidance during walking. J Mot Behav. 2003;35:53–63. doi: 10.1080/00222890309602121. [DOI] [PubMed] [Google Scholar]
- 133.Gage WH, Sleik RJ, Polych MA, McKenzie NC, Brown LA. The allocation of attention during locomotion is altered by anxiety. Exp Brain Res. 2003;150:385–394. doi: 10.1007/s00221-003-1468-7. [DOI] [PubMed] [Google Scholar]
- 134.Shkuratova N, Morris ME, Huxham F. Effects of age on balance control during walking. Arch Phys Med Rehabil. 2004;85:582–588. doi: 10.1016/j.apmr.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 135.Beauchet O, Dubost V, Herrmann FR, Kressig RW. Stride-to-stride variability while backward counting among healthy young adults. J Neuroengineering Rehabil. 2005;2:26. doi: 10.1186/1743-0003-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Regnaux JP, Robertson J, Ben Smail D, Daniel O, Bussel B. Human treadmill walking needs attention. J Neuroengineering Rehabil. 2006;3:19. doi: 10.1186/1743-0003-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture. 2007;26:113–119. doi: 10.1016/j.gaitpost.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Faulkner KA, Redfern MS, Rosano C, et al. Reciprocal influence of concurrent walking and cognitive testing on performance in older adults. Gait Posture. 2006;24:182–189. doi: 10.1016/j.gaitpost.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 139.van Iersel MB, Ribbers H, Munneke M, Borm GF, Rikkert MG. The effect of cognitive dual tasks on balance during walking in physically fit elderly people. Arch Phys Med Rehabil. 2007;88:187–191. doi: 10.1016/j.apmr.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 140.de Hoon EW, Allum JH, Carpenter MG, et al. Quantitative assessment of the stops walking while talking test in the elderly. Arch Phys Med Rehabil. 2003;84:838–842. doi: 10.1016/s0003-9993(02)04951-1. [DOI] [PubMed] [Google Scholar]
- 141.Wellmon R, Pezzillo K, Eichhorn G, Lockhart W, Morris J. Changes in dual-task voice reaction time among elders who use assistive devices. J Geriatr Phys Ther. 2006;29:74–80. doi: 10.1519/00139143-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 142.Rochester L, Hetherington V, Jones D, et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch Phys Med Rehabil. 2005;86:999–1006. doi: 10.1016/j.apmr.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 143.Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res. 2006 doi: 10.1007/s00221-006-0676-3. [DOI] [PubMed] [Google Scholar]
- 144.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 145.Camicioli R, Bouchard T, Licis L. Dual-tasks and walking fast: Relationship to extra-pyramidal signs in advanced Alzheimer disease. J Neurol Sci. 2006 doi: 10.1016/j.jns.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 146.Allali G, Kressig RW, Assal F, Herrmann FR, Dubost V, Beauchet O. Changes in gait while backward counting in demented older adults with frontal lobe dysfunction. Gait Posture. 2007 doi: 10.1016/j.gaitpost.2006.12.011. epub. [DOI] [PubMed] [Google Scholar]
- 147.Pettersson AF, Olsson E, Wahlund LO. Effect of divided attention on gait in subjects with and without cognitive impairment. J Geriatr Psychiatry Neurol. 2007;20:58–62. doi: 10.1177/0891988706293528. [DOI] [PubMed] [Google Scholar]
- 148.Bowen A, Wenman R, Mickelborough J, Foster J, Hill E, Tallis R. Dual-task effects of talking while walking on velocity and balance following a stroke. Age Ageing. 2001;30:319–323. doi: 10.1093/ageing/30.4.319. [DOI] [PubMed] [Google Scholar]
- 149.Cockburn J, Haggard P, Cock J, Fordham C. Changing patterns of cognitive-motor interference (CMI) over time during recovery from stroke. Clin Rehabil. 2003;17:167–173. doi: 10.1191/0269215503cr597oa. [DOI] [PubMed] [Google Scholar]
- 150.Regnaux JP, David D, Daniel O, Smail DB, Combeaud M, Bussel B. Evidence for cognitive processes involved in the control of steady state of walking in healthy subjects and after cerebral damage. Neurorehabil Neural Repair. 2005;19:125–132. doi: 10.1177/1545968305275612. [DOI] [PubMed] [Google Scholar]
- 151.Lord SE, Rochester L, Weatherall M, McPherson KM, McNaughton HK. The effect of environment and task on gait parameters after stroke: A randomized comparison of measurement conditions. Arch Phys Med Rehabil. 2006;87:967–973. doi: 10.1016/j.apmr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 152.Hyndman D, Ashburn A, Yardley L, Stack E. Interference between balance, gait and cognitive task performance among people with stroke living in the community. Disabil Rehabil. 2006;28:849–856. doi: 10.1080/09638280500534994. [DOI] [PubMed] [Google Scholar]
- 153.Yang YR, Chen YC, Lee CS, Cheng SJ, Wang RY. Dual-task-related gait changes in individuals with stroke. Gait Posture. 2006 doi: 10.1016/j.gaitpost.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 154.Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry. 2000;69:479–486. doi: 10.1136/jnnp.69.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Catena RD, van Donkelaar P, Chou LS. Cognitive task effects on gait stability following concussion. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0596-2. [DOI] [PubMed] [Google Scholar]
- 156.Parker TM, Osternig LR, van Donkelaar P, Chou LS. Gait stability following concussion. Med Sci Sports Exerc. 2006;38:1032–1040. doi: 10.1249/01.mss.0000222828.56982.a4. [DOI] [PubMed] [Google Scholar]
- 157.Vallee M, McFadyen BJ, Swaine B, Doyon J, Cantin JF, Dumas D. Effects of environmental demands on locomotion after traumatic brain injury. Arch Phys Med Rehabil. 2006;87:806–813. doi: 10.1016/j.apmr.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 158.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 159.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 160.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]


