Abstract
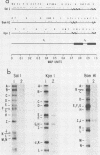
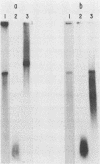
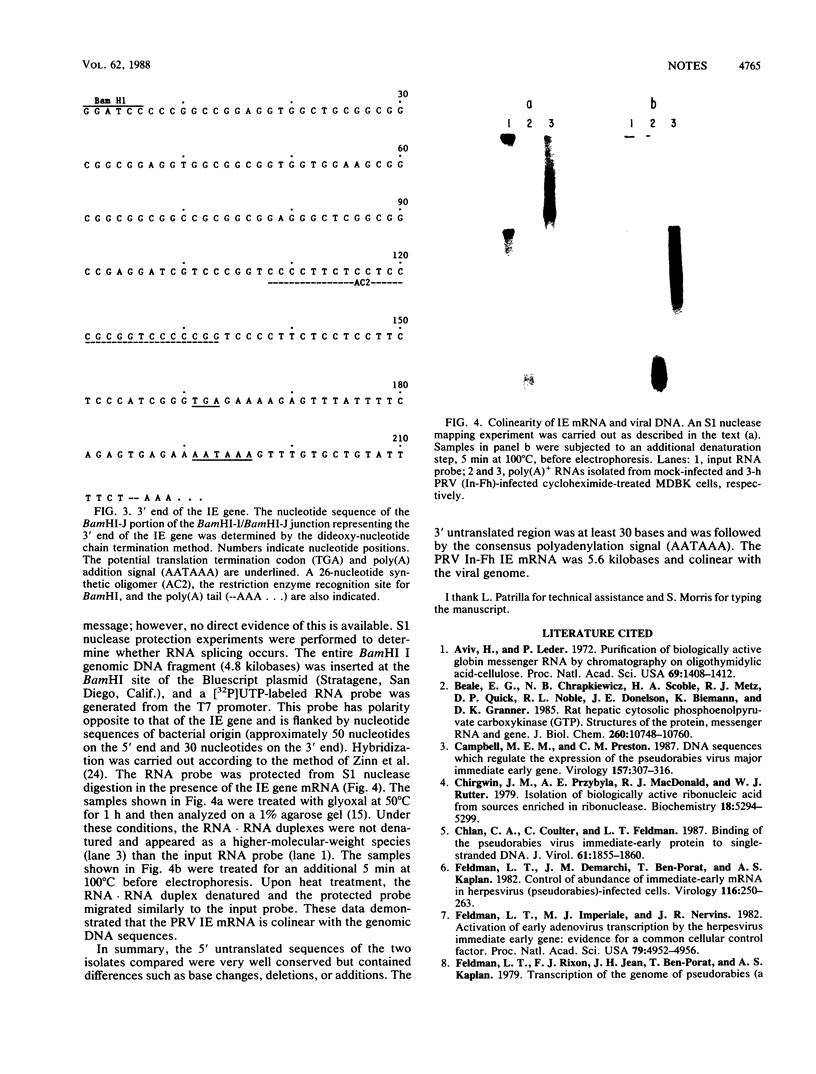
The immediate-early gene transcript of pseudorabies virus was found to be 5.6 kilobases and colinear with the DNA genome. Transcription initiation started in the BamHI E fragment, 45 nucleotides from the BamHI-E/BamHI-I junction. The first AUG codon was located in BamHI-I, 260 nucleotides from the mRNA initiation site, and a potential TGA termination codon was located in BamHI-J, 161 nucleotides from the BamHI-I/BamHI-J junction. The AATAAA polyadenylation signal was 30 bases from the stop codon, while the actual polyadenylation site was 18 bases further downstream.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Campbell M. E., Preston C. M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987 Apr;157(2):307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chlan C. A., Coulter C., Feldman L. T. Binding of the pseudorabies virus immediate-early protein to single-stranded DNA. J Virol. 1987 Jun;61(6):1855–1860. doi: 10.1128/jvi.61.6.1855-1860.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Demarchi J. M., Ben-Porat T., Kaplan A. S. Control of abundance of immediate-early mRNA in herpesvirus (pseudorabies)-infected cells. Virology. 1982 Jan 15;116(1):250–263. doi: 10.1016/0042-6822(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Feldman L. T., Imperiale M. J., Nevins J. R. Activation of early adenovirus transcription by the herpesvirus immediate early gene: evidence for a common cellular control factor. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4952–4956. doi: 10.1073/pnas.79.16.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L., Rixon F. J., Jean J. H., Ben-Porat T., Kaplan A. S. Transcription of the genome of pseudorabies virus (A herpesvirus) is strictly controlled. Virology. 1979 Sep;97(2):316–327. doi: 10.1016/0042-6822(79)90343-x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Ihara S., Feldman L., Watanabe S., Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983 Dec;131(2):437–454. doi: 10.1016/0042-6822(83)90510-x. [DOI] [PubMed] [Google Scholar]
- Jean J. H., Ben-Porat T., Kaplan A. S. Early functions of the genome of herpesvirus. 3. Inhibition of the transcription of the viral genome in cells treated with cycloheximide early during the infective process. Virology. 1974 Jun;59(2):516–523. doi: 10.1016/0042-6822(74)90461-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P. S., Mengeling W. L., Pirtle E. C. Differentiation of pseudorabies (Aujeszky's disease) virus strains by restriction endonuclease analysis. Arch Virol. 1982;73(2):193–198. doi: 10.1007/BF01314727. [DOI] [PubMed] [Google Scholar]
- Pirtle E. C., Wathen M. W., Paul P. S., Mengeling W. L., Sacks J. M. Evaluation of field isolates of pseudorabies (Aujeszky's disease) virus as determined by restriction endonuclease analysis and hybridization. Am J Vet Res. 1984 Oct;45(10):1906–1912. [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Himeno M., Kaplan A. S. Early functions of the genome of herpesvirus. I. Characterization of the RNA synthesized in cycloheximide-treated, infected cells. Virology. 1971 Dec;46(3):877–889. doi: 10.1016/0042-6822(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Characterization and mapping of a nonessential pseudorabies virus glycoprotein. J Virol. 1986 Apr;58(1):173–178. doi: 10.1128/jvi.58.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]