Abstract
The interaction between v-SNAREs on transport vesicles and t-SNAREs on target membranes is required for membrane traffic in eukaryotic cells. Here we identify Vti1p as the first v-SNARE protein found to be required for biosynthetic traffic into the yeast vacuole, the equivalent of the mammalian lysosome. Certain vti1-ts yeast mutants are defective in alkaline phosphatase transport from the Golgi to the vacuole and in targeting of aminopeptidase I from the cytosol to the vacuole. VTI1 interacts genetically with the vacuolar t-SNARE VAM3, which is required for transport of both alkaline phosphatase and aminopeptidase I to the vacuole. The v-SNARE Nyv1p forms a SNARE complex with Vam3p in homotypic vacuolar fusion; however, we find that Nyv1p is not required for any of the three biosynthetic pathways to the vacuole. v-SNAREs were thought to ensure specificity in membrane traffic. However, Vti1p also functions in two additional membrane traffic pathways: Vti1p interacts with the t-SNAREs Pep12p in traffic from the TGN to the prevacuolar compartment and with Sed5p in retrograde traffic to the cis-Golgi. The ability of Vti1p to mediate multiple fusion steps requires additional proteins to ensure specificity in membrane traffic.
INTRODUCTION
In eukaryotic cells protein traffic between different organelles is mediated by transport vesicles budding from the donor compartment and fusing with the acceptor compartment (Rothman, 1994). The yeast Saccharomyces cerevisiae has emerged as a powerful model system to study membrane traffic. Genetic screens have identified numerous genes involved in specific trafficking steps. In addition, the complete sequencing of the yeast genome gives an inventory of all yeast proteins and allows for the identification of new members of protein families and proteins with homologues in higher eukaryotes.
Proteins enter the secretory pathway via translocation into the lumen of the endoplasmic reticulum (ER). After transport from the ER to the cis-Golgi, proteins passage through the Golgi stacks and arrive in the trans-Golgi network (TGN). In the TGN proteins targeted for the plasma membrane or for secretion are sorted into secretory vesicles away from those destined for the vacuole. Two different pathways that lead from the TGN to the vacuole, the equivalent of the mammalian lysosome, have been identified to date (Bryant and Stevens, 1998; Odorizzi et al., 1998). Genetic screens have identified >50 genes as being required for transport of the soluble vacuolar hydrolase carboxypeptidase Y (CPY) from the Golgi to the vacuole, including the VPS, PEP, and VAM genes (Jones, 1977; Bankaitis et al., 1986; Rothman and Stevens, 1986; Bryant and Stevens, 1998). CPY and most other vacuolar enzymes are packaged into transport vesicles, which fuse with the prevacuolar/endosomal compartment (PVC; Vida et al., 1993). The PVC also receives proteins that are endocytosed from the plasma membrane (Vida et al., 1993; Piper et al., 1995). A second fusion step is required for transport from the PVC to the vacuole. It is unclear whether this occurs via a maturation process or is mediated by transport vesicles budding from the PVC.
The vacuolar membrane protein alkaline phosphatase (ALP) does not follow the same pathway as CPY from the Golgi to the vacuole but instead is transported in vesicles separate from those carrying CPY (Cowles et al., 1997; Piper et al., 1997). ALP does not pass through the PVC, whereas CPY does transit this organelle. It is not known yet whether ALP reaches the vacuole directly or through an as yet unidentified intermediate compartment. The adaptor complex AP3 is required for ALP but not for CPY transport to the vacuole (Cowles et al., 1997; Stepp et al., 1997). Several proteins have been identified as being involved in both ALP and CPY transport to the vacuole, for example, Vam3p, Vam7p and a vacuolar protein complex consisting of Vps18p, Vps11p, Vps16p, and Vps33p (Darsow et al., 1997; Piper et al., 1997; Wada et al., 1997; Rieder and Emr, 1997; Sato et al., 1998; Srivastava and Jones, 1998).
Aminopeptidase I (API) is transported from the cytosol to the vacuole without passing through either the ER or the Golgi apparatus (Harding et al., 1995; Klionsky, 1997). API oligomerizes in the cytosol and is enclosed in a double membrane to form cytosol to vacuole transport (CVT) vesicles. A number of genes (CVT) have been identified that are required for API transport, and some CVT genes are identical to genes involved in autophagy or to genes required for vacuolar fusion in the CPY and ALP pathways (Klionsky, 1998). It is unclear whether traffic of API, CPY, and ALP converge at a common compartment before transport to the vacuole or whether membranes from these pathways fuse directly with vacuolar membranes. Recent EM studies suggest that the outer membranes of the double membranes that surround autophagosomes and CVT vesicles fuse with the vacuole (Baba et al., 1997).
According to the soluble NSF attachment protein receptor (SNARE) hypothesis the specific recognition between a transport vesicle and its target membrane is achieved through complexes of specific SNARE proteins (Rothman, 1994; Götte and Fischer von Mollard, 1998). Members of the target membrane-associated SNARE (t-SNARE) family are localized on the target membrane and mark this compartment for different types of incoming transport vesicles. Transport vesicles contain vesicle-associated SNAREs (v-SNAREs), which bind a specific t-SNARE and are responsible for specificity in membrane traffic. The interaction between v- and t-SNAREs leads to changes in their structure, which probably drives membrane fusion (Hanson et al., 1997).
Eight syntaxin-related t-SNAREs have been identified in the yeast genome (Götte and Fischer von Mollard, 1998; Holthuis et al., 1998a). Ufe1p is needed for retrograde traffic to the ER and for ER homotypic fusion (Lewis and Pelham, 1996; Patel et al., 1998). Sed5p is involved in anterograde and retrograde traffic to the cis-Golgi compartment (Hardwick and Pelham, 1992). Sso1p and Sso2p are required for secretion (Aalto et al., 1993). Tlg1p and Tlg2p are required for endocytosis, maintenance of wild-type levels of TGN proteins, and the correct localization of chitin synthase III (Holthuis et al., 1998a,b). Tlg2p has been implicated either in the internalization (Abeliovich et al., 1998) or degradation (Séron et al., 1998) of endocytic markers. Some CPY is secreted in tlg1Δ and tlg2Δ cells (Abeliovich et al., 1998; Nichols et al., 1998). Tlg2 has been localized to the TGN (Holthuis et al., 1998a), to the TGN and endosomes (Abeliovich et al., 1998), and to endosomes (Séron et al., 1998), and Tlg1p has been localized to the putative early endosome and to the chitosome (Holthuis et al., 1998a,b). Pep12p is needed for traffic from the Golgi to the PVC (Becherer et al., 1996). The vacuolar Vam3p is involved in homotypic vacuolar fusion and in the trafficking of CPY, ALP, and API along three different biosynthetic routes to the vacuole (Darsow et al., 1997; Nichols et al., 1997; Piper et al., 1997; Wada et al., 1997; Srivastava and Jones, 1998). Recently, the SNAP-25-related t-SNARE Vam7p has been characterized (Sato et al., 1998; Ungermann and Wickner, 1998). Vam7p forms a vacuolar subcomplex with Vam3p and is required for homotypic vacuolar fusion and for transport of CPY, ALP, and API into the vacuole.
Several v-SNAREs with functions in traffic from the ER to the Golgi apparatus or in retrograde traffic within the Golgi apparatus have been identified in yeast: Sec22p/Sly2p, Bet1p/Sly12p, Bos1p, Sft1p, Ykt6p, and Gos1p (Newman et al., 1990; Ossig et al., 1991; McNew et al., 1997; Holthuis et al., 1998a). The v-SNAREs Snc1p and Snc2p interact with Sso1p and Sso2p in secretion (Protopopov et al., 1993). Nyv1p forms a complex with Vam3p in homotypic vacuolar fusion (Nichols et al., 1997). Vti1p is the only other v-SNARE known to be involved in endosomal or vacuolar trafficking steps. Vti1p functionally interacts with Pep12p in traffic from the Golgi to the PVC and with Sed5p in retrograde traffic to the cis-Golgi (Fischer von Mollard et al., 1997; Lupashin et al., 1997). Allele-specific differences among several vti1-ts mutants allowed us to distinguish between these traffic steps (Fischer von Mollard et al., 1997). vti1-1 and vti1-2 cells exhibit defects in TGN to PVC transport at the nonpermissive temperature. vti1-11 cells display a block in traffic to the PVC and an additional defect in retrograde traffic to the cis-Golgi. Genetic interactions of VTI1 with YKT6 and SFT1 confirm a role for Vti1p in retrograde traffic to the cis-Golgi (Lupashin et al., 1997). A complex of Vti1p, Ykt6p, and Sft1p may bind to Sed5p to ensure specificity in this trafficking step. Vti1p can also bind to Vam3p, Tlg1p, and Tlg2p, although the functional relevance of these complexes identified by coimmunoprecipitations has not yet been determined (Holthuis et al., 1998a).
Here we report that Vti1p interacts functionally with Vam3p in two different trafficking steps, in transport of ALP from the Golgi to the vacuole and in traffic of API from the cytoplasm to the vacuole. Nyv1p was found not to be required for biosynthetic pathways into the vacuole and did not interact genetically with VTI1 in these trafficking steps. The involvement of Vti1p in multiple trafficking steps is unexpected and poses the question of how specificity in membrane traffic is controlled.
MATERIALS AND METHODS
Materials
Reagents were used from the following sources: enzymes for DNA manipulation from New England Biolabs (Beverly, MA) and Boehringer Mannheim (Indianapolis, IN); secondary antibodies from Promega (Madison, WI), Amersham (Arlington Heights, IL), and Jackson ImmunoResearch (West Grove, PA); 35S-Express label and ECL solution from New England Nuclear (Boston, MA); fixed Staphylacoccus aureus cells (IgGsorb) from The Enzyme Center (Malden, MA); Oxalyticase from Enzogenetics (Corvallis, OR), Glusulase from DuPont (Boston, MA); and Zymolyase from Seikagaku (Tokyo, Japan). All other reagents were purchased from Sigma (St. Louis, MO).
Plasmid manipulations were performed in the Escherichia coli strains MC1061 or XL1Blue using standard media.
Yeast strains (Table 1) were grown in rich media (1% yeast extract, 1% peptone, 2% dextrose; YEPD) or standard minimal medium (SD) with appropriate supplements. To induce expression from the GAL1 promoter, dextrose was replaced by 2% raffinose and 2% galactose.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 mel | (Robinson et al., 1988) |
| SEY6211 | MATa leu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel | (Robinson et al., 1988) |
| RPY10 | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 | (Piper et al., 1994) |
| RPY95 | MATα ura3-52 his4-519 ade6 gal2 vam3Δ∷LEU2 | (Piper et al., 1997) |
| FvMY7 | MATa leu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel- vti1-1 | (Fischer von Mollard et al., 1997) |
| FvMY21 | MATa leu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel- vti1-11 | (Fischer von Mollard and Stevens, 1998) |
| FvMY24 | MATa leu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel- vti1-2 | This study |
| FvMY22 | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 vti1-2 | This study |
| FvMY36 | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 pho8-Δ329∷LEU2∷GAL1-PHO8 vti1-2 | This study |
| Δnyv-A | leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 suc2-Δ9 mel- nyv1Δ∷HIS5p | (Nichols et al., 1997) |
| FvMY33 | MATa leu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel- vti1-1 nyv1Δ∷HIS5p | This study |
| FvMY34 | MATaleu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel- vti1-11 nyv1Δ∷HIS5p | This study |
| FvMY35 | MATa leu2-3,112 ura3-52 his3-Δ200 ade2-101 trp1-Δ901 suc2-Δ9 mel- vti1-2 nyv1Δ∷HIS5p | This study |
Plasmids and Strains
Yeast strains used in this study are listed in Table 1. To integrate the vti1-2 allele into the yeast genome, vti1-2 DNA from pFvM93 was subcloned into the integration vector pRS306 (Sikorski and Hieter, 1989). FvMY22 and FvMY24 were constructed by integration of these plasmids linearized by XbaI digestion into RPY10 (SF838–9D background) and SEY6211, respectively, and looping out the wild-type VTI1 on 5-FOA plates (Boeke et al., 1984). To express PHO8 encoding ALP under the control of the GAL1 promoter, the plasmid pRCP132 was linearized with NcoI and integrated into FvMY22. This yielded FvMY36 carrying the vti1-2 and pho8-Δ329::LEU2::GAL1-PHO8 mutations. NYV1 was deleted in the vti1 mutant strains FvMY7, FvMY21, and FvMY24 by transformation with the PCR-amplified disruption construct nyv1Δ::HIS5p (HIS5 from Schizosaccharomyces pombe) generated with genomic DNA isolated from the yeast strain Δnyv-A and oligonucleotides binding 450 nt upstream and 350 nt downstream of the NYV1 ORF. The double mutant strains are FvM33 (vti1-1 nyv1Δ), FvMY34 (vti1-11 nyv1Δ), and FvMY35 (vti1-2 nyv1Δ).
Plasmids used in this study are listed in Table 2. pFvM125 contains a PCR-amplified 1.4-kb fragment encoding VAM3 with EcoRI and BamHI ends in YEp352. pFvM137 was generated by PCR amplification of NYV1 with 450-nt upstream and 350-nt downstream sequences and cloned into YEp352 with EcoRI and BamHI ends.
Table 2.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pFvM93 | vti1-2 in pRS314 (CEN6-TRP1) | (Fischer von Mollard et al., 1997) |
| pRCP132 | GAL1-PHO8 integrating plasmid in pRS305 | (Piper et al., 1997) |
| pFvM89 | SED5 in YEp351 (2μ-LEU2) | (Fischer von Mollard et al., 1997) |
| pFvM125 | VAM3 in YEp352 (2μ-URA3) | This study |
| pFvM137 | NYV1 in YEp352 (2μ-URA3) | This study |
| pRCP110 | PEP12 in YEp351 (2μ-LEU2) | (Fischer von Mollard et al., 1997) |
Immunoprecipitation of 35S-labeled Proteins
CPY, ALP, and API were immunoprecipitated as described earlier (Klionsky et al., 1992; Vater et al., 1992; Nothwehr et al., 1993). For CPY immunoprecipitations, log-phase growing yeast cells were labeled for 10 min with 35S-Express label (10 μl/0.5 OD unit of cells) followed by a 30-min chase with cysteine and methionine. The medium was separated and the cell pellet, spheroplasted, and lysed. CPY was isolated from the medium and cellular extracts. For ALP immunoprecipitations yeast cells were labeled for 7 min and chased for the indicated periods. To investigate API traffic at 36°C, 0.25 units OD of yeast cells in 500 μl of medium were labeled with 10 μl of 35S-Express label for each time point. After a 10-min pulse, cells were chased for the indicated periods and spheroplasted. Yeast cells (0.5 OD unit per time point) were labeled with 20 μl of 35S-Express label for 15 min to follow API traffic at 24°C. Extracts were prepared by boiling in 50 μl of 50 mM NaPO4, pH 7.0, 1% SDS, and 3 M urea and dilution with 950 μl of 50 mM Tris, pH 7.5, 0.5% Triton X-100, 150 mM NaCl, and 0.1 mM EDTA. The API antiserum was kindly provided by D. Klionsky (University of California, Davis, CA). Immunoprecipitates were analyzed by SDS-PAGE and autoradiography. A phosphorimager was used for quantification.
Immunofluorescence Microscopy
Indirect immunofluorescence microscopy was performed as previously described (Raymond et al., 1992; Piper et al., 1997). FvMY36 cells (GAL1-PHO8 vti1-2) were grown at 24°C in YEP and 2% raffinose. Synthesis of ALP was induced for 1 h by addition of 2% galactose at 24°C or simultaneously with a shift to 35°C. Cells were fixed, spheroplasted, and permeabilized with 1% SDS in 1.2 M sorbitol for 2 min. Cells were transferred to coverslips, blocked with 2% goat serum in PBS, and incubated overnight at 4°C with ALP antiserum (Raymond et al., 1992), which had been preabsorbed against pho8Δ cells; 1:100 diluted biotin-conjugated goat anti-rabbit IgG (heavy and light chains) and 1:100 diluted FITC-conjugated streptavidin were used for detection.
RESULTS
Vti1p Interacts with Vam3p in ALP Transport to the Vacuole
Newly synthesized vacuolar proteins are transported from the Golgi apparatus to the vacuole through two different pathways. CPY and most other vacuolar proteins reach the vacuole via the PVC. The vacuolar membrane protein ALP is transported to the vacuole without passage through the PVC (Bryant and Stevens, 1998).
Traffic of ALP to the vacuole requires the vacuolar t-SNARE Vam3p (Darsow et al., 1997; Piper et al., 1997). The v-SNARE involved in this step has not yet been identified, but it has been shown that Vam3p binds to Vti1p (Holthuis et al., 1998a). Allele-specific differences between different vti1-ts mutants revealed that Vti1p serves as a v-SNARE in two different membrane-trafficking pathways (Fischer von Mollard et al., 1997). vti1-1 and vti1-2 mutant cells are completely blocked in transport of CPY from the TGN to the PVC, but in contrast to vti1-11 cells, do not exhibit a defect in retrograde traffic to the cis-Golgi (Fischer von Mollard et al., 1997, see Figure 3).
Figure 3.
The CPY sorting defect in vti1-1 and vti1-2 cells is suppressed by overproduction of Pep12p but not by Vam3p. Cells were grown at 24°C, shifted to 36°C (A and B) or 31°C (C) for 15 min, labeled for 10 min at that temperature, and chased for 30 min. CPY was immunoprecipitated from cellular extracts (I) and extracellular fractions (E) and analyzed by SDS-PAGE. (A) In vti1 mutant cells the Golgi-modified p2CPY accumulated within the cells (I) and was secreted (E). Overproduction of Pep12p led to the production of mature CPY (mCPY) in vti1-1 cells (A) and in vti1-2 cells (C) at a semipermissive temperature (31°C). At 36°C the CPY sorting defect could not be suppressed in vti1-2 cells (B). Overproduction of Vam3p had no effect on CPY sorting in either vti1-1 or vti1-2 cells.
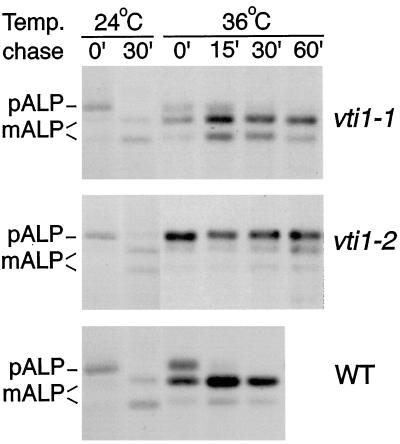
To determine whether Vti1p functions in the alternative (ALP) pathway to the vacuole, we tested whether the various temperature-sensitive vti1 mutants display ALP processing defects. Wild-type, vti1-1, and vti1-2 cells were pulse–chase labeled with 35S-Met/-Cys at 24 or at 36°C after a 15-min preincubation at 36°C. ALP was immunoprecipitated from these cells, and the precursor form of ALP (pALP) and the mature vacuolar ALP (mALP) separated by SDS-PAGE. ALP was delivered to the vacuole and proteolytically processed with wild-type kinetics in vti1-1 cells at both 24 and 36°C, as indicated by the appearance of mALP (Figure 1, compare upper and lower panels). By contrast, vti1-2 cells exhibited a strong block in ALP processing at the nonpermissive temperature but transported ALP normally at the permissive temperature (Figure 1, middle panel). ALP processing was partially blocked in yeast cells carrying either of two other vti1 alleles that exhibit a complete block in CPY processing at the nonpermissive temperature (our unpublished results).
Figure 1.
ALP traffic is defective in vti1-2 but not in vti1-1 cells at the restrictive temperature. ALP transport was followed in wild-type, vti1-1, and vti1-2 cells at 24 and 36°C after a 15-min preincubation at 36°C. Cells were labeled for 7 min with 35S-Met/-Cys and chased for 0–60 min. ALP was immunoprecipitated from cellular extracts and separated by SDS-PAGE. In vti1-1 cells pALP is processed to the vacuolar mALP at both temperatures with wild-type kinetics. ALP matures in vti1-2 cells at 24°C. By contrast, at 36°C pALP predominates even after a 60-min chase in vti1-2 cells, indicating that ALP traffic to the vacuole is blocked.
These data indicate that Vti1p is required for an additional trafficking step, transport of ALP to the vacuole. The different vti1 mutants exhibited allele-specific differences in their effect on ALP traffic, even though all mutants showed a tight block in CPY traffic at the high temperature.
Next we tested whether the ALP processing defect in vti1-2 cells could be suppressed by overexpression of different yeast t-SNAREs (Figure 2). Overproduction of the vacuolar t-SNARE Vam3p in vti1-2 cells resulted in the appearance of a significant amount of mALP. The suppression of the ALP transport defect indicates that VTI1 and VAM3 interact functionally and suggests that Vti1p and Vam3p form a SNARE complex in transport of ALP to the vacuole. Both Sed5p and Pep12p interact with Vti1p in retrograde traffic to the Golgi and in traffic from the Golgi to the PVC, respectively (Fischer von Mollard et al., 1997). Overexpression of the cis-Golgi t-SNARE Sed5p or the prevacuolar t-SNARE Pep12p did not have an effect on ALP processing in vti1-2 cells. This indicates that the suppression is specific for Vam3p, the t-SNARE that acts in vacuolar transport of ALP.
Figure 2.
Overproduction of Vam3p specifically suppresses the ALP processing defect in vti1-2 cells. The t-SNAREs Vam3p (vacuole), Pep12p (PVC), or Sed5p (cis-Golgi) were overproduced in vti1-2 cells by introducing multicopy plasmids encoding these proteins. ALP transport was compared with vti1-2 cells without a multicopy plasmid (−). Cells were preincubated for 15 min and labeled for 7 min at 36°C. Aliquots were removed after 0-, 10-, 20-, and 30-min chase periods. ALP was immunoprecipitated and analyzed by SDS-PAGE. Overproduction of Vam3p resulted in the appearance of vacuolar ALP (mALP), indicating that VAM3 overexpression suppressed the ALP traffic defect in vti1-2 cells. By contrast, overproduction of Pep12p and Sed5p had no effect on ALP processing.
Vam3p is also required for delivery of CPY from the PVC to the vacuole (Darsow et al., 1997). We next tested whether overexpression of VAM3 in vti1 mutant cells had an effect on CPY sorting. CPY traffic was followed by pulse–chase labeling with 35S and immunoprecipitation. In vti1-1 cells overproduction of Vam3p did not suppress the CPY sorting defect (Figure 3A). As described before, overexpression of Pep12p resulted in a suppression of the CPY sorting defect, whereas overexpression of Sed5p had no effect (Fischer von Mollard et al., 1997; Figure 3A). This experiment confirms that suppression is specific and that elevated levels of Vam3p cannot restore traffic through the PVC in vti1-1 cells.
The CPY sorting defect in vti1-2 cells was not suppressed by overproduction of either Pep12p or Vam3p at 36°C (Figure 3B). By contrast, at 31°C overproduction of Pep12p suppressed the partial CPY sorting defect quite efficiently. These results suggest that the vti1-2 protein retains partial folding and activity at 31°C, which enables Vti1p to function in the presence of high levels of Pep12p, but that this partial function is lost at 36°C. These data also indicate that vti1-2 cells are defective in Golgi-to-PVC traffic, which hinders analysis of CPY traffic from the PVC to the vacuole.
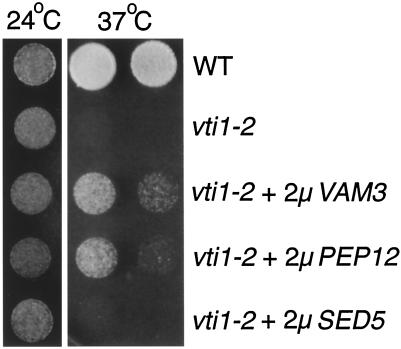
Deletion of vps genes necessary for fusion of transport vesicles with the PVC or required for fusion of membranes derived from the PVC and from the ALP pathway with the vacuole results in a growth defect at 37°C (Piper et al., 1994; Becherer et al., 1996; Rieder and Emr, 1997). vti1-1 cells do not have a growth defect at the nonpermissive temperature. By contrast, vti1-2 cells displayed a growth defect at 37°C (Figure 4). This defect was more pronounced in strains with an integrated copy of vti1-2 than in the strains with the vti1-2 allele on a centromeric plasmid used in the earlier study (Fischer von Mollard et al., 1997). The temperature-sensitive growth defect in vti1-2 cells was partially suppressed by overexpression of either Vam3p or Pep12p. Overproduction of Sed5p in vti1-2 cells did not suppress the growth defect at 37°C, even though overproduction of Sed5p allows for growth at 30°C in the complete absence of Vti1p (vti1Δ), because the Golgi traffic block is overcome (Fischer von Mollard et al., 1997; our unpublished data). The partial suppression of the growth defect in vti1-2 cells by both Vam3p and Pep12p indicates that some traffic either through the ALP pathway or through the PVC is restored and that this is sufficient for slow growth of vti1-2 cells at 37°C.
Figure 4.
The temperature-sensitive growth defect of vti1-2 cells is partially suppressed by overproduction of Vam3p or Pep12p. Dilutions of wild-type (WT), vti1-2, and vti1-2 cells overexpressing VAM3, PEP12, or SED5 were grown at 24 or 37°C on plates with rich media. vti1-2 cells grew very slowly at 37°C. Overproduction of either Pep12p or Vam3p allowed vti1-2 cells to grow somewhat at 37°C. Overproduction of Sed5p did not suppress the temperature-sensitive growth defect in vti1-2 cells.
Indirect immunofluorescence was used as an independent method to study traffic of ALP in vti1-2 cells. Expression of PHO8 (encoding ALP) was placed under control of the GAL1 promoter. vti1-2 cells were grown at 24°C in the absence of galactose. Synthesis of ALP was induced at 24°C or concomitant with a shift to 35°C for 1 h. The localization of this newly synthesized ALP was determined by indirect immunofluorescence. At the permissive temperature ALP reached the vacuoles, as indicated by the ring-like staining that colocalized with the vacuolar indentation seen in the differential interference contrast picture (Figure 5, middle panel). This staining was similar to ALP staining in wild-type cells (Figure 5, top panel). In approximately half of the cells examined the vacuolar membranes were not stained homogeneously. Intensely stained dots outlined the vacuolar membrane, indicating that labeling was restricted or concentrated in parts of the vacuolar membrane. Greater than 90% of the cells exhibited vacuolar staining. By contrast, the ALP staining at the nonpermissive temperature appeared very diffuse (Figure 5, bottom panel). ALP clearly had not traveled to the vacuole, and the staining pattern is consistent with an accumulation of ALP in nonvacuolar vesicular or membrane intermediates in 60% of the cells. In addition, some vacuolar staining was observed in ∼40% of the cells, especially after induction of ALP synthesis for 2 h at 35°C, indicating that the vti1-2 allele is leaky. This is consistent with the presence of some mALP observed in immunoprecipitations of newly synthesized ALP after long times (see Figure 1). The immunofluorescence results confirm that vti1-2 cells have a temperature-sensitive defect in ALP transport to the vacuole and indicate that ALP accumulates in nonvacuolar vesicular or membrane intermediates.
Figure 5.
In vti1-2 cells newly synthesized ALP localizes to the vacuole at 24°C but remains in vesicles at 35°C. Expression of ALP was placed under the control of the GAL1 promoter in vti1-2 cells. ALP expression was induced for 1 h at 24°C or simultaneously with a shift to 35°C, and cells were prepared for indirect immunofluorescence. At 24°C ALP antibodies stained ring-like structures, which were identified as vacuoles by the indentations in the differential interference contrast picture (DIC, arrowheads). At 35°C ALP staining was not vacuolar but very diffuse, which is a pattern typical of vesicles. For comparison, ALP staining in wild-type cells (WT) is shown.
Vti1p Interacts with Vam3p in Traffic of API to the Vacuole
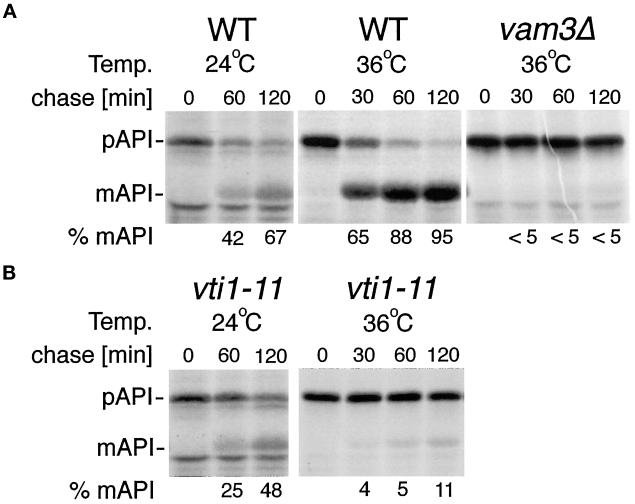
API reaches the vacuole from the cytoplasm independently of the secretory pathway (Klionsky, 1997, 1998). API is synthesized in the cytoplasm and packaged into vesicles with a double membrane, which fuse with the vacuole in a Vam3p-dependent reaction (Darsow et al., 1997). Therefore, we tested whether Vti1p is required for API transport to the vacuole. vti1-11 cells were used because they exhibit the strongest trafficking defects. vti1-11 cells are blocked in retrograde traffic to the cis-Golgi and in traffic of CPY from the TGN to the vacuole at the nonpermissive temperature (Fischer von Mollard et al., 1997). Cells were pulse labeled with 35S-Met/-Cys at the indicated temperatures and chased for 0, 30, 60, or 120 min with unlabeled cysteine and methionine. API was immunoprecipitated, and pAPI was separated from the vacuolar mAPI by SDS-PAGE. In wild-type cells 67% of the immunoprecipitated API was processed to mAPI after a 120-min chase period at 24°C (Figure 6A). In vti1-11 cells mAPI represented 48% of the total API after a 120-min chase at 24°C (Figure 6B), indicating that API matured with a slight kinetic delay in vti1-11 cells at the permissive temperature. At 36°C API matured with a half-time of <30 min in wild-type cells. By contrast, in vti1-11 cells processing of API was almost completely blocked at the nonpermissive temperature (Figure 6B). This block was almost as tight as the block in vam3Δ cells (Darsow et al., 1997; Figure 6A).
Figure 6.
API traffic is defective in vti1-11 cells. Cells were grown and labeled 15 min at 24°C, or shifted to 36°C for 15 min, labeled for 10 min, and chased for the indicated length of time. API was immunoprecipitated and separated by SDS-PAGE. A phosphorimager was used for quantification. (A) In wild-type cells (WT) pro-API transport to the vacuole and processing to mAPI was completed after a 120-min chase at 36°C, whereas it was tightly blocked in vam3Δ cells. (B) API traffic in vti1-11 cells was only slightly slower than in wild-type cells at 24°C. Processing of API was severely inhibited in vti1-11 cells at the restrictive temperature.
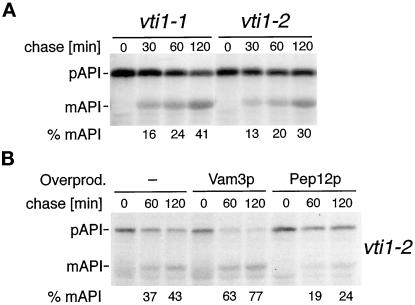
API processing was partially but reproducibly blocked in vti1-1 and vti1-2 cells at 36°C (Figure 7A). Whereas API was almost fully processed after a 60- or 120-min chase period in wild-type cells (>80 or >90% mAPI, respectively; see Figure 6A), only 24% of the total API was processed after 60 min, and 41% was processed after 120 min in vti1-1 cells at the restrictive temperature. An average of 26% mAPI was found after a 60-min chase period, and 34% was found after a 120-min chase period in vti1-2 cells (four independent experiments). Considerably higher levels of mAPI were found in vti1-2 cells overproducing Vam3p (Figure 7B). In four independent experiments an average of 46% mAPI was observed after a 60-min chase period, and 62% mAPI was observed after a 120-min chase period. By contrast, overproduction of Pep12p did not improve API processing in vti1-2 cells. This partial but t-SNARE–specific suppression of the API processing defect in vti1-2 cells by overproduction of Vam3p indicates that Vti1p is directly involved in an additional traffic step and that Vam3p and Vti1p form a SNARE complex in API traffic to the vacuole. VAM3 overexpression had no effect on API processing in vti1-11 cells (our unpublished results), suggesting that the suppression was allele specific and not a result of bypass suppression. Even though both ALP and API transport to the vacuole require Vam3p and Vti1p, the severity of the block induced by different vti1 alleles varied in the different membrane transport pathways. vti1-1 cells processed ALP with wild-type kinetics, whereas vti1-2 cells were completely blocked in ALP traffic. By contrast, both vti1-1 and vti1-2 cells displayed partial blocks in API transport. The trafficking defects in different vti1-ts mutants are summarized in Table 3.
Figure 7.
API traffic in vti1-2 cells can be partially restored by overproduction of Vam3p but not of Pep12p. API transport was analyzed as described in Figure 6. (A) API delivery to the vacuole was partially blocked in vti1-1 and vti1-2 cells at 36°C. (B) The API processing defect in vti1-2 cells was partially suppressed by overexpression of VAM3 with a multicopy plasmid. Overexpression of PEP12 was without effect.
Table 3.
Transport defects in different vti1-ts mutants
| vti1-1 | vti1-2 | vti1-11 | |
|---|---|---|---|
| Retrograde to cis-Golgi | +a | + | ts |
| TGN to PVC (CPY) | ts | ts | ts |
| TGN to vacuole (ALP) | + | ts | nd |
| Cytosol to vacuole (API) | ts | ts | ts |
+, no defect; ts, transport defect at 36°C; nd, not determined, because pALP that accumulated could be due to a block in traffic either to the cis-Golgi or from the TGN to the PVC.
Very slight block at 37°C.
Role of Nyv1p in Biosynthetic Pathways to the Vacuole
The only known v-SNAREs in vacuolar and endosomal traffic are Vti1p and Nyv1p. Nyv1p forms a SNARE complex with Vam3p in homotypic vacuolar fusion (Nichols et al., 1997). It has been reported that nyv1Δ cells contain mature CPY and mature ALP under steady-state conditions, but the kinetics of vacuolar transport were not studied (Nichols et al., 1997). We therefore investigated the kinetics of CPY, ALP, and API transport to the vacuole in nyv1Δ cells. We also explored possible genetic interactions between NYV1 and VTI1 by creating nyv1Δ vti1-ts double mutants and by overproduction of Nyv1p in the various vti1-ts mutant cells.
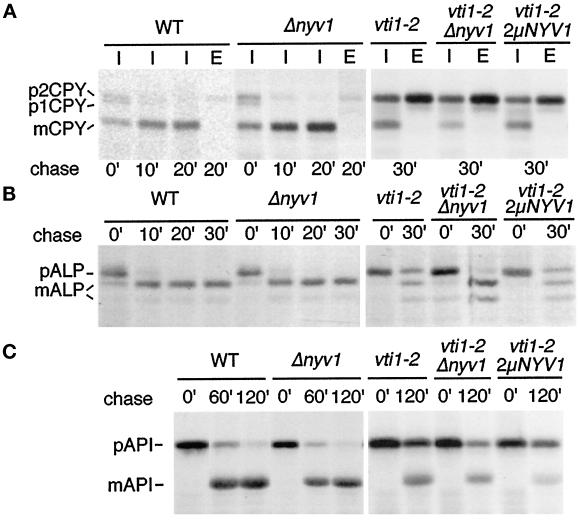
CPY sorting was analyzed in wild-type and nyv1Δ cells after a 10-min pulse followed by a 0-, 10-, or 20-min chase period (Figure 8A). Even directly after the pulse period (0-min chase) most of the immunoprecipitated CPY was vacuolar mCPY in nyv1Δ cells. CPY delivery to the vacuole and processing was almost completed after a 10-min chase period in both wild-type and nyv1Δ cells. Only small amounts of CPY were secreted into the medium (20-min chase; Figure 8E) in nyvΔ cells, and this secretion level was comparable with that of wild-type cells. These results demonstrate that CPY was delivered to the vacuole with normal kinetics in nyv1Δ cells. To investigate genetic interactions in CPY traffic, vti1-2 and nyv1Δ vti1-2 cells were grown at 24°C, preincubated for 15 min at 31°C, and radiolabeled at 31°C as before. A semipermissive temperature was used, because this is the most sensitive condition in which to identify weak genetic interactions. The absence of Nyv1p slightly but reproducibly worsened the partial CPY trafficking defect in vti1-2 cells at 31°C (Figure 8A, middle). By contrast, neither nyv1Δ vti1-2 nor vti1-2 cells secreted CPY at 24°C (our unpublished results). vti1-2 cells were transformed with a multicopy plasmid encoding NYV1 to assess any effects of Nyv1p overproduction. By Western blot analysis this strain contained at least 10-fold more Nyv1p than wild-type cells (our unpublished results). Overproduction of Nyv1p did not suppress the CPY sorting defect in vti1-2 cells (Figure 8A). Deletion of nyv1 or overproduction of Nyv1p had no effect on CPY traffic in either vti1-1 or vti1-11 cells (our unpublished results). Therefore, we conclude that there is no significant direct interaction of VTI1 and NYV1 in CPY traffic.
Figure 8.
Nyv1p is not required for transport of CPY, ALP, or API into the vacuole. Transport was analyzed in wild-type cells, nyv1Δ cells, vti1-2 cells, vti1-2 nyv1Δ double mutant cells, and vti1-2 cells overexpressing NYV1 from a 2μ plasmid. (A) CPY traffic was followed by pulse–chase immunoprecipitations at 31°C. CPY was transported to the vacuole and processed in nyv1Δ cells (mCPY). CPY traffic at the semipermissive temperature was affected to a similar degree in vti1-2, vti1-2 nyv1Δ double mutant and vti1-2 cells overexpressing NYV1. (B) ALP traffic was analyzed at 31°C by pulse–chase immunoprecipitations. ALP was delivered to the vacuole with normal kinetics in nyv1Δ cells. Deletion or overexpression of NYV1 did not change ALP traffic in a vti1-2 background. (C) API traffic was studied by pulse–chase immunoprecipitations at 36°C. API transport was unaffected in nyv1Δ cells. Deletion or overexpression of NYV1 did not influence API processing in a vti1-2 background. These data indicate that NYV1 is not required for CPY, ALP, or API traffic, and that VTI1 and NYV1 do not interact genetically.
nyv1Δ cells processed ALP with normal kinetics compared with wild-type cells (Figure 8B). The absence of Nyv1p did not aggravate the ALP transport defect in vti1-2 cells at the semipermissive temperature. By contrast, ALP processing was slightly more efficient in nyv1Δ vti1-2 cells compared with vti1-2 cells in four independent experiments. As seen at 31°C (Figure 8B), a slight improvement of ALP processing was also observed in vti1-2 cells upon deletion of nyv1 at 36°C. Overexpression of NYV1 did not suppress the ALP sorting defect of vti1-2 cells at any temperature. Deletion of NYV1 in vti1-1 cells did not result in an ALP processing defect (our unpublished results). Therefore, Nyv1p is not required for ALP transport to the vacuole.
The effect of the nyv1Δ mutation on API transport was also investigated. nyv1Δ as well as wild-type cells contained predominantly mAPI after a 60-min chase period, indicating that nyv1Δ cells processed API with normal kinetics (Figure 8C). The absence of Nyv1p did not worsen the defect in API traffic in vti1-2 cells (Figure 8C). Overexpression of NYV1 did not improve API processing and by contrast slightly aggravated the traffic block in some experiments. Similar results were obtained in vti1-1 and vti1-11 strains. Therefore, Nyv1p is not required for API transport to the vacuole. Deletion of NYV1 did not change the growth rates of vti1-1, vti1-2 or vti1-11 cells at either 24 or 37°C (our unpublished results). These experiments demonstrate that Nyv1p does not play a demonstrable role in any of the pathways for biosynthetic delivery of proteins to the vacuole. In addition, there is no evidence for any genetic interaction between VTI1 and NYV1 in these biosynthetic trafficking pathways. By contrast, both NYV1 (Nichols et al., 1997; Ungermann et al., 1998) and VTI1 (Ungermann, Fischer von Mollard, Stevens, and Wickner, unpublished results) are required for homotypic vacuolar fusion and form a SNARE complex. The interaction of NYV1 and VTI1 in homotypic fusion may indirectly cause the slight worsening of CPY transport at semipermissive temperature in vti1-2 cells and the improvement of ALP transport in vti1-2 cells upon deletion of nyv1. Therefore, NYV1 seems to function exclusively in homotypic vacuolar fusion (Nichols et al., 1997; Ungermann et al., 1998).
DISCUSSION
This study identifies Vti1p as a v-SNARE required for both the API and the ALP membrane traffic pathways to the yeast vacuole, indicating that Vti1p is required for all three biosynthetic pathways to the vacuole. Here we demonstrate that a single v-SNARE, yeast Vti1p, is required for at least four transport steps and is able to interact functionally with three different t-SNAREs. Given the assumption that v-SNAREs determine specificity in membrane traffic, these results raise serious questions about how transport vesicles recognize the correct target membrane.
Implication for Vacuolar Membrane Traffic
Here we show that Vti1p interacts functionally with the vacuolar t-SNARE Vam3p in at least two different biosynthetic pathways to the vacuole (Figure 9). Vti1p is required for transport of ALP from the Golgi apparatus to the vacuole. In addition, Vti1p is involved in traffic of API from the cytosol to the vacuole through the CVT pathway. We conclude that Vti1p is directly involved in these trafficking steps for two reasons. First, processing of ALP and API is rapidly blocked upon shift to nonpermissive temperature, thus greatly minimizing the possibility of the indirect effects seen in deletion mutants. API transport could still be affected indirectly if efficient traffic to the PVC, which is blocked in vti1-ts mutants, is required for API transport. For example, the PVC could serve as the unknown membrane source for CVT vesicles. Second, overproduction of the t-SNARE Vam3p partially but reproducibly suppressed the ALP and API processing defects in certain vti1-ts mutants. This suppression was specific because overproduction of the prevacuolar t-SNARE Pep12p was without effect. These data indicate that Vti1p acts in the same step as Vam3p and is probably part of a SNARE complex required for fusion with the vacuole. The third biosynthetic pathway into the vacuole, traffic from the prevacuole to the vacuole, could not be assayed directly, because Vti1p is also required for traffic of the reporter protein CPY from the Golgi to the prevacuole.
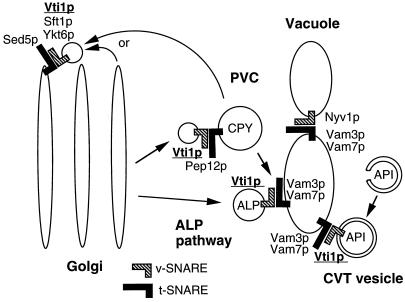
Figure 9.
Model for Vti1p function in membrane traffic. Sed5p forms a SNARE complex with Vti1p, Sft1p, and Ykt6p in retrograde traffic to the cis-Golgi compartment. Vti1p binds to Pep12p in transport of CPY from the TGN to the PVC. Vti1p interacts with Vam3p in transport of ALP from the Golgi to the vacuole and in transport of API from the cytoplasm to the vacuole. Nyv1p is only required for homotypic vacuolar fusion and forms a SNARE complex with Vam3p and Vam7p. Vti1p has been coimmunoprecipitated with Tlg1p and Tlg2p, but the functional relevance is not yet known.
A SNARE complex containing Vam3p, Vam7p, and Nyv1p is required for homotypic vacuolar fusion (Nichols et al., 1997; Ungermann and Wickner, 1998). Recently, Vti1p has been identified as a fourth component of the SNARE complex involved in homotypic vacuolar fusion (Ungermann, Fischer von Mollard, Stevens, and Wickner, unpublished data). Our study indicates that Nyv1p is not required for any of the biosynthetic pathways into the vacuole. Therefore, the SNARE complex involved in trafficking of ALP and API to the vacuole contains Vam3p (Darsow et al., 1997; Piper et al., 1997), Vam7p (Sato et al., 1998) and Vti1p but probably not Nyv1p. This means that different SNARE complexes form in homotypic vacuolar fusion and in biosynthetic traffic to the vacuole.
Our data indicate that the SNARE complexes involved in ALP and API transport to the vacuole are different from each other. Yeast cells carrying the temperature-sensitive allele vti1-2 display a complete block in ALP processing at the nonpermissive temperature. By contrast, ALP is delivered to the vacuole with normal kinetics in vti1-1 cells at the nonpermissive temperature for CPY transport. Delivery of API to the vacuole is partially blocked and affected to a similar degree in both vti1-1 and vti1-2 cells at the nonpermissive temperature. These allele-specific differences suggest that different sets of additional proteins are required for ALP versus API traffic in addition to Vti1p and Vam3p. These results also imply that the ALP and API pathways do not converge before fusion with the vacuole. Instead, membranes from either pathway form separate SNARE complexes with Vam3p in fusion with the vacuolar membrane.
In the CVT pathway a double membrane engulfs oligomerized cytosolic API to form CVT vesicles (Klionsky, 1997, 1998). Because Vti1p is required for their vacuolar delivery, we assume that Vti1p is localized on the CVT vesicles. However, it is still unclear from which compartment the membranes forming the CVT vesicles originate. Vti1p has to be targeted to this membrane source. It also remains to be established whether ALP travels to the vacuole directly in Golgi-derived transport vesicles or through an intermediate compartment. Because Vti1p is localized to the Golgi, it could be incorporated into ALP-containing vesicles at this point. The role of Vti1p in fusion of transport vesicles with the vacuolar membrane means that Vti1p itself is transported to the vacuole. Under normal conditions little Vti1p is localized in the vacuole, even though Vti1p is a very stable protein (Fischer von Mollard et al., 1997; Bryant et al., 1998). Only upon overexpression does a significant amount of Vti1p redistribute to the vacuole (our unpublished observations). This suggests that Vti1p may be able to recycle from vacuolar membranes to participate in new rounds of vesicle transport. We have recently discovered a retrograde traffic pathway out of the vacuole to the PVC (Bryant et al., 1998). Vti1p accumulates in the vacuole in vac7 mutant cells, indicating that Vti1p indeed is transported to the vacuole and that Vac7p is involved in retrograde traffic out of the vacuole in addition to its role in vacuolar inheritance (Bonangelino et al., 1997; Bryant et al., 1998).
Implication for the Role of v-SNAREs in Specificity
This study demonstrates that Vti1p interacts functionally with the vacuolar t-SNARE Vam3p in two different biosynthetic pathways to the vacuole. Earlier we demonstrated that Vti1p interacts with Pep12p in traffic from the Golgi to the prevacuole and with Sed5p in retrograde traffic to the cis-Golgi compartment (Fischer von Mollard et al., 1997). Different trafficking steps are affected in various vti1-ts mutants. vti1-1 mutants are completely blocked in traffic from the TGN to the PVC and partially in API traffic to the vacuole. The vti1-1 protein contains the amino acid exchanges E145K and G148R (Fischer von Mollard and Stevens, 1998). vti1-2 mutants are blocked in traffic from the Golgi to the TGN, in traffic of ALP to the vacuole, and partially in API traffic to the vacuole. The amino acid alterations are S130P and I151T in the vti1-2 protein. The vti1-11 protein is defective in interactions with Sed5p, Pep12p, and Vam3p and contains the amino acid exchanges E145G and L155F. These amino acid exchanges are all clustered in a short, evolutionary conserved domain. This domain is predicted to form an α-helical structure, and all mutations are localized on the hydrophobic face of the predicted helix (Fischer von Mollard and Stevens, 1998). This domain probably represents the interaction site between Vti1p and different t-SNAREs. Amino acid exchanges on this surface seem to affect interactions with different t-SNAREs in specific ways. This hypothesis is strengthened by the recent finding that the equivalent domains in the synaptic SNARE complex form a four-helix bundle with leucine zipper-like layers and a central ionic interaction between an arginine and three glutamine residues (Sutton et al., 1998).
It was also shown that Vti1p is able to bind to five of the eight syntaxin-related t-SNAREs identified in the yeast genome (Fischer von Mollard et al., 1997; Lupashin et al., 1997; Holthuis et al., 1998). Vti1p does not interact with Sso1p/Sso2p in secretory traffic from the Golgi to the plasma membrane and does not interact with Ufe1p in retrograde traffic to the ER. Vti1p binds Tlg1p and Tlg2p in addition to Sed5p, Pep12p, and Vam3p (Holthuis et al., 1998a). Both Tlg1p and Tlg2p are t-SNAREs required for endocytosis and for maintenance of normal levels of TGN proteins (Abeliovich et al., 1998; Holthuis et al., 1998a; Séron et al., 1998). Tlg2p has been localized to the TGN and endosomes, whereas Tlg1p was found in a novel compartment that may represent the early endosome in yeast. These data indicate that Vti1p may function in additional steps in post-Golgi/endosomal/vacuolar traffic.
A single v-SNARE or combinations of different v-SNAREs may be required for interactions with different t-SNAREs. Aside from Vti1p, Sec22p/Sly2p is the only other v-SNARE implicated in more than one fusion step. Sec22p interacts with Sed5p in traffic from the ER to the Golgi. Efficient binding of Sec22p to Sed5p requires the presence of the v-SNAREs Bet1p/Sly12p and Bos1p (Sacher et al., 1997; Stone et al., 1997). Sec22p is also required for retrograde traffic to the ER and binds to the ER t-SNARE Ufe1p. Bos1p and Bet1p were not found in the Sec22p–Ufe1p complex (Lewis et al., 1997). Therefore a Sec22p–Bet1p–Bos1p complex could serve as a targeting signal for anterograde traffic to the cis-Golgi, whereas Sec22p alone could direct retrograde traffic to the ER.
Vti1p interacts genetically with Sft1p (Lupashin et al., 1997). Sft1p is a v-SNARE involved in retrograde traffic within the Golgi apparatus and binds to Sed5p (Banfield et al., 1995). In addition, Vti1p interacts both genetically and physically with Ykt6p (Lupashin et al., 1997). The v-SNARE Ykt6p is also found in a complex with Sed5p (Sogaard et al., 1994). Therefore, it is possible that Vti1p requires the presence of both Sft1p and Ykt6p for functional interaction with Sed5p in retrograde traffic to the cis-Golgi, despite the fact that recombinant Sed5p and Vti1p bind each other.
The presence of additional v-SNAREs could explain specificity in the binding interactions between Vti1p and the other t-SNAREs. Nyv1p is the only other known v-SNARE localized to endosomal or vacuolar membranes. Nyv1p is involved in homotypic vacuolar fusion (Nichols et al., 1997). In this study we demonstrate that Nyp1p is not required for any of the three biosynthetic pathways into the vacuole and that Nyv1p does not interact with Vti1p in these pathways. Therefore, Nyv1p seems to be involved specifically in a single fusion step, homotypic vacuolar fusion.
Binding has been detected between Tlg1p and Snc1p (Holthuis et al., 1998a) and between Tlg2p and Snc2p (Abeliovich et al., 1998). Snc1p and Snc2p are v-SNAREs that are localized on secretory vesicles and interact with the plasma membrane t-SNAREs Sso1p and Sso2p in secretion (Protopopov et al., 1993). The functional relevance of the interactions between Tlg1p and Vti1p, between Tlg1p and Snc1p, and between Tlg2p and Snc2p are not yet known. Still, not enough other v-SNAREs have been identified that could contribute to specificity in interactions of Vti1p with several t-SNAREs. It is possible that v-SNAREs were missed by sequence comparisons, because v-SNAREs share less amino acid identity with each other, and they lack a clearly identifiable conserved sequence motif in contrast to t-SNAREs. Therefore, it seems more likely that Vti1p has a role as a general fusion protein and is only one factor contributing to specificity in membrane traffic. Whether Vti1p is unique in this capacity cannot be determined from the data currently available.
Members of the Ypt/rab family of small GTPases are thought to contribute to specificity in membrane traffic (Lazar et al., 1997). A recent study demonstrates that Ypt1p is required for membrane binding of the protein Uso1p (Cao et al., 1998). Uso1p acts before the assembly of the SNARE complex and probably tethers ER-derived transport vesicles to Golgi membranes (Sapperstein et al., 1996). Recently, the TRAPP protein complex was identified on cis-Golgi membranes (Sacher et al., 1998). This protein complex may also be involved in tethering, because genetic interactions with SNAREs required for ER to cis-Golgi traffic and with USO1 were found. The tethering step is followed by SNARE-dependent docking and fusion. Therefore, it is possible that specificity in membrane traffic could be achieved through the sequential reactions of Ypt-regulated initial tethering and SNARE-dependent docking. Several Ypt proteins are involved in endosomal and vacuolar transport pathways. Vps21p/Ypt51p has been implicated in Golgi to prevacuolar and in endosomal traffic. Ypt7p is needed for traffic to the vacuole and for homotypic vacuolar fusion. Ypt6p is probably involved in retrograde traffic to the Golgi apparatus. Two additional Ypt proteins, Ypt10p and Ypt11p, were identified by sequence homology. Their localization and function has not yet been determined. Deletion of the YPT10 or YPT11 gene does not cause the severe phenotypes expected for proteins required in the secretory pathway, making it possible that Ypt10p and Ypt11p also play a role in endosomal or vacuolar trafficking (Lazar et al., 1997). Therefore, Ypt/rab proteins are good candidates to contribute to specificity in Vti1p dependent trafficking steps.
In conclusion, our work reveals that Vti1p regulates multiple vesicle transport steps to different organelles. Therefore, trafficking of Vti1p itself must be very complex and highly regulated.
ACKNOWLEDGMENTS
We are grateful to Dan Klionsky for his generous gift of API antiserum. Benjamin J. Nichols and Hugh R.B. Pelham (Medical Research Council, Cambridge, United Kingdom) are acknowledged for providing the nyv1Δ yeast strain. We thank Nia J. Bryant and Elizabeth Conibear for stimulating discussions and critical reading of the manuscript. This work was supported by a postdoctoral fellowship from the American Heart Association, Oregon Affiliate (to G.F.v.M.) and by National Institutes of Health grant GM-32448 (to T.H.S.).
Abbreviations used:
- ALP
alkaline phosphatase
- API
aminopeptidase I
- CPY
carboxypeptidase Y
- CVT
cytosol-to-vacuole transport
- ER
endoplasmic reticulum
- mALP
mature vacuolar ALP
- pALP
precursor ALP
- PVC
prevacuolar/endosomal compartment
- TGN
trans-Golgi network
- VPS
vacuolar protein sorting
REFERENCES
- Aalto MK, Ronne H, Keranen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. doi: 10.1074/jbc.273.19.11719. [DOI] [PubMed] [Google Scholar]
- Baba M, Osumi M, Scott SV, Klionsky DJ, Ohsumi Y. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J Cell Biol. 1997;139:1687–1695. doi: 10.1083/jcb.139.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Pelham HR. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Johnson LM, Emr SD. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci USA. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homolog, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phospahte decarboxylase activity in yeast:5-fluororotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bonangelino CJ, Catlett NL, Weisman LS. VAc7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17:6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Weisman LS, Stevens TH. Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol. 1998;142:651–664. doi: 10.1083/jcb.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. (1998). Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SE. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Nothwehr SF, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stevens TH. A Human homolog can functionally replace the yeast v-SNARE Vti1p in two vesicle transport pathways. J Biol Chem. 1998;273:2624–2630. doi: 10.1074/jbc.273.5.2624. [DOI] [PubMed] [Google Scholar]
- Götte M, Fischer von Mollard G. A new beat for the SNARE drum. Trends Cell Biol. 1998;8:215–218. doi: 10.1016/s0962-8924(98)01272-0. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Heuser JE, Jahn R. Neurotransmitter release—four years of SNARE complexes. Current Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- Harding TM, Morano KA, Scott SV, Klionsky Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HR. SED5 encodes a 39-kDa integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system in yeast. EMBO J. 1998a;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Pelham HRB. The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol Biol Cell. 1998b;9:3383–3397. doi: 10.1091/mbc.9.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EW. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977;85:23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ. Protein transport from the cytoplasm into the vacuole. J Membr Biol. 1997;157:105–115. doi: 10.1007/s002329900220. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. Nonclassical protein sorting to the vacuole. J Biol Chem. 1998;273:10807–10810. doi: 10.1074/jbc.273.18.10807. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Cueva R, Yaver DS. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J Cell Biol. 1992;119:287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar T, Götte M, Gallwitz D. Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell. Trends Biochem Sci. 1997;22:468–472. doi: 10.1016/s0968-0004(97)01150-x. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Rayner JC, Pelham HRB. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol Biol Cell. 1997;8:2659–2676. doi: 10.1091/mbc.8.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JA, Søgaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Söllner TH. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- Newman AP, Shim J, Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990;10:3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Holthuis JCM, Pelham HRB. The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur J Cell Biol. 1998;77:263–268. doi: 10.1016/s0171-9335(98)80084-8. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Roberts CJ, Stevens TH. Membrane protein retention in the yeast Golgi apparatus: dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J Cell Biol. 1993;121:1197–1209. doi: 10.1083/jcb.121.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte HH, Schmitt HD, Gallwitz D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–546. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Hong Y, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, postGolgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JH, Stevens TH. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates JR, Abeliovich H, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Stone S, Ferro-Novick S. The synaptobrevin-related domains of Bos1p and Sec22p bind to the syntaxin-like region of Sed5p. J Biol Chem. 1997;272:17134–17138. doi: 10.1074/jbc.272.27.17134. [DOI] [PubMed] [Google Scholar]
- Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Darsow T, Emr SD. Vam7p, a SNAP-25-like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol Cell Biol. 1998;18:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séron K, et al. A yeast t-SNARE involved in endocytosis. Mol Biol Cell. 1998;9:2873–2889. doi: 10.1091/mbc.9.10.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Jones EW. Pth1/Vam3p is the syntaxin homolog at the vacuolar membrane of Saccharomyces cerevisiae required for the delivery of vacuolar hydrolases. Genetics. 1998;148:85–98. doi: 10.1093/genetics/148.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S, Sacher M, Mao Y, Carr C, Lyons P, Quinn AM, Ferro-Novick S. Bet1p activates the v-SNARE Bos1p. Mol Biol Cell. 1997;8:1175–1181. doi: 10.1091/mbc.8.7.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HRB, Wickner W. A vacuole v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Wickner W. Vam7p, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater CA, Raymond CK, Ekena K, Howald SI, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Huyer G, Emr SD. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J Cell Biol. 1993;121:1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]