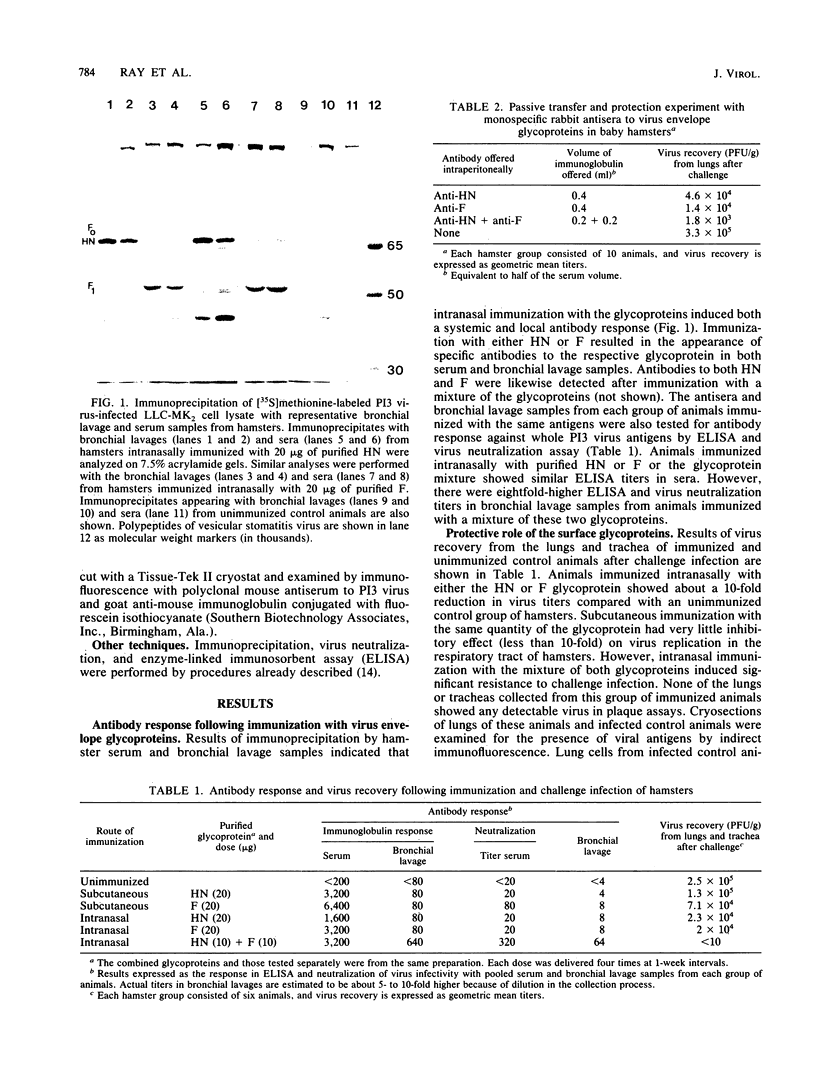
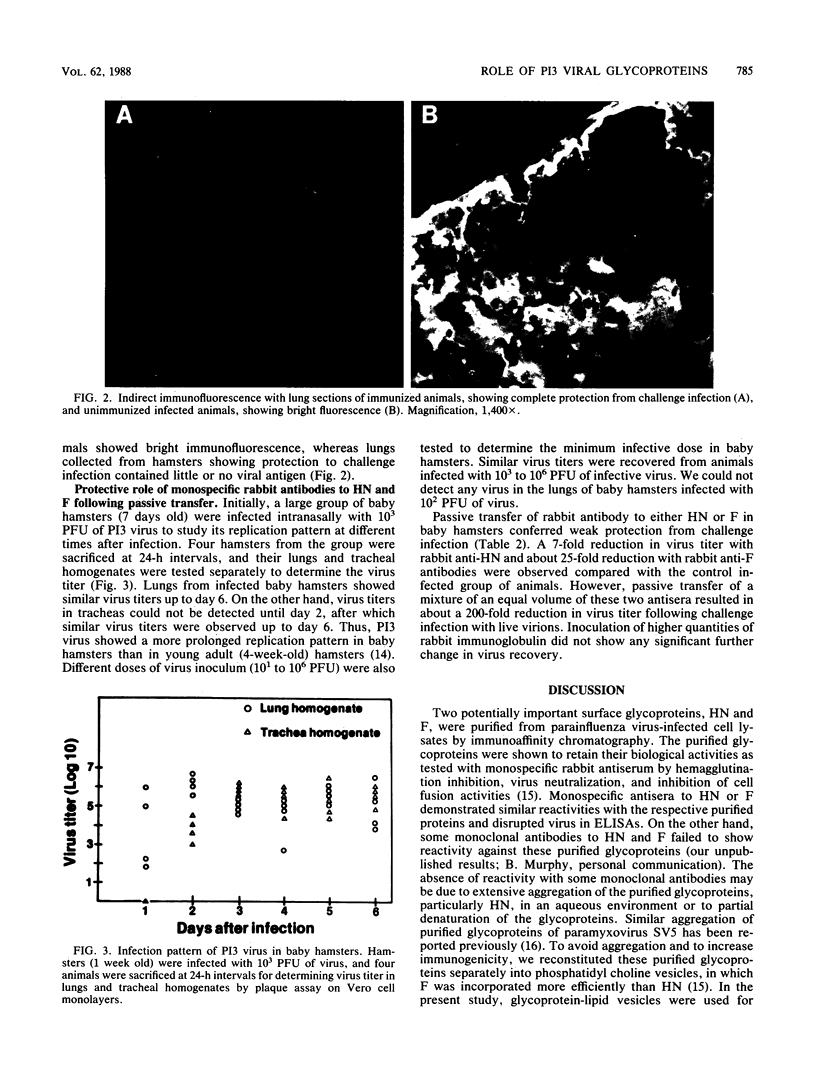
Abstract
Affinity-purified hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins of human parainfluenza virus type 3 (P13 virus) were used to investigate their role in the induction of a protective immune response following immunization of hamsters. The efficacy of immunization with the glycoprotein antigens was tested by challenge infection. Results of virus recovery from lungs and trachea demonstrated that although immunization with HN or F alone induced an antibody response to the respective glycoproteins, it did not provide a significant level of protection. However, immunization with a mixture of both purified glycoproteins induced higher virus-neutralizing activity in bronchial lavages and afforded complete protection from challenge infection. Similarly, incomplete protection was observed after passive transfer of monospecific rabbit antibody to the purified HN or F in baby hamsters. On the other hand, passive transfer of a mixture of antibodies to HN and F conferred a higher level of protection. Thus, the presence of antibody to both glycoproteins of P13 virus may be essential for protective immunity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANOCK R. M., PARROTT R. H. ACUTE RESPIRATORY DISEASE IN INFANCY AND CHILDHOOD: PRESENT UNDERSTANDING AND PROSPECTS FOR PREVENTION. Pediatrics. 1965 Jul;36:21–39. [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Loda F. A., Clyde W. A., Jr, Senior R. J., Sheaffer C. I., Conley W. G., Denny F. W. Epidemiologic patterns of acute lower respiratory disease of children in a pediatric group practice. J Pediatr. 1971 Mar;78(3):397–406. doi: 10.1016/s0022-3476(71)80218-4. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Jr, Olmsted R. A., Prince G. A., Murphy B. R., Alling D. W., Walsh E. E., Collins P. L. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987 Oct;61(10):3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasel J. A., Frank A. L., Keitel W. A., Taber L. H., Glezen W. P. Acquisition of serum antibodies to specific viral glycoproteins of parainfluenza virus 3 in children. J Virol. 1984 Dec;52(3):828–832. doi: 10.1128/jvi.52.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Krause H. E., Mocega H. E., Dawson F. W. Viruses, Mycoplasma pneumoniae and bacteria associated with lower respiratory tract disease among infants. Am J Epidemiol. 1970 Feb;91(2):192–202. doi: 10.1093/oxfordjournals.aje.a121128. [DOI] [PubMed] [Google Scholar]
- Norrby E., Utter G., Orvell C., Appel M. J. Protection against canine distemper virus in dogs after immunization with isolated fusion protein. J Virol. 1986 May;58(2):536–541. doi: 10.1128/jvi.58.2.536-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A., Moss B., Murphy B. R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987 Jun;61(6):1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Brown V. E., Compans R. W. Glycoproteins of human parainfluenza virus type 3: characterization and evaluation as a subunit vaccine. J Infect Dis. 1985 Dec;152(6):1219–1230. doi: 10.1093/infdis/152.6.1219. [DOI] [PubMed] [Google Scholar]
- Ray R., Compans R. W. Glycoproteins of human parainfluenza virus type 3: affinity purification, antigenic characterization and reconstitution into lipid vesicles. J Gen Virol. 1987 Feb;68(Pt 2):409–418. doi: 10.1099/0022-1317-68-2-409. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Ball L. A., Young K. K., Furze J., Wertz G. W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986 Nov;60(2):607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Hall C. B., Briselli M., Brandriss M. W., Schlesinger J. J. Immunization with glycoprotein subunits of respiratory syncytial virus to protect cotton rats against viral infection. J Infect Dis. 1987 Jun;155(6):1198–1204. doi: 10.1093/infdis/155.6.1198. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Stott E. J., Young K. K., Anderson K., Ball L. A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987 Feb;61(2):293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Murphy B. R., Collins P. L., Lebacq-Verheyden A. M., Battey J. F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987 Oct;160(2):465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]